Abstract
Object: The purpose of this study was to determine whether supplementation with L-arginine, a substrate used in the production of nitric oxide, had an effect on adiponectin concentration in rats fed a high-fat diet. The influence of L-arginine on insulin resistance was also evaluated. Materials and methods: The experiment was performed using 36 Wistar rats divided into three groups: group 1 was fed a standard diet, group 2 a high-fat (HF) diet, group 3 a HF diet supplemented with L-arginine. After 42 days, serum levels of lipids, glucose, insulin, NO, and adiponectin were measured. Insulin resistance (IR) was estimated by the Homeostasis Model Assessment (HOMA). Results: Body mass was equal in all 3 groups, at the beginning as well as at the end of the study, however, in group 2 the amount of visceral fat was greater after 42 days. In group 3, there was a tendency for visceral fat to decrease. An increase in cholesterol, triglycerides, insulin and HOMA-IR, as well as a decrease in NO and adiponectin were seen in group 2, while in group 3, L-arginine supplementation ameliorated these disturbances. Conclusions: Our study shows that L-arginine supplementation in rats fed a HF diet is associated with an increase in insulin sensitivity. Our findings suggest that the underlying mechanism could be at least partially related to an increase in adiponectin concentration.
Keywords: High-fat (HF) diet, nitric oxide, L-arginine supplementation, insulin resistance, serum adiponectin
Introduction
During the past few decades, nitric oxide (NO) has been recognized as a messenger molecule, involved in the regulation of the cardiovascular system [1,2], the nervous system [3], and gene transcription [4]. NO acts as a neurotransmitter in the central and peripheral nervous systems and mediates physiological functions such as learning, memory and neurogenesis [3]. In the cardiovascular system, NO plays a central role in the control of vascular tone, cardiac contractility, regulation of arterial blood pressure and atherogenesis [1,5,6]. Consequently, a disturbance in NO production in the cardiovascular system may play a role as a pathogenic factor in many diseases. Hypertension, diabetes mellitus, hypercholesterolemia, hyperhomocysteinemia, kidney failure, and smoking are conditions that are associated with reduced NO biosynthesis [7-9].
The normal L-arginine plasma concentration is about 100 µmol/L, although the concentration sufficient enough to saturate endothelial NO synthase (eNOS) is much smaller, only about 3 µmol/L [10,11]. Supplementation of L-arginine in healthy subjects does not increase NO production [12]. Numerous scientific reports have shown positive results in terms of the efficacy and safety of L-arginine supplementation, especially in patients with diseases characterized by endothelial dysfunction [7-9]. There is evidence describing NO mediated effects associated with L-arginine supplementation, despite the fact that NOS is theoretically saturated at the physiological concentration of L-arginine, a phenomenon known as the L-arginine paradox [13]. The effect of L-arginine supplementation is an intensely explored topic. The influence of L-arginine on NO production and insulin resistance is also observed in our study.
Adiponectin is a cytokine predominantly produced and secreted by adipocytes. Although produced in adipose tissue, plasma adiponectin is paradoxically reduced in obese subjects [14]. In lean healthy individuals, circulating adiponectin level ranges from 2 to 30 mg/L [15]. Hypoadiponectinemia is observed in patients with diabetes mellitus, insulin resistance [16,17], coronary artery disease [18] and hypertension [19]. In contrast, in patients with chronic diseases, such as chronic heart failure [20] and chronic renal failure [21], an increased level of circulating adiponectin is observed. Adiponectin has been shown to have anti-inflammatory [22] and anti-fibrotic properties [23].
Some studies demonstrate that adiponectin can regulate NO production via eNOS and inducible NOS (iNOS). There are three different isoforms of the NOS enzyme which are able to generate NO in mammals; endothelial (eNOS), cytokine-inducible (iNOS) and neuronal (nNOS) [24]. The isoform eNOS results in NO release from the endothelium of blood vessels and causes vasodilation via cyclic guanosine monophosphate (cGMP) [25]. The expression of iNOS can be induced by bacteria, cytokines and other agents. This isoform produces large amounts of NO, which allows NO to react with superoxide (forming peroxynitrite), that in turn can lead to cell toxicity and death [26]. Endothelial cells have the capacity to express iNOS under some conditions, for example during an inflammatory state. Augmented myocardial iNOS expression is associated with chronic heart failure [24]. It has been found that iNOS markedly increases in adiponectin knockout rats after ischemia/reperfusion (I/R) injury, and that exogenous administration of adiponectin significantly reduces iNOS expression, but elevates eNOS activity [27]. Adiponectin exhibits a dual role in cardioprotection against I/R injury through stimulating eNOS and inhibiting iNOS [27,28]. The influence of adiponectin on NO production leads to the next question; whether or not supplementation of a substrate of NO can influence adiponectin levels in subjects with an elevated cardiovascular risk. The purpose of this study was to determine whether L-arginine had an effect on adiponectin concentration, and to evaluate the potential influence of L-arginine on insulin resistance in rats fed a high fat diet.
Materials and methods
The protocol of the study was approved by the local bioethical commission in Poznan (approval no. 20/2011). The animal experiment conforms to institutional standards.
Laboratory animals
The experiment was performed using 18 male and 18 female Wistar rats (8 weeks old, weighing 160 ± 12 g), purchased from the Department of Toxicology at the Medical University of Poznan, Poland. All rats were housed individually in carbonate cages in a temperature and humidity controlled environment, on a 12-hr light and 12-hr dark cycle, designed for the purpose of the study. The temperature inside the rat cages was 21 ± 2°C, relative humidity was 60 ± 5% and consecutive light-dark cycles lasted 12 hours. All rats were provided ad libitum diet and distilled water for 42 days. Food intake was measured daily and weight changes were monitored weekly. The rats were under veterinary supervision during the whole course of the experiment. Although individual housing may seem like a stressful habitat, all of the rats were subjected to the same conditions, and tests assessing the influence of stress on the rats were performed.
Experimental design
After a 5 day period of adaptation to laboratory conditions, the rats were randomly divided into three equal groups (male to female ratio 1:1):
Group 1 (control-K; n = 12): untreated rats, were allowed free access to a standard diet (Table 1).
Table 1.
Ingredient and nutrient composition of diets (grams per kilogram diet)
| Ingredient | Standard diet | High fat diet |
|---|---|---|
| Casein | 140 | 140 |
| Wheat starch | 625 | 430 |
| Sucrose | 100 | 100 |
| Potato starch | 50 | 50 |
| Vitamin mixture* | 10 | 10 |
| Mineral mixture** | 35 | 35 |
| Sunflower oil | 40 | 40 |
| Lard | - | 160 |
| Sodium chloride | - | 35 |
| Total energy (kcal/100 g diet) | 420 | 515 |
| Total protein (% of energy) | 18 | 18 |
| Total fat (% of energy) | 9 | 39 |
Composition of vitamin mixture (g/kg mix): nicotinic acid (3), Ca pantothenate (1.6), pyridoxine (0.7), thiamin (0.6), riboflavin (0.6), folic acid (0.2), biotin (0.02), vitamin B12 (0.003); vitamin E (500 IU/g), vitamin A (500,000 IU/g), vitamin D3 (400,000 IU/g), vitamin K1 (0.08), choline bitartrate (200), powdered sucrose (777.15).
Composition of mineral mixture (g/kg mix): calcium carbonate (357), potassium phosphate monobasic (250), potassium citrate (28), sodium chloride (74), potassium sulfate (46.6), magnesium oxide (24), ferric citrate (6.06), zinc carbonate (1.65), sodium meta-silicate×9H2O (1.45), manganous carbonate (0.64), cupric carbonate (0.30), chromium chloride×6H2O (0.147), boric acid (0.0816), sodium fluoride (0.0635), nickel chloride×6H2O (0.0578), lithium sulfate×H2O (0.0263), sodium selenate anhydrous (0.0103), potassium iodate (0.010), ammonium paramolybdate×4H2O (0.0795), ammonium vanadate (0.066), powdered sucrose (209.758).
Group 2 (high-fat diet-F; n = 12): were fed a high-fat diet.
Group 3 (arginine group-A; n = 12): were fed a high-fat diet supplemented with L-arginine (Curtis Healthcare, Warsaw, Poland) 20 g/kg diet; the appropriate value of wheat starch was reduced in the diet. Arginine is generally safe and non-toxic when used as a dietary supplement [29]. There is some evidence that suggests that an excess of arginine, due to an amino acid imbalance, can cause severe adverse effects, such as impaired growth and even death [30].
The rats were fed either a standard semisynthetic diet based on the AIN-93M diet [31] or a high-fat (HF) diet with modified amounts of fat and sodium chloride. The full composition of the diets is presented in Table 1.
Collection of material
At the end of the experiment (day 42), having been starved for 16 hours, the rats were weighed, anesthetized by an intraperitoneal injection of thiopental (40 mg/kg body mass) and killed by cardiac puncture. The blood samples were collected in serum separation tubes, and subsequently used for biochemical studies. The coagulated blood was left to clot at room temperature for 30 min, next it was centrifuged for 15 min at 2,500 r.p.m. at a temperature of 4°C; and finally the supernatant fluid was separated. Serum samples used for analysis were stored at -70°C. Visceral perirenal fat was dissected and weighed.
Examined parameters
Selected nutritional parameters in rats
In all rats, weight gain and the absolute and relative weight of visceral fat was determined. The relative weight of visceral fat was defined as the percentage of body weight.
Laboratory measurements
Serum lipid profiles, including total cholesterol (TCH) and triglycerides (TG) were measured by an enzymatic method (Siemens Healthcare Diagnostics Inc., Erlangen, German). Serum fasting glucose concentration was analyzed by an enzymatic method involving hexokinase and glucose-6-phosphate dehydrogenase (Siemens Healthcare Diagnostics Inc., Erlangen, Germany).
The NO concentration in serum was determined by means of a spectrophotometric method using a testing set from Oxis (Oxis International Inc., Foster City, USA).
The plasma level of adiponectin was determined by an enzyme-linked immunoassay, in strict accordance with the manufacturer’s instructions (R&D Systems, Minneapolis, USA).
The plasma concentration of insulin was determined by an enzyme-linked immunoassay, also in strict accordance with the manufacturer’s instructions (Demenditec Diagnostic GmbH, Kiel-Wellsee, Germany).
Insulin resistance was estimated by the Homeostasis Model Assessment (HOMA), according to the formula: Insulin resistance index = fasting insulin (μg/L) × fasting glucose (mg/dl)/405.
Statistical analysis
Results are shown as mean ± SEM. Continuous variables were assessed for normal distribution with the use of the Shapiro-Wilk test. Differences between means of three groups for diet intake, initial body mass, weight gain, visceral body fat, TCH, TG, glucose, insulin, HOMA-IR, NO, adiponectin were analyzed by One-way ANOVA followed by Tukey’s post hoc test. A value of P<0.05 was regarded as a significant. Statistics were performed with STATISTICA version 10 (data analysis software system), StatSoft, Inc. (www.statsoft.com).
Results
The average dietary intake, initial body weight, and weight gain were comparable in all groups (Table 2). The absolute and relative mass of visceral fat (as percentage of body mass) was significantly higher in rats fed a HF diet, as compared to the control group. In rats fed a HF diet supplemented with L-arginine, a tendency towards a decrease in visceral fat was observed.
Table 2.
Effects of dietary L-arginine on initial body mass, weight gain and visceral fat tissue
| Groups | Parameters | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| n | Dietary intake (g/day) | Initial body mass (g) | Weight gain (g) | Visceral body fat (g)/percentage of body mass (%) | ||
| Group 1 | control | 12 | 21.5 ± 1.8 | 190.1 ± 12.2 | 153.5 ± 17.8 | 3.0 ± 0.2/0.84a |
| Group 2 | high fat diet | 12 | 20.5 ± 1.2 | 188.8 ± 12.8 | 157.2 ± 19.2 | 3.5 ± 0.2/1.05b |
| Group 3 | arginine | 12 | 20.8 ± 1.5 | 190.7 ± 12.5 | 142.5 ± 20.1 | 3.1 ± 0.3/0.9a,b |
Values are calculated as means ± SEM for twelve rats. Means in the same column with different superscripts are significantly different (P<0.05).
The results presented in Table 3 demonstrate that six weeks of a HF diet resulted in a statistically significant increase (P<0.05) in TCH and TG, while L-arginine supplementation significantly ameliorated the increase in both TCH and TG.
Table 3.
Effects of dietary L-arginine on total cholesterol concentration (TCH), triglycerides (TG) and glucose (Glu) concentration in high fat diet rats
| Groups | Parameters | |||
|---|---|---|---|---|
|
| ||||
| TCH (mg/dL) | TG (mg/dL) | Glu (mg/dL) | ||
| Group 1 | control | 65.2 ± 5.7a | 35.2 ± 8.1a | 102.2 ± 13.0 |
| Group 2 | High-fat diet | 73.6 ± 8.0b | 43.6 ± 7.2b | 106.0 ± 13.5 |
| Group 3 | arginine | 67.2 ± 9.8a,b | 33.2 ± 6.7a | 100.7 ± 10.9 |
Values are calculated as means ± SEM for twelve rats. Means in the same column with different superscripts are significantly different (P<0.05).
From the results in Figures 1, 2, it is clear that there was a significant increase (P<0.05) in serum insulin and HOMA-IR level in rats fed a HF diet. L-arginine supplementation partially restored the insulin concentration and HOMA-IR to control levels.
Figure 1.
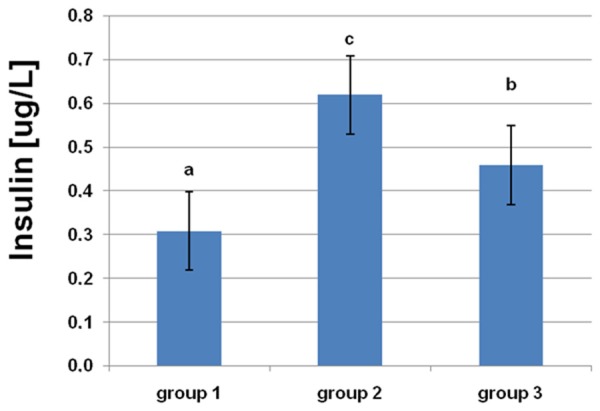
Effects of dietary L-arginine supplementation on serum insulin levels. Means with different superscripts (a-c) are significantly different (P<0.05).
Figure 2.
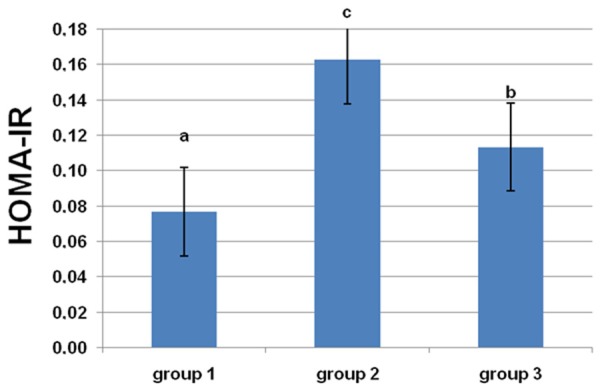
Effects of dietary L-arginine supplementation on insulin resistance index. Means with different superscripts (a-c) are significantly different (P<0.05).
From the results in Figures 3, 4, it is apparent that there were significant decreases in NO and adiponectin concentrations in rats fed a HF diet. L-arginine supplementation protected against a decrease in adiponectin and significantly increased the concentration of NO.
Figure 3.
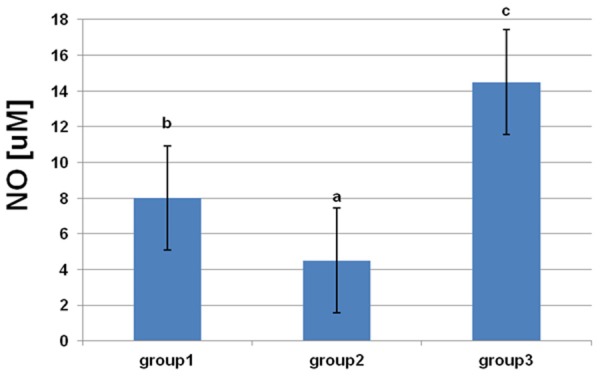
Effects of dietary L-arginine supplementation on serum nitric oxide levels. Means with different superscripts (a-c) are significantly different (P<0.05).
Figure 4.
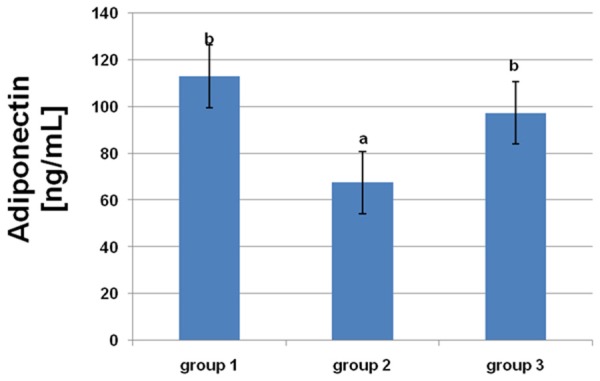
Effects of dietary L-arginine supplementation on serum adiponectin levels. Means with different superscripts (a, b) are significantly different (P<0.05).
Discussion
The results of our study confirm the favorable effect of L-arginine on the level of insulin resistance. The significant impact of L-arginine supplementation on adiponectin concentration, in rats fed a HF diet, is a new finding demonstrated by our study.
The study provided a useful model of environmentally induced visceral obesity. In our trial, the rats fed a HF diet did not gain any more weight than the rats fed the standard diet; although the duration of the study was most likely too brief to be able to reveal significant differences between the two groups. After 42 days of consuming a different diet, despite the similarities in bodyweight, changes in body composition were observed. Rats fed a HF diet had a significantly higher absolute and relative mass of visceral fat as compared to the control group. The results of our study demonstrated that the short interval of being fed a HF diet was responsible for an increase in visceral fat, even though it did not affect body weight. Surprisingly, in rats on a HF diet supplemented with L-arginine, a tendency towards a decrease in visceral fat was observed. Some previous studies have shown that L-arginine therapy decreases white fat mass in obese rats [32].
Many studies have shown that the amount of visceral fat is closely associated with the level of serum insulin and is linked to many metabolic defects with an underlying mechanism related to insulin resistance. In support of this view, serum insulin and HOMA-IR levels were found to be higher in the rats fed a HF diet, than in those from the control group which were fed the standard diet. L-arginine supplementation partially restored the insulin concentration and HOMA-IR to control levels. The beneficial effects of dietary L-arginine supplementation on both insulin and HOMA-IR may result from increased insulin sensitivity. The potential influence of L-arginine on insulin resistance is still being explored, heretofore the results of several studies on this topic are inconsistent and generally inconclusive [33-37]. The beneficial influence of L-arginine therapy on insulin sensitivity has been described in several animal and human trials. A study by Bogdański [38] evaluated insulin sensitivity in obese patients after 6 months of L-arginine supplementation using the hyperinsulinemic-euglycemic clamp technique. It was found that six months of L-arginine treatment significantly increased the M value and decreased the insulin concentration, whereas no changes in the M value or the insulin level were observed in the placebo group in the aforementioned trial.
It seems that NO production is strictly associated with insulin resistance [39]. The underlying molecular mechanisms are still being examined. In our study, the results suggest that one of the potential mechanisms responsible for the favorable effect of L-arginine supplementation on insulin resistance could be the increase in adiponectin concentration. We found that L-arginine is an effective stimulus for adiponectin release. Serum adiponectin levels correlate negatively with body mass index (BMI) and hypoadiponectinemia is a typical feature of obesity [14]. In many trials, a significant increase in serum adiponectin level was observed following weight reduction [40]. In our study, the influence of the change in body weight on the level of adiponectin was excluded. In all three groups of rats, we found different levels of serum adiponectin, whereas the body weight was similar in all groups. Rats fed a HF diet had lower adiponectin levels than those fed the standard diet. L-arginine supplementation in rats fed a HF diet was effective in increasing both adiponectin and NO concentrations. Some trials suggest a close relationship between endothelial function and adiponectin release. A defect in NO bioavailability associated with increased endothelin-1 activity is observed in patients with metabolic syndrome [41,42]. Hattori [43] examined the mechanism by which adiponectin levels were reduced in rats, noting that chronic inhibition of NO synthesis by N-omega-nitro-L-arginine-methyl ester (L-NAME) in rats induced a state of hypoadiponectinemia. Adiponectin mRNA level was also reduced. Treatment with peroxisome proliferator-activated receptor gamma (PPARγ) agonist (pioglitazone), increases adiponectin levels in L-NAME treated rats, most likely by means of transcriptional activation of the adiponectin gene promoter in adipose tissue via the PPAR response element (PPRE) [44]. Activation of angiotensin II and angiotensin converting enzyme (ACE) has also been demonstrated in L-NAME treated rats, suggesting a pathogenic role of oxidative stress in hypoadiponectinemia. A study by Hattori [43] has confirmed that oxidative stress in rat adipose tissue is mediated by xanthine oxidase. In line with these findings, they suggest that NO may modulate adiponectin release. Adiponectin stimulates phosphorylation of adenosine monophosphate-activated protein kinase (AMPK), which results in downstream phosphorylation of eNOS in the ischemic/reperfused myocardium. Phosphorylation of eNOS at residue Ser1177 is associated with increased enzyme activity and increased production of NO [32]. The observations made by Gonon [28] suggest that the cardioprotective effect of adiponectin is mediated via NO by phosphorylation of eNOS.
We found abnormal lipid profiles in the rats fed the HF diet, which is a typical consequence of a HF diet and insulin resistance. The critical role of insulin resistance in the pathogenesis of lipid disorders is well-documented. Our results show the positive influence of L-arginine on the concentrations of total cholesterol and triglycerides. Similar results were obtained in other experimental and clinical studies [45,46]. It has been suggested that the hypolipemic effect of L-arginine is associated, at least in part, with an increase in NO level and a decrease in fatty acid oxidation [47]. The results of the study by Tan [48] indicate that L-arginine regulates the expression of lipid-metabolic genes in skeletal muscle and in white adipose tissue, therefore favoring lipogenesis in muscle along with lipolysis in adipose tissue.
A limitation of the present study is the relatively small number of studied rats, nevertheless, we have reached our goal of demonstrating a statistically significant increase in insulin, and HOMA-IR, as well as a decrease in adiponectin and NO in rats fed a HF diet. The results were also statistically significant in rats fed a HF diet combined with L-arginine, demonstrating the positive effects of L-arginine supplementation. In a study consisting of a larger group of rats, the test subjects could be divided by gender and analyzed separately to avoid the influence of estrous cycles on metabolism. In our study, male and female rats were not analyzed separately; but in all studied subgroups, both genders were represented equally. A further limitation of our study is the inability to fully determine the level of insulin sensitivity. We assessed the serum insulin and glucose concentration and calculated HOMA-IR. However, the results should also be evaluated by the test-clamp technique, oral glucose tolerance test, and tissue analysis.
In conclusion, our study shows that L-arginine supplementation in rats fed a HF diet is associated with improvement in insulin sensitivity. Our findings suggest that the underlying mechanism could at least be partly related to an increased concentration of adiponectin.
Disclosure of conflict of interest
None.
References
- 1.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 2.Llorens S, Jordan J, Nava E. The nitric oxide pathway in the cardiovascular system. J Physiol Biochem. 2002;58:179–188. doi: 10.1007/BF03179855. [DOI] [PubMed] [Google Scholar]
- 3.Zhou L, Zhu DY. Neuronal nitric oxide synthase: structure, subcellular localization, regulation, and clinical implications. Nitric Oxide. 2009;20:223–230. doi: 10.1016/j.niox.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Gudi T, Hong GK, Vaandrager AB, Lohmann SM, Pilz RB. Nitric oxide and cGMP regulate gene expression in neuronal and glial cells by activating type II cGMP-dependent protein kinase. FASEB J. 1999;13:2143–2152. [PubMed] [Google Scholar]
- 5.Alheid U, Frolich JC, Forstermann U. Endothelium-derived relaxing factor from cultured human endothelial cells inhibits aggregation of human platelets. Thromb Res. 1987;47:561–571. doi: 10.1016/0049-3848(87)90361-6. [DOI] [PubMed] [Google Scholar]
- 6.Arndt H, Smith CW, Granger DN. Leukocyte-endothelial cell adhesion in spontaneously hypertensive and normotensive rats. Hypertension. 1993;21:667–673. doi: 10.1161/01.hyp.21.5.667. [DOI] [PubMed] [Google Scholar]
- 7.Drexler H, Zeiher AM, Meinzer K, Just H. Correction of endothelial dysfunction in coronary microcirculation of hypercholesterolaemic patients by L-arginine. Lancet. 1991;338:1546–1550. doi: 10.1016/0140-6736(91)92372-9. [DOI] [PubMed] [Google Scholar]
- 8.Imaizumi T, Hirooka Y, Masaki H, Harada S, Momohara M, Tagawa T, Takeshita A. Effects of L-arginine on forearm vessels and responses to acetylcholine. Hypertension. 1992;20:511–517. doi: 10.1161/01.hyp.20.4.511. [DOI] [PubMed] [Google Scholar]
- 9.Pieper G, Siebeneich W, Dondlinger L. Short-term oral administration of L-arginine reverses defective endothelium-dependent relaxation and cGMP generation in diabetes. Eur J Pharmacol. 1996;317:317–320. doi: 10.1016/s0014-2999(96)00831-x. [DOI] [PubMed] [Google Scholar]
- 10.Closs EI, Scheld JS, Sharafi M, Forstermann U. Substrate supply for nitric-oxide synthase in macrophages and endothelial cells: role of cationic amino-acid transporters. Mol Pharmacol. 2000;57:68–74. [PubMed] [Google Scholar]
- 11.Pollock JS, Forstermann U, Mitchell JA, Warner TD, Schmidt HH, Nakane M, Murad F. Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc Natl Acad Sci U S A. 1991;88:10480–10484. doi: 10.1073/pnas.88.23.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alvares TS, Conte-Junior CA, Silva JT, Paschoalin VM. Acute L-arginine supplementation does not increase nitric oxide production in Healthy subjects. Nutr Metab (Lond) 2012;9:54. doi: 10.1186/1743-7075-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bode-Boger SM, Scalera F, Ignarro LJ. The L-arginine paradox: importance of the L-arginine/asymmetrical dimethylarginine ratio. Pharmacol Ther. 2007;114:295–306. doi: 10.1016/j.pharmthera.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Maraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 15.Shimada K, Miyazaki T, Daida H. Adiponectin and atherosclerotic disease. Clin Chim Acta. 2004;344:1–12. doi: 10.1016/j.cccn.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 16.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda N, Nishida M, Kihara S, Sakai N, Nakaijma T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T, Matsuzawa Y. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 17.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 18.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 19.Iwashima Y, Katsuya T, Ishikawa K, Ouchi N, Ohishi M, Sugimoto K, Fu Y, Motone M, Yamamoto K, Matsuo A, Ohashi K, Kihara S, Funahashi T, Rakugi H, Matsuzawa Y, Ogihara T. Hypoadiponectinemia is an independent risk factor for hypertension. Hypertension. 2004;43:1318–1323. doi: 10.1161/01.HYP.0000129281.03801.4b. [DOI] [PubMed] [Google Scholar]
- 20.Kistorp C, Faber J, Galatius S, Gustafsson F, Frystyk J, Flyvbjerg A, Hildebrandt P. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005;112:1756–1762. doi: 10.1161/CIRCULATIONAHA.104.530972. [DOI] [PubMed] [Google Scholar]
- 21.Iwashima Y, Horio T, Kumada M, Suzuki Y, Kihara S, Rakugi H, Kawano Y, Funahashi T, Ogihara T. Adiponectin and renal function, and implication as a risk of cardiovascular disease. Am J Cardiol. 2006;98:1603–1608. doi: 10.1016/j.amjcard.2006.07.039. [DOI] [PubMed] [Google Scholar]
- 22.Kumada M, Kihara S, Ouchi N, Kobayashi H, Okamoto Y, Ohashi K, Maeda K, Nagaretani H, Kishida K, Maeda N, Nagasawa A, Funahashi T, Matsuzawa Y. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation. 2004;109:2046–2049. doi: 10.1161/01.CIR.0000127953.98131.ED. [DOI] [PubMed] [Google Scholar]
- 23.Fujita K, Maeda N, Sonoda M, Ohashi K, Hibuse T, Nishizawa H, Nishida M, Hiuge A, Kurata A, Kihara S, Shimomura I, Funahashi T. Adiponectin protects against angiotensin II-induced cardiac fibrosis through activation of PPAR-alpha. Arterioscler Thromb Vasc Biol. 2008;28:863–870. doi: 10.1161/ATVBAHA.107.156687. [DOI] [PubMed] [Google Scholar]
- 24.Dobutović B, Smiljanić K, Soskić S, Düngen HD, Isenović ER. Nitric oxide and its role in cardiovascular diseases. The Open Nitric Oxide Journal. 2011;3:65–71. [Google Scholar]
- 25.Bourgoin F, Bachelard H, Badeau M, Melancon S, Pitre M, Lariviere R, Nadeau A. Endothelial and vascular dysfunctions and insulin resistance in rats fed a high-fat, high-sucrose diet. Am J Physiol Heart Circ Physiol. 2008;295:H1044–1055. doi: 10.1152/ajpheart.00516.2008. [DOI] [PubMed] [Google Scholar]
- 26.Shaker O, Ghallab Na, Hamdy E, Sayed S. Inducible nitric oxide synthase (iNOS) in gingivial tissues of chronic peridontitis with and without diabetes immunohistochemistry and RT-PCR study. Arch Oral Biol. 2013;58:1397–1406. doi: 10.1016/j.archoralbio.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Tao L, Gao E, Jiao X, Yuan Y, Li S, Christopher TA, Lopez BL, Koch W, Chan L, Goldstein BJ, Ma XL. Adiponectin cardioprotection after myocardial ischemia/reperfusion involves the reduction of oxidative/nitrative stress. Circulation. 2007;115:1408–1416. doi: 10.1161/CIRCULATIONAHA.106.666941. [DOI] [PubMed] [Google Scholar]
- 28.Gonon AT, Widegren U, Bulhak A, Salehzadeh F, Persson J, Sjoquist PO, Pernow J. Adiponectin protects against myocardial ischaemia-reperfusion injury via AMP-activated protein kinase, Akt and nitric oxide. Cardiovasc Res. 2008;78:116–122. doi: 10.1093/cvr/cvn017. [DOI] [PubMed] [Google Scholar]
- 29.Flynn NE, Meininger CJ, Haynes TE, Wu G. The metabolic basis of arginine nutrition and pharmacotherapy. Biomed Pharmacother. 2002;56:427–438. doi: 10.1016/s0753-3322(02)00273-1. [DOI] [PubMed] [Google Scholar]
- 30.Edmonds MS, Gonyou HW, Baker DH. Effect of excess levels of methionine, tryptophan, arginine, lysine or threonine on growth and dietary choice in the pig. J Anim Sci. 1987;65:179–185. doi: 10.2527/jas1987.651179x. [DOI] [PubMed] [Google Scholar]
- 31.Reeves PG. Components of the AIN-93 diets as improvements in the AIN-76A diet. J Nutr. 1997;127:838S–841S. doi: 10.1093/jn/127.5.838S. [DOI] [PubMed] [Google Scholar]
- 32.Jobgen W, Meininger CJ, Jobgen SC, Li P, Lee MJ, Smith SB, Spencer TE, Fried SK, Wu G. Dietary L-arginine supplementation reduces white fat gain and enhances skeletal muscle and brown fat masses in diet-induces obese rats. J Nutr. 2009;139:230–237. doi: 10.3945/jn.108.096362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bogdański P, Suliburska J, Grabańska K, Musialik K, Cieślewicz A, Skołuda A, Jabłecka A. Effect of 3 months L-arginine supplementation on insulin resistance and tumor necrosis factor activity in patients with visceral obesity. Eur Rev Med Pharmacol Sci. 2012;16:816–823. [PubMed] [Google Scholar]
- 34.De Castro Barbosa T, Lourenço Poyares L, Fabres Machado U, Nunes MT. Chronic oral administration of arginine induces GH gene expression and insulin resistance. Life Sci. 2006;79:1444–1449. doi: 10.1016/j.lfs.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Kawano T, Nomura M, Nisikado A, Nakaya Y, Ito S. Supplementation of L-arginine improves hypertension and lipid metabolism but not insulin resistance in diabetic rats. Life Sci. 2003;73:3017–3026. doi: 10.1016/j.lfs.2003.06.004. [DOI] [PubMed] [Google Scholar]
- 36.Lucotti P, Setola E, Monti LD, Galluccio E, Costa S, Sandoli EP, Fermo I, Rabaiotti G, Gatti R, Piatti P. Beneficial effects of a long-term oral L-arginine treatment added to a hypocaloric diet and exercise training program in obese, insulin-resistant type 2 diabetic patients. Am J Physiol Endocrinol Metab. 2006;291:E906–12. doi: 10.1152/ajpendo.00002.2006. [DOI] [PubMed] [Google Scholar]
- 37.Lucotti P, Monti L, Setola E, La Canna G, Castiglioni A, Rossodivita A, Pala Mg, Formica F, Paolini G, Catapano Al, Bosi E, Alfieri O, Piatti P. Oral L-arginine supplementation improves endothelial function and ameliorates insulin sensitivity and inflammation in cardiopathic nondiabetic patients after an aortocoronary bypass. Metabolism. 2009;58:1270–1276. doi: 10.1016/j.metabol.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 38.Bogdanski P, Szulinska M, Suliburska J, Pupek-Musialik D, Jablecka A, Witmanowski H. Supplementation with L-arginine favorably influences plasminogen activator inhibitor type 1 concentration in obese patients. A randomized, double blind trial. J Endocrinol Invest. 2013;36:221–226. doi: 10.3275/8467. [DOI] [PubMed] [Google Scholar]
- 39.Claybaugh T, Decker S, Mcall K, Slyvka Y, Steimle J, Wood A, Schaefer M, Thuma J, Inman S. L-arginine supplementation in type II diabetic rats preserves renal function and improves insulin sensitivity by altering the nitric oxide pathway. Int J Endoc. 2014;2014:171–546. doi: 10.1155/2014/171546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang WS, Lee WJ, Funahashi T, Tanaka S, Matsuzawa Y, Chao CL, Chen CL, Tai TY, Chuang LM. Weight reduction increases plasma levels of an adipose-derived anti-inflammatory protein, adiponectin. J Clin Endocrinol Metab. 2001;86:3815–3819. doi: 10.1210/jcem.86.8.7741. [DOI] [PubMed] [Google Scholar]
- 41.Cardillo C, Campia U, Bryant MB, Panza JA. Increased activity of endogenous endothelin in patients with type II diabetes mellitus. Circulation. 2002;106:1783–1787. doi: 10.1161/01.cir.0000032260.01569.64. [DOI] [PubMed] [Google Scholar]
- 42.Piatti PM, Monti LD, Zavaroni I, Valsecchi G, Van Phan C, Costa S, Conti M, Sandoli Ep, Solerte B, Pozza G, Pontiroli AE, Reaven G. Alterations in nitric oxide/cyclic-GMP pathway in nondiabetic siblings of patients with type 2 diabetes. J Clin Endocrinol Metab. 2000;85:2416–2420. doi: 10.1210/jcem.85.7.6667. [DOI] [PubMed] [Google Scholar]
- 43.Hattori S, Hattori Y, Kasai K. Hypoadiponectinemia is caused by chronic blockade of nitric oxide synthesis in rats. Metabolism. 2005;54:482–487. doi: 10.1016/j.metabol.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 44.Iwaki M, Matsuda M, Maeda N, Funahashi T, Matsuzawa Y, Makishima M, Shimomura I. Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes. 2003;52:1655–1663. doi: 10.2337/diabetes.52.7.1655. [DOI] [PubMed] [Google Scholar]
- 45.Míguez I, Mariño G, Rodríguez B, Taboada C. Effects of dietary L-arginine supplementation on serum lipids and intestinal enzyme activities in diabetic rats. J Physiol Biochem. 2004;60:31–37. doi: 10.1007/BF03168218. [DOI] [PubMed] [Google Scholar]
- 46.Saleh AI, Abdel Maksoud SM, El-Maraghy SA, Gad MZ. Protective effect of L-arginine in experimentally induced myocardial ischemia: comparison with aspirin. J Cardiovasc Pharmacol Ther. 2011;16:53–62. doi: 10.1177/1074248410378506. [DOI] [PubMed] [Google Scholar]
- 47.Tripathi P, Misra MK. Therapeutic role of L-arginine on free radical scavenging system in ischemic heart diseases. Indian J Biochem Biophys. 2009;46:498–502. [PubMed] [Google Scholar]
- 48.Tan B, Yin Y, Liu Z, Tang W, Xu H, Kong X, Li X, Yao K, Gu W, Smith SB, Wu G. Dietary L-arginine supplementation differentially regulates expression of lipid-metabolic genes in porcine adipose tissue and skeletal muscle. J Nutr Biochem. 2011;22:441–445. doi: 10.1016/j.jnutbio.2010.03.012. [DOI] [PubMed] [Google Scholar]


