Abstract
Transarterial chemoembolization (TACE) plus percutaneous ethanol injection (PEI) have been used for patients with unresectable hepatocellular carcinoma (HCC). However, whether the combination therapy of TACE plus PEI is better than TACE or PEI alone in the treatment of HCC remains controversial. Thus, we conducted this meta-analysis to assess the efficacy of combined therapy for unresectable HCC compared with that of TACE or PEI alone. Randomized controlled trials (RCTs) published from Pubmed, Embase, Web Of Science, Chinese Biomedical Literature database (SinoMed), China National Knowledge Infrastructure (CNKI), and Wanfang database, were systematically reviewed to assess the survival benefits and tumor recurrence for HCC patients treated with TACE plus PEI. Pooled risk ratio (RR) with 95% confidence intervals (95% CIs) for survival rate and tumor recurrence rate were calculated using a random-effects or fixed-effects model, depending on the heterogeneity between the included studies. 19 RCTs met the inclusion criteria were included in this meta-analysis with a total number of 1948 patients. The pooled results showed that the combination therapy of TACE plus PEI significantly improved 1, 2, 3-year survival rate [RR1-year = 1.24, 95% CI: 1.17-1.31, P = 0.000; RR2-year = 1.64, 95% CI: 1.44-1.87, P = 0.000; RR3-year = 2.27, 95% CI: 1.93-2.67, P = 0.000] compared with that of TACE or PEI alone. The local tumor recurrence rate in HCC patients treated with TACE plus PEI was lower than that of monotherapy (RR = 0.53, 95% CI: 0.29-0.96; P = 0.035). The combined therapy of TACE with PEI also significantly reduced the AFP level (RR = 1.40, 95% CI: 1.19-1.66, P = 0.000) and tumor size (>50%) (RR = 1.61, 95% CI: 1.40-1.85, P = 0.000). This meta-analysis confirms the benefits of TACE + PEI in the treatment of unresectable HCC, with an improvement in survival rate, and a reduction in local tumor recurrence, AFP level, and tumor size.
Keywords: Transarterial chemoembolization, percutaneous ethanol injection, hepatocellular carcinoma, meta-analysis
Introduction
The incidence of hepatocellular carcinoma (HCC) has increased dramatically in the past decade, making HCC the sixth most common cancer in the world currently [1]. Although more patients with HCC are being diagnosed at an earlier period by surveillance, most of them are at intermediate or advanced stages [2-4]. About 30% of patients with early-stage may benefit from curative therapies, such as surgical resection, liver transplantation [5,6]. Moreover, for patients with large lesions or live dysfunction, surgical resection is not recommend as the first-treatment choice, whereas the palliative care, including transcatheter arterial chemoembolization (TACE) and percutaneous ethanol injection (PEI), are widely used for HCC patients to relieve suffering and improve quality of life. TACE has been proved to prolong the survival in intermediate-stage HCC patients, especially for those with large and multiple lesions [7]. And, PEI as a thermal in situ destruction technique has been shown to be more effective in the treatment of small HCC patients [8,9].
Accumulating evidence suggests that combined treatment of TACE and PEI may play a synergistic effect in HCC patients, especially for larger lesions that do not respond adequately to either procedure alone. This survival benefit of combination therapy have been observed in one clinical trial conducted by Carlo Bartolozzi [10]. However, in another study assessing the effectiveness of TACE plus PEI compared with TACE alone, the over survival in both arms showed no statistically difference, indicating conflicting reports [11]. We therefore conducted this meta-analysis to compare the efficacy of combination therapy of TACE plus PEI with TACE monotherapy for the treatment of patients with HCC.
Material and methods
Literature search
We performed a comprehensive search to identify studies focusing on the treatment benefits of TACE plus with PEI in HCC patients. Database, including Pubmed, Embase, Web Of Science, Chinese Biomedical Literature (SinoMed), China National Knowledge Infrastructure (CNKI), and Wanfang, were searched up to January 19, 2015, using “transcatheter arterial chemoembolization” or “TACE”, “percutaneous ethanol injection” or “PEI”, “hepatocellular carcinoma” or “primary liver carcinoma”, “randomized controlled trials”. There were no language restrictions in the search strategy. We also manually searched the reference lists of published articles and reviews to complete the database. When multiple articles for one single trial were present, we chose the latest articles, or the one with most complete data.
Study inclusion and exclusion criteria
The studies were reviewed by two independent investigators. We identified those studies that met the following inclusion criteria: (1) each trial was conducted by a randomized controlled method; (2) the HCC patients were treated with TACE and/or PEI; (3) available data of efficacy were presented (survival rate, tumor recurrence rate). These studies were excluded if they met the following exclusion criteria: (1) non-randomized controlled studies; (2) not scheduled to apply any of the two treatment procedures (TACE and PEI); (3) no outcome data of interest were provided. We also exclude abstracts, reviews and meta-analysis without original data, and case reports.
Review strategy
Endnote bibliographic software was used to create an electronic library of citations indentified in the literature searches. The searches of Pubmed, Embase and Web of Science, SinoMed, CNKI, and Wanfang were performed by using of Endnote. After the duplicate records were deleted, two independent investigators trained to perform the abstract review and thereafter full text review. Any disagreements between two investigators were resolved through consensus and discussion. Data were extracted using a standardized data extraction form. Two investigators independently extracted the following information from the studies included: the first author, patients’ age, sample size (TACE-PEI, TACE), region, male/female, baseline characteristics, the number of survived patients in both TACE-PEI group and TACE group, the number of patients with tumor recurrence in TACE + PEI group and monotherapy group.
Quality assessment
The quality of included studies was scored by two independent investigators using validated Jadad 5 point scale. The scale consists of three items describing randomization (0-2 points), blinding (0-2 points), and dropouts and withdrawals (0-1 point) in the report of randomized controlled trial [12]. A score of one is given for each of the points described. A further point is obtained where the method of randomization and/or blinding is given and is appropriate, and a point is deducted where it is not appropriate. The quality scale ranges from 0 to 5 points. Higher scores indicate better reporting. Articles with ≥3 score are said to be of high quality [13].
Statistical analysis
We assessed the overall efficacy of TACE plus PEI in the treatment of HCC patients based on the data from the studies included. For the dichotomous variables (survival rate, recurrence rate, decrease rates of AFP level and tumor size), the number of survival patients and total number of patients were extracted from the included studies. Thereafter, the risk ratio (RR) with 95% confidence intervals (CIs) were calculated. I 2 statistic was used to test the heterogeneity between the studies. Studies with an I 2 value of 25% to 50% were considered to have low heterogeneity; those with an I 2 value of 50% to 75% were considered to have moderate heterogeneity, and those with an I 2 value of larger than 75% were considered to have high heterogeneity [14]. Pooled estimates of RR was calculated using a fixed-effects model (Mantel-Haenszel method) [15] or random-effects model (DerSimonian-Laird method) [16] depending on the significant heterogeneity (I 2>10%). The presence of publication bias was evaluated by using the Begg and Egger tests [17,18]. A P value less than 0.05 was considered statistically significant. All statistical analyses were conducted by using of STATA software version 12.0 (Stata Corporation, College Station, TX, USA).
Results
Identification of eligible studies
The initial search identified 514 relevant publications from Pubmed, Embase, Web of Science, SinoMed, CNKI and Wanfang. 85 articles were excluded for duplicate records, and 410 were excluded for various reasons in the process of abstract and full text screening (Figure 1). Finally 19 studies with a total number of 1948 that met the inclusion criteria were included in this meta-analysis [10,11,19-35].
Figure 1.
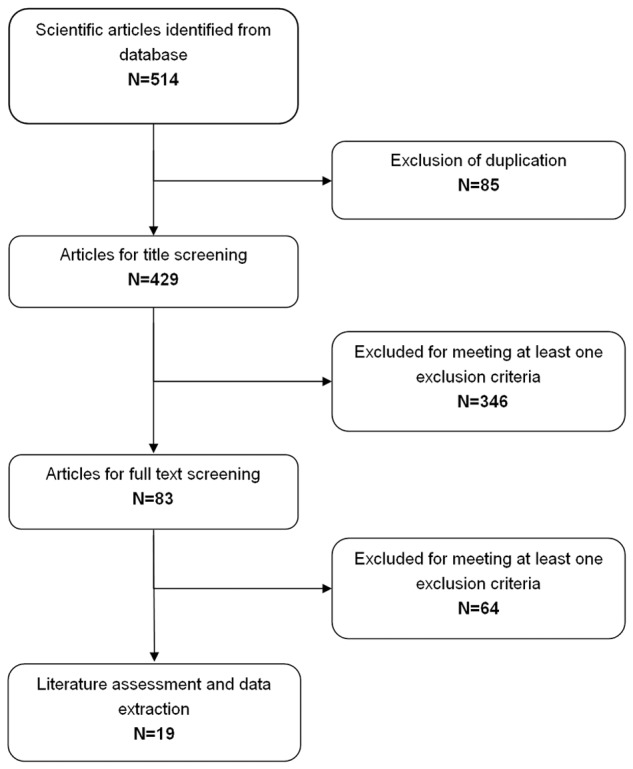
Identification of eligible randomized-controlled trials.
Characteristics, quality of eligible studies
The main characteristics of the 19 randomized controlled trials (RCTs) included in this meta-analysis are described in Table 1. Of the 19 studies, two were performed in Italy [10,20], one in Germany [11], and the left sixteen studies were conducted in China [19,21-35]. The sample size of the RCTs ranged from 45 to 655. All trials included both men and women. Follow-up ranged from 8 to 39 months. All patients at first received TACE treatment, and then those assigned to TACE-PEI group additionally received PEI treatment 10 to 20 days after TACE. Of the 19 studies, three RCTs were conducted to compare with TACE-EPI with PEI alone [25,27,30], and the remaining 16 RCTs were conducted to compare TACE-PEI with TACE alone [10,11,19-24,26,28,29,31-35].
Table 1.
Baseline characteristics of the trials included in the meta-analysis
| Study | Treatment | No. of patients | Age (mean ± SD, year) | Male/Female | Region | Child-Pugh class (A/B/C) | Tumor size (mean ± SD, mm) | Number of tumors (1/≥2) | Jadad Scale |
|---|---|---|---|---|---|---|---|---|---|
| Carlo Bartolozzi [10] | TACE + PEI | 26 | 65.3 ± 6.2 | 19/7 | Italy | 14/12/0 | 48.4 ± 14.4 | 18/8 | 2 |
| TACE | 27 | 66.1 ± 4.9 | 22/5 | 11/16/0 | 50.9 ± 13.6 | 14/13 | |||
| Gerhild Becker [11] | TACE + PE | 27 | 64 (47-76) | 20/7 | Germany | 17/10/0 | >50 (largest):170 | 13/14 | 3 |
| TACE | 25 | 63.6 (48-79) | 21/4 | 22/3/0 | >50 (largest):170 | 9/16 | |||
| Wu PH [19] | TACE + PEI | 50 | 55 ± l8 | 47/3 | China | 40/8/2 | 52.0 ± 23.0 | less than 3 | 2 |
| TACE | 52 | 55 ± 16 | 49/3 | 40/9/3 | 52.0 ± 21.0 | less than 3 | |||
| Francesco SF [20] | TACE + PEI | 33 | 67.8 ± 6.8 | 27/6 | Italy | 27/6/0 | 25.9 ± 8.3 | 28/5 | 2 |
| TACE | 18 | 67.8 ± 6.8 | 14/4 | 14/4/0 | 30.1 ± 7.0 | 16/2 | |||
| Xu GH [21] | TACE + PEI | 23 | NR | NR | China | NR | NR | NR | 2 |
| TACE | 22 | NR | NR | NR | NR | NR | |||
| Chen DL [22] | TACE + PEI | 31 | ≤60/>60:6/25 | 17/14 | China | 17/14/0 | NR | NR | 2 |
| TACE | 30 | ≤60/>60:3/27 | 15/15 | 19/11/0 | NR | NR | |||
| Li XJ [23] | TACE + PEI | 34 | 50.5 | 28/6 | China | 24/10/0 | 23-84 | 26/8 | 2 |
| TACE | 30 | 51.5 | 24/6 | 21/9/0 | 21-85 | 22/8 | |||
| Wang QL [24] | TACE + PEI | 21 | 55.3 | NR | China | NR | 18-118 | 12/9 | 2 |
| TACE | 46 | 54.3 | NR | NR | 18-118 | 10/36 | |||
| Yu HP [25] | TACE + PEI | 32 | 45.4 ± 11.2 | 29/3 | China | NR | NR | NR | 2 |
| PEI | 36 | 49.3 ± 12.5 | 32/4 | NR | NR | NR | |||
| Wang YH [26] | TACE + PEI | 32 | 49 | 27/5 | China | NR | NR | NR | 2 |
| TACE | 32 | 49 | 26/6 | NR | NR | NR | |||
| Shen CL [27] | TACE + PEI | 48 | 18-70 | 36/12 | China | NR | NR | NR | 2 |
| PEI | 48 | 18-69 | 34/14 | NR | NR | NR | |||
| Tian YT [28] | TACE + PEI | 36 | 43-76 | 34/3 | China | NR | NR | NR | 2 |
| TACE | 36 | 42-77 | 33/5 | NR | NR | NR | |||
| Cao WF [29] | TACE + PEI | 44 | 64.3 ± 15.5 | 38/6 | China | NR | NR | NR | 2 |
| TACE | 44 | 65.4 ± 16.7 | 37/7 | NR | NR | NR | |||
| Ma SY [30] | TACE + PEI | 38 | 46.3 | 28/10 | China | NR | NR | NR | 2 |
| PEI | 38 | 46.4 | 29/9 | NR | NR | NR | |||
| Liu DX [31] | TACE + PEI | 39 | 53 ± 13 | 35/4 | China | 33/6/0 | 70 ± 19 | NR | 2 |
| TACE | 39 | 53 ± 11 | 32/7 | 30/9/0 | 69 ± 22 | NR | |||
| Li GF [32] | TACE + PEI | 36 | 53 | 34/2 | China | 17/11/8 | 100-160 | NR | 2 |
| TACE | 32 | 53 | 31/1 | 15/8/9 | 100-160 | NR | |||
| Li GS [33] | TACE + PEI | 34 | 32-79 | 23/11 | China | 21/13 | 70-140 | NR | 2 |
| TACE | 33 | 32-79 | 23/10 | 22/11 | 70-140 | NR | |||
| Chen XP [34] | TACE + PEI | 169 | 18-72 | 163/6 | China | 100/69/0 | NR | NR | 3 |
| TACE | 486 | 18-72 | 463/23 | 296/190/0 | NR | NR | |||
| Huang ZY [35] | TACE + PEI | 68 | 45.2 | 56/12 | China | NR | 40-230 | NR | 2 |
| TACE | 53 | 45.2 | 46/7 | NR | 40-230 | NR |
The quality of the included studies was assessed by Jadad scale and the results were shown in Table 1. The mean Jadad score for the trials was 2 (ranged from 2 to 3). Only two trials [11,34] revealed a high quality scale (≥3 points).
6-month survival rate
Among the 19 RCTs included in the meta-analysis, 4 studies reported data on 6 month survival rate [24,28,30,31]. Two of them demonstrated that TACE plus PEI had significantly survival benefit compared with that of monotherapy [28,31], whereas the remaining two demonstrated that the 6-month survival rate between the two arms were similar [24,30]. I 2 statistic showed that there was significant heterogeneity among the trials (I 2 = 84.1%, P = 0.000), thus a random-effects model was used to pool data. The pooled RR for the 6-month survival rate in the included studies was 1.23 (95% CI: 0.99-1.53; P = 0.0058), indicating that HCC patients treated with TACE-PEI had no better survival benefit at 6 month compared with those treated with monotherapy (Figure 2). We tried to perform the subgroup analyses based on tumor size, tumor stage and etiology of cirrhosis to find any differences in specific subgroup; however, no sufficient data were provided by these included studies. Since the number of included studies was less than 10, assessment of publication bias was not performed.
Figure 2.
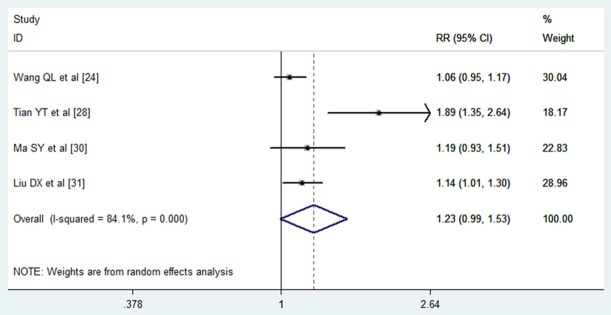
Comparison of combination therapy with monotherapy for unresectable hepatocellular carcinoma patients in terms of 6 month survival rate.
1-year survival rate
Among the 19 RCTs included in the meta-analysis, 17 studies reported data on 1-year survival rate [10,11,19,20,22-26,28-35]. 8 of them demonstrated that TACE plus PEI had survival benefit compared with that of monotherapy [19,20,23,26,28,31,34,35], whereas the remaining 9 demonstrated that the 1-year survival rate between the two arms were similar [10,11,22,24,25,29,30,32,33]. I 2 statistic showed that there was no significant heterogeneity among the trials (I 2 = 28.7%, P = 0.130), thus a fixed-effects model was used to pool data. The pooled RR for the 1-year survival rate in the included studies was 1.24 (95% CI: 1.17-1.31; P = 0.00), indicating that HCC patients treated with TACE-PEI had better survival benefit at 1 year compared with those with monotherapy (Figure 3). We tried to perform the subgroup analyses based on tumor size, tumor stage and etiology of cirrhosis to find any differences in specific subgroup; however, only two studies [11,20] presented relevant data among the included trials. Thus, we did not carry out the subgroup analyses of survival rate at 1-year. The Egger’s test (P = 0.101) and Begg’s test (P = 0.232) revealed that there was no publication bias among the included studies.
Figure 3.
Comparison of combination therapy with monotherapy for unresectable hepatocellular carcinoma patients in terms of 1 year survival rate.
2-year survival
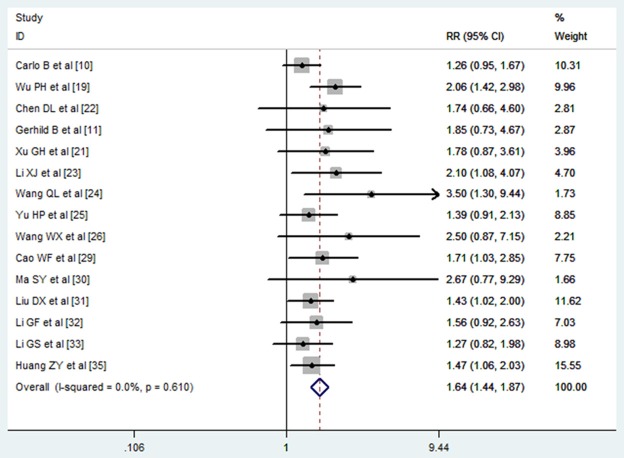
Among the 19 RCTs included in the meta-analysis, 15 presented the results of 2-year survival [10,11,19,21,23-26,29-33,35]. 6 of the 15 trials showed that HCC patients treated with TACE plus PEI had better 2-year survival rate when compared with those treated with monotherapy [19,23,24,29,31,35], while the remaining 12 studies showed that the 2 year survival rate between the two arms were similar [10,11,21,22,25,26,30,32,33]. Meta-analysis using fixed-effects model showed that combination treatment significantly improved the survival rate at 2 year when compared with monotherapy (RR = 1.64, 95% CI: 1.44-1.87; P = 0.000) (Figure 4). The Egger’s test (P = 0.5) and Begg’s test (P = 0.38) revealed that there was no publication bias among the included studies.
Figure 4.
Comparison of combination therapy with monotherapy for unresectable hepatocellular carcinoma patients in terms of 2 year survival rate.
3-year survival
The data of 3-year survival was reported in 10 trials included in the meta-analysis [10,19-21,23,25,29,32,34,35]. 5 studies showed that TACE plus PEI significantly improved the 3-year survival rate in HCC patients when compared with monotherapy [10,19,29,34,35], whereas the remaining 5 studies showed that the 3-year survival rate between the two arms were similar [20,21,23,25,32]. Pooled analysis using fixed-effects model showed that a significant difference in 3-year survival rate between HCC patients treated with TACE-PEI and those treated with monotherapy (RR = 2.27, 95% CI: 1.93-2.67; P = 0.000) (Figure 5). The Egger’s test (P = 0.078) and Begg’s test (P = 0.050) revealed that there was no publication bias among the included studies.
Figure 5.
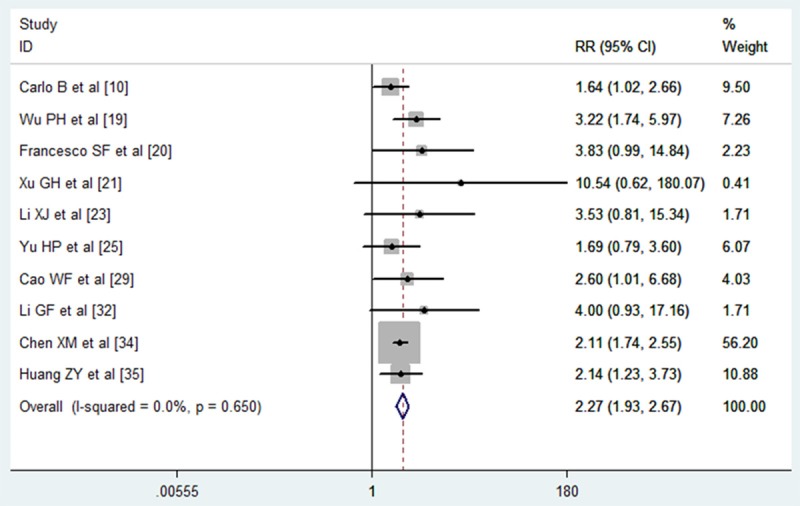
Comparison of combination therapy with monotherapy for unresectable hepatocellular carcinoma patients in terms of 3 year survival rate.
Recurrence rate
Among the 19 trials included in the meta-analysis, four of them reported data on local recurrence [10,19,20,35]. Three trials showed that HCC patients treated with TACE plus PEI had a lower local tumor recurrence rate than those treated with TACE alone [10,19,35], whereas the remaining one showed that the local recurrence rate between the TACE-PEI group and TACE or PEI group was similar [20]. The pooled analysis using a fixed-effects model showed a statistical difference in local recurrence rate between patients treated with combination therapy and those with monotherapy (RR = 0.53, 95% CI: 0.29-0.96; P = 0.035) (Figure 6). The new tumor recurrence rate was described in only two studies [10,20], both of which showed that new recurrence rate was almost similar in the two groups. The pooled results suggested that HCC patients treated with TACE-PEI had no advantage in new tumor recurrence rate over those treated with monotherapy (RR = 2.52, 95% CI: 0.28-22.64; P = 0.409) (Figure 6). Since only four trials were pooled for meta-analysis, publication bias analysis for local tumor recurrence analysis was not performed.
Figure 6.
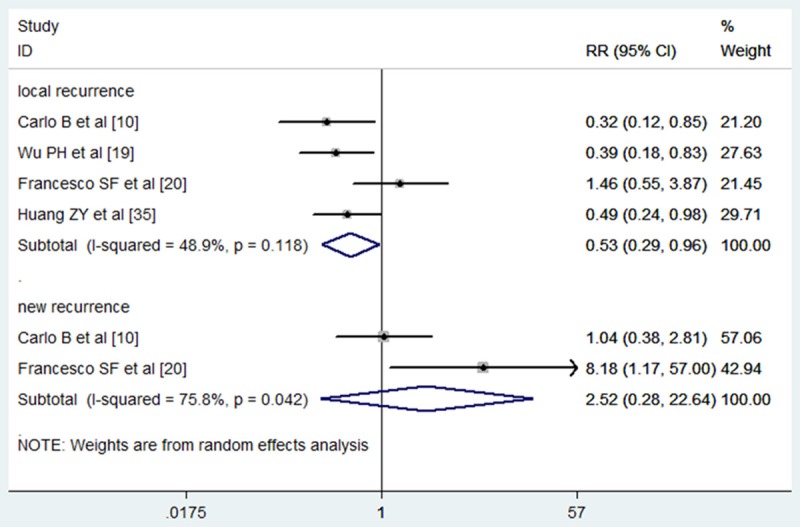
Comparison of combination therapy with monotherapy for unresectable hepatocellular carcinoma patients in terms of local and new tumor recurrence rate.
The decrease rates of AFP level and tumor size
Five studies reported the data of the decrease rates of AFP level [26,28,31,32,35]. Only one of the five trials showed that combination therapy of TACE and PEI significantly reduced the AFP level in HCC patients [35], whereas the remaining three did not. Pooled all studies using a fixed-effects model showed a significantly reduction in AFP level in patients who were under the combined therapy of TACE and PEI (RR = 1.40, 95% CI: 1.19, 1.66, P = 0.00) (Figure 7). There was no significantly heterogeneity between each studies (I 2 = 0.0%, P = 0.834).
Figure 7.
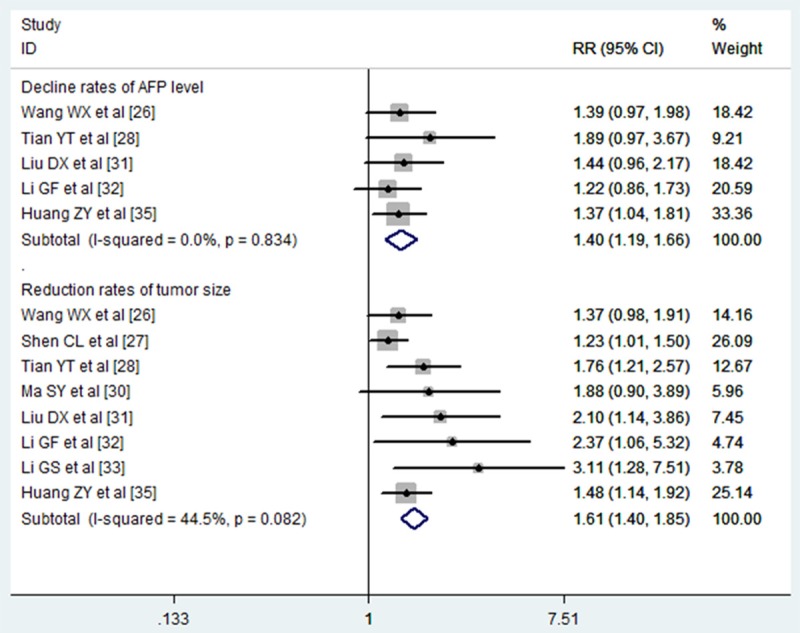
Comparison of combination therapy with monotherapy for unresectable hepatocellular carcinoma patients in terms of decline rates of AFP level and reduction rates of tumor size.
The reduction of tumor size data were reported in eight studies [26-28,30-33,35]. The pooled analysis using a fixed-effect model showed that patients who were treated with the combined therapy of TACE and PEI had a significantly decrease in tumor size (RR = 1.61, 95% CI: 1.40, 1.85, P = 0.00) (Figure 7). There was no significantly heterogeneity between each studies (I 2 = 44.5%, P = 0.082).
Sensitivity analysis
We performed sensitivity analysis based on sample size, and omitting one study in each turn. In the final analysis for 1 year survival, exclusion of two studies with a modest sample size [10,11] did not essentially change the pooled results (RR = 1.26, 95% CI: 1.18-1.33, P = 0.000). Further exclusion of any single study did not alter the results (data not shown). Next, the same analysis was perform for 2,3-year survival, local recurrence rate, and the final results remained unchanged, which suggested that the pooled results were stable.
Discussion
The present meta-analysis from 19 randomized controlled trials demonstrated that the combined therapy of TACE and PEI significantly improved the survival rate at 1, 2, 3-year, and decreased the local tumor recurrence rate, AFP level and tumor size. The pooled results for survival rate at 1, 2, 3-year were 1.24 (95% CI: 1.17-1.31, P = 0.000), 1.64 (95% CI: 1.44-1.87, P = 0.000) and 2.27 (95% CI: 1.93-2.67, P = 0.000), respectively, and for local tumor recurrence rate was 0.53 (95% CI: 0.29-0.98). Our findings identified the therapeutic benefits of TACE plus PEI for unresectable HCC patients in terms of survival and local tumor recurrence.
HCC is distributed unevenly around the world, and morbidity and mortality are particular high in Asia, including China, Korea and Japan [36-38]. Antibiotics were administrated intravenously according to the usual prophylactic protocol, whereas routine anticoagulation therapy was not used [39,40]. Locoregional treatment options, including transcatheter arterial embolization, thermal ablation, TACE and PEI, have been widely used in the treatment of HCC patients. TACE is more effective for large lesions, whereas PEI is appreciate for use of small lesions, since large ones is difficult for ethanol to permeate into. Hence, the combined therapy of TACE and PEI would be more effective for either large HCCs or small lesions.
Our results were consistency with a recent meta-analysis conducted by Ni Jiayan [41]. In this meta-analysis, the authors assessed the effectiveness of the combination of TACE and percutaneous ablation (including PEI, radiofrequency ablation) with that of monotherapy in the treatment of HCC. They found that TACE plus percutaneous ablation had beneficial effects on 1- and 3-year overall survival rate (OR1-year = 4.61, 95% CI: 2.26, 9.42; P<0.0001; OR3-year = 2.79, 95% CI: 1.69, 4.61; P<0.0001) and 3-year recurrence-free survival (OR = 3.0, 95% CI: 1.75, 5.13; P<0.0001). However, due to the limited number of studies, the subgroup analysis comparing the efficacy of TACE plus PEI with monotherapy was not performed. Another meta-analysis conducted by Wang Na [42] found similar results with ours. In that meta-analysis, the authors estimated the combination of TACE and PEI compared with TACE alone on overall survival rate and side effects. The pooled results showed that, the 6- month, 1-, 2-, 3-year survival rate were significantly higher in patients with combination group than TACE group. However, in current meta-analysis, we found that the 6 month survival rate between the TACE-PEI group and monotherapy group was not significantly different.
Compared to these prior meta-analysis, the current study has several unique features. First, we had enlarged sample size, which gave great power to evaluate this effect. Second, we also assessed the local and new recurrence rate in the HCC patients, which had not been discussed in the prior meta-analysis. Third, in the meta-analysis conducted by Wang Na [42], the authors did not perform the assessment of publication bias. Thus, the pooled results may be confounded by unpublished studies. In this meta-analysis, we found no existence of publication bias among these included studies. Fourth, we also performed sensitivity analysis, and exclusion of any single study did not materially alter the pooled results, which adds robustness to our findings.
Numerous studies have examined the efficacy of TACE combined with PEI in the treatment of unresectable HCC. A prospective randomized control trial conducted by Gerhild Becker [11] showed that TACE plus PEI did not significantly improve the overall survival for HCC patients. However, the survival benefits was observed in patients with HCC Okuda stage I under combination therapy, indicating that only patients with good liver function could gain a better survival benefit from the combination therapy. It is recognized that the degree of liver dysfunction contributes greatly to the prognosis of HCC patients. Repeated procedures of TACE or PEI would damage non-cancerous hepatocyte functions and may have potential impact on the therapeutic effect [43]. In other RCTs performed by Koda [44] and Kamada [45], TACE combined with PEI significantly improved survival for patients with HCC when compared with those treated with TACE alone. Interestingly, in this study patients with Child-Pugh class C liver cirrhosis were also included. These contrary results may be explained by the following factors: (1) In Becker’s study, they excluded patients with complete portal vein thrombosis, whereas other studies excluded patients with any evidence of thrombosis. (2) In Becker’s study, about 50% liver disease was caused by alcohol, whereas in other studies, most patients had chronic hepatitis B or C. The comparison of different treatment outcomes suggested that combined therapy of TACE and PEI may be less effective in patients with alcohol-induced cirrhosis than those with HBV-or HCV-induced cirrhosis.
Tumor recurrence is the leading risk factor that affects the prognosis of HCC patients. Also, the intrahepatic recurrence after TACE plus PEI is the main cause of late death of HCC patients. In this meta-analysis, the pooled results suggested that the local tumor recurrence rate was lower after TACE plus PEI than after TACE alone.
Some potential limitations in this meta-analysis should be acknowledged when interpreting our results. First, the characteristics of population (age, tumor size, number of tumors, aetiology of liver disease, Child-Pugh class) varied greatly between the included studies, which might have a potential impact on the consistency of the results. Second, no enough data for subgroup analysis of survival can be pooled for meta-analysis among the included studies. The etiological factors of HCC, including virus-induced or alcoholic-induced hepatitis, were not well presented by the included studies. Thus, whether TACE plus PEI would be more effectiveness in patients with virus-induced hepatitis than that with alcoholic-induced hepatitis is needed to be identified in the further study. Third, this meta-analysis was conducted based on very limited number of high quality RCTs. In fact, only two studies was scaled 3 or above by Jadad score, which indicting high quality. The main reason for this is that it is very difficult to implement the randomization allocation and double blinding, given the condition of performance of TACE and PEI. Finally, we did not assess the cost-effectiveness of TACE plus PEI in this meta-analysis. Cost-effectiveness analysis could provide valuable information for the allocation of health care resources across a broad range of conditions and interventions [46,47]. Further studies can analyze the cost-effectiveness of TACE plus PEI in the treatment of patients with HCC.
In conclusion, this meta-analysis suggests that combined therapy of TACE and PEI is more effective than monotherapy in the treatment of patients with unresectable HCC, with significantly higher survival rates at 1, 2, 3-year and lower local tumor recurrence rate, as well as decrease rates of AFP and tumor size. However, considering the heterogeneity among study designs and limited number of RCTs, further multi-centre, RCTs are needed to verify these findings.
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Llovet JM. HCC surveillance: who is the target population? Hepatology. 2003;37:507–509. doi: 10.1053/jhep.2003.50142. [DOI] [PubMed] [Google Scholar]
- 3.Kudo M. Japan’s Successful Model of Nationwide Hepatocellular Carcinoma Surveillance Highlighting the Urgent Need for Global Surveillance. Liver Cancer. 2012;1:141–3. doi: 10.1159/000342749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim DY, Han KH. Epidemiology and surveillance of hepatocellular carcinoma. Liver Cancer. 2012;1:2–14. doi: 10.1159/000339016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519–24. doi: 10.1053/jhep.2002.32089. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–17. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 7.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–42. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 8.Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, Ishikawa T, Koike Y, Yoshida H, Kawabe T, Omata M. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129:122–30. doi: 10.1053/j.gastro.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Brunello F, Veltri A, Carucci P, Pagano E, Ciccone G, Moretto P, Sacchetto P, Gandini G, Rizzetto M. Radiofrequency ablation versus ethanol injection for early hepatocellular carcinoma: a randomized controlled trial. Scand J Gastroenterol. 2008;43:727–35. doi: 10.1080/00365520701885481. [DOI] [PubMed] [Google Scholar]
- 10.Bartolozzi C, Lencioni R, Caramella D, Vignali C, Cioni R, Mazzeo S, Carrai M, Maltinti G, Capria A, Conte PF. Treatment of large HCC: transcatheter arterial chemoembolization combined with percutaneous ethanol injection versus repeated transcatheter arterial chemoembolization. Radiology. 1995;197:812–8. doi: 10.1148/radiology.197.3.7480761. [DOI] [PubMed] [Google Scholar]
- 11.Becker G, Soezgen T, Olschewski M, Laubenberger J, Blum HE, Allgaier HP. Combined TACE and PEI for palliative treatment of unresectable hepatocellular carcinoma. World J Gastroenterol. 2005;11:6104–9. doi: 10.3748/wjg.v11.i39.6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 13.Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med. 2001;135:982–989. doi: 10.7326/0003-4819-135-11-200112040-00010. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 16.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 17.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 18.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu PH, Li L, Zhang YM. Transcathrter arterial chemoembolization combined with CT-guided percutaneous intratumoral injection of lipiodol-ethanol for the treatment of hepatocellular carcinoma. Chin J Oncol. 1998;5:391–3. [PubMed] [Google Scholar]
- 20.Ferrari FS, Stella A, Pasquinucci P, Vigni F, Civeli L, Pieraccini M, Magnolfi F. Treatment of small hepatocellular carcinoma: a comparison of techniques and long-term results. Eur J Gastroenterol Hepatol. 2006;18:659–72. doi: 10.1097/00042737-200606000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Xu GH, Wen HC, Li ZW, et al. Evaluation of hepatic chemoembolization and percutaneous ethanol injection in the treatment of HCC. Chin J Radiology. 2002;1:66–8. [Google Scholar]
- 22.Chen DL, Xu XM, et al. Transcatheter arterial chemoembolization combined with intratumoral injection of iodized oil and ethonol for middle-advanced stage hepatocellular carcinoma. Journal of Practical Oncology. 2012;27:171–4. [Google Scholar]
- 23.Li XJ, Rao RS. Hypovascular liver career treated by PElT (Idic oil injection therapy) combined with TACE (Transcatheter hepatic arterial chemoembilizatlon) Haninan Medical Journal. 2010;21:16–18. [Google Scholar]
- 24.Wang QL, Guo YT, Liu HY. Comparison of the effect of TACE and Tace combined with PEI and RF on liver metastasis. Chin J Curr Adv Gen Surg. 2006;9:230–4. [Google Scholar]
- 25.Yu HP, Ding SZ, Yang HL, et al. Combined TACE and PEI for palliative treatment of advanced hepatocellular carcinoma. Chin Hosp Pharm J. 2005;25:856–8. [Google Scholar]
- 26.Wang WX, Yue HZ, Zhou GX. Combination of transcatheter arterial chemoembolization and CT-guided Percutaneous ethanol injection therapy in treating advanced hepatocellular carcinoma. China Journal of Modern Medicine. 2005;15:2845–50. [Google Scholar]
- 27.Shen CL. The efficacy of transcatheter arterial chemoembolization combined with Percutaneous ethanol injection in the treatment of hepatocellular carcinoma. Guide of China Medicine. 2012;10:170–2. [Google Scholar]
- 28.Tian YT. The efficacy of transcatheter arterial chemoembolization combined with Percutaneous ethanol injection in the treatment of patients with large hepatocellular carcinoma. Chin J Digst Med Imageol (Electronic Edition) 2012;2:179–81. [Google Scholar]
- 29.Cao WF, Bao W, Xu Y. CT-guided Percutaneous ethanol injection combined with transcatheter arterial chemoembolization in the treatment of massive liver cancers. Chin J Curr Adv Gen Surg. 2013;16:628–31. [Google Scholar]
- 30.Ma SY. TACE combined with PEI sequential therapy for primary hepatocellular carcinoma. Clinical and Trial Journal of Medicine. 2006;5:673–5. [Google Scholar]
- 31.Liu DX, Li HD, Li XF, et al. Evaluation of TACE combined with RFA and PEI in treating advanced hepatocellular carcinoma. J Intervent Radiol. 2009;18:389–93. [Google Scholar]
- 32.Li GF, Sun H, Luo YY, et al. The efficacy of transcatheter arterial chemoembolization combined with percutaneous ethanol injection in the treatment of massive liver cancers. Chinese Clinical Oncology. 2001;6:74–7. [Google Scholar]
- 33.Li GS, Ma HQ, Huang HX, et al. Evaluation of TACE combined with PEI in the treatment of patients with massive liver cancers. Journal of Guangxi Medical University. 2001;18:836–7. [Google Scholar]
- 34.Chen CM, Luo PF, Hin HH, Zhou ZJ, Shao PJ, Fu L, Li WK. Long-term result of combination of transcatheter arterial chemoembolization and percutaneous ethanol injection for treatment of hepatocellular carcinoma. Chinese Journal of cancer. 2004;23:829–32. [PubMed] [Google Scholar]
- 35.Huang JY, Yang CZ, Jiang N, et al. Treatment of primary hepatic carcinoma with transcatheter arterial chemoembolization and CT-guided percutaneous ethanol injection. China Oncology. 2000;10:71–2. [Google Scholar]
- 36.Li GC, Ye QH, Dong QZ, Ren N, Jia HL, Qin LX. TGF beta1 and related-Smads contribute to pulmonary metastasis of hepatocellular carcinoma in mice model. J Exp Clin Cancer Res. 2012;31:93. doi: 10.1186/1756-9966-31-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.You N, Liu W, Tang L, Zhong X, Ji R, Zhang N, Wang D, He Y, Dou K, Tao K. Tg737 signaling is required for hypoxia-enhanced invasion and migration of hepatoma cells. J Exp Clin Cancer Res. 2012;31:75. doi: 10.1186/1756-9966-31-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shojaei F, Scott N, Kang X, Lappin PB, Fitzgerald AA, Karlicek S, Simmons BH, Wu A, Lee JH, Bergqvist S, Kraynov E. Osteopontin induces growth ofmetastatic tumors in a preclinical model of non-small lung cancer. J Exp Clin Cancer Res. 2012;31:26. doi: 10.1186/1756-9966-31-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu XC, Xu M, Xu SF, Song RX. Long-term outcomes of epiphyseal preservation and reconstruction with inactivated bone for distal femoral osteosarcoma of children. Orthop Surg. 2012;4:21–7. doi: 10.1111/j.1757-7861.2011.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu YC, Ji JT, Lun DX. Intraoperative microwave inactivation in situ of malignant tumors in the scapulas. Orthop Surg. 2011;3:229–35. doi: 10.1111/j.1757-7861.2011.00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jia-yan Ni, Shan-shan Liu, Ni JY, Liu SS, Xu LF, Sun HL, Chen YT. Transarterial chemoembolization combined with percutaneous radiofrequency ablation versus TACE and PRFA monotherapy in the treatment for hepatocellular carcinoma: a meta-analysis. J Cancer Res Clin Oncol. 2013;139:653–659. doi: 10.1007/s00432-012-1369-x. [DOI] [PubMed] [Google Scholar]
- 42.Na Wang, Quanlin Guan, Wang K, Zhu B, Yuan W, Zhao P, Wang X, Zhao Y. TACE combined with PEI versus TACE alone in the treatment of HCC: a meta-analysis. Med Oncol. 2011;28:1038–1043. doi: 10.1007/s12032-010-9620-2. [DOI] [PubMed] [Google Scholar]
- 43.Chua TC, Liauw W, Saxena A, et al. Systematic review of neoadjuvant transarterial chemoembolization for resectable hepatocellular carcinoma. Liver Int. 2009;30:166–74. doi: 10.1111/j.1478-3231.2009.02166.x. [DOI] [PubMed] [Google Scholar]
- 44.Koda M, Murawaki Y, Mitsuda A, Oyama K, Okamoto K, Idobe Y, Suou T, Kawasaki H. Combination therapy with transcatheter arterial chemoembolization and percutaneous ethanol injection compared with percutaneous ethanol injection alone for patients with small hepatocellular carcinoma. Cancer. 2001;92:1516–24. doi: 10.1002/1097-0142(20010915)92:6<1516::aid-cncr1477>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 45.Kamada K, Kitamoto M, Aikata H, Kawakami Y, Kono H, Imamura M, Nakanishi T, Chayama K. Combination of transcatheter arterial chemoembolization using cisplatinlipiodol suspension and percutaneous ethanol injection for treatment of advanced small hepatocellular carcinoma. Am J Surg. 2002;184:284–290. doi: 10.1016/s0002-9610(02)00933-9. [DOI] [PubMed] [Google Scholar]
- 46.Doubilet P, Weinstein MC, McNeil BJ. Use and misuse of the term ‘cost-effective’ in medicine. N Engl J Med. 1986;314:253–256. doi: 10.1056/NEJM198601233140421. [DOI] [PubMed] [Google Scholar]
- 47.Russell LB, Gold MR, Siegel JE, Daniels N, Weinstein MC. The role of costeffectiveness analysis in health and medicine. JAMA. 1996;276:1172–1177. [PubMed] [Google Scholar]