Abstract
This study investigated the effects of sericin on the testicular growth hormone (GH)/insulin-like growth factor-1 (IGF-1) axis in rats with type 2 diabetes mellitus. Forty rats were randomly assigned to normal control, type 2 diabetes mellitus, sericin and metformin treated groups. Type 2 diabetes was established by repeated intraperitoneal injection of streptozotocin, and identified by blood glucose ≥16.7 mmol/L at 1 week. The diabetic rats were given no other treatment, these rats in the sericin group were intragastrically perfused with 2.4 g/kg sericin and the metformin treated rats were intragastrically perfused with 55.33 mg/kg Metformin daily for 35 consecutive days. Enzyme-linked immunosorbent assays were used to determine serum testosterone, growth hormone and IGF-1 levels. Immunohistochemical staining, western blotting and reverse transcription-PCR were used to determine testicular growth hormone, growth hormone receptor and IGF-1 expression. The sericin significantly reduced serum growth hormone levels, downregulated growth hormone expression, increased serum testosterone and IGF-1 levels, and upregulated testicular growth hormone receptor and IGF-1 expression. Moreover, there were no significant differences in any of the parameters between the sericin and metformin treated groups. These findings indicated that sericin improved spermatogenic function through regulating the growth hormone/IGF-1 axis, thereby protecting reproductive function against diabetes-induced damage.
Keywords: Sericin, growth hormone, insulin-like growth factor-1, testis, type 2 diabetes
Introduction
With the rise in the rate of diabetes mellitus and the decrease in the age of onset, considerable research has focused on diabetes mellitus-related reproductive function injury. Diabetic damage to male reproduction is commonly characterized by pathological testicular changes, diabetes-related erectile dysfunction and related endocrine changes [1,2]. The growth hormone (GH)/insulin-like growth factor-1 (IGF-1) axis has been shown to interact with the hypothalamic-pituitary-testicular axis to both directly and indirectly influence testicular endocrine function and reproductive function through regulating gonadotropin-releasing hormone and gonadotropic hormone release [3,4].
The cocoon of the silkworm is composed of fibroin and sericin. Silkworm cocoons, mainly the fibroin, have been used in China for thousands of years, but the sericin, accounting for 30% of the floss, is discarded during silk reeling. Sericin is a water-soluble polymer protein that can easily be extracted from the cocoons by soaking them in boiling water. It has been used in cosmetology, skin care, nutrition, anti-oxidation, and cancer treatment [5-7]. Silkworm cocoons soaked in water have also been used for controlling blood glucose levels. Previous studies have confirmed that sericin can effectively reduce blood glucose concentrations and improve metabolic blood fat disorder in rats fed with a high-fat diet and with type 2 diabetes mellitus (T2DM) [8-10]. The present study investigated the effects of sericin on the testicular GH/IGF-1 axis and the possible underlying mechanism in T2DM rats to provide scientific support for the application of sericin in the prevention and treatment of diabetes mellitus and chronic complications.
Materials and methods
Drugs and reagents
Silkworm cocoons were soaked in water. The water was extracted, filtrated, and condensed to prepare sericin. Streptozotocin (STZ) was purchased from Sigma (St Louis, MO). Blood glucose detection kits were purchased from Baoding Changcheng Clinical Reagent Co. Ltd. (Hebei, China). ELISA kits for serum testosterone, GH and IGF-1 were purchased from USCN Life Sciences, Inc. (Wuhan, China), and Rb was purchased from Wuhan Boster Biological Technology, Ltd. (Wuhan, China). Mouse anti-GH antibody and mouse anti-β-actin monoclonal antibodies were purchased from Santa Cruz (Santa Cruz, CA). Rabbit anti-GH receptor (GHR) polyclonal antibody was purchased from Beijing Biosynthesis Biotechnology (Beijing, China). Rabbit anti-IGF-1 polyclonal antibody was purchased from Wuhan Boster. Ready-to-use horseradish peroxidase-labeled goat anti-rabbit and goat anti-mouse IgG were purchased from KPL (Gaithersburg, MD). Trizol was purchased from Invitrogen (CA, USA). GH, GHR and IGF-1 primers were synthesized by Shanghai Sangon Bioengineering (Shanghai, China). One-step reverse transcription-PCR kits were purchased from Dalian Takara (Dalian, China). Prestained (blue) protein 600 bp DNA Ladder was purchased from Beijing Taigemei Science and Technology (Beijing, China).
Animals grouping and treatment
Forty male Sprague-Dawley rats weighing 200-250 g were provided by the Laboratory Animal Center of Hebei Medical University (license No. 712024). The food intake of rats were strictly controlled, and each rat were given fodder about 15 g/d. Ten rats were randomly selected as normal controls and were raised with no specific treatment. The remaining 30 rats were intraperitoneally injected with 2% STZ (25 mg/kg) once daily for 3 days to establish T2DM [11]. Establishment of T2DM was deemed successful if the blood glucose concentration reached more than 16.7 mmol/L after 1 week [12]. The diabetic rats were randomly assigned to T2DM, sericin and metformin treated groups, with 10 animals in each group. The rats in the sericin group were intragastrically perfused with 2.4 g/kg sericin daily for 35 consecutive days [13]. The rats in the metformin treated group were intragastrically perfused with 55.33 mg/kg Metformin and those in the T2DM group were perfused with normal saline of the same volume for 35 consecutive days [14].
Blood glucose, testosterone, GH and IGF-1
The body weight of rats was measured every day. After the final drug administration, after 12 hours fasting, all rats were anesthetized by intraperitoneal injection of 4% chloral hydrate. Blood was harvested from the posterior orbital venous plexus via the medial angle of eye, centrifuged at 3000 rpm for 20 minutes using a LD4-2A centrifuge (Jingli, Beijing, China), and the serum was collected. Blood glucose was determined by glucose oxidase method using an automatic clinical biochemical analyzer (Hitachi, Japan). Serum testosterone, GH and IGF-1 levels were measured with ELISA kits.
Testicular GH, GHR, and IGF-1 protein expression
After blood sampling, the rats were sacrificed and bilateral testes were immediately harvested. One testis was fixed in Bouin’s solution, and the other was placed in liquid nitrogen. Immunohistochemical staining for IGF-1 protein expression and quantitative analysis was performed as follows: The testis fixed in Bouin’s solution was embedded in paraffin and sectioned into 5 μm sections using a Leica paraffin microtome. Sections were immunohistochemically stained for testicular IGF-1 protein (1:50) expression. The staining was visualized with diaminobenzidine and sections were counterstained with hematoxylin, dehydrated, cleared and mounted. The negative control was treated with PBS rather than a primary antibody. Positive reactions were identified by brown yellow or dark brown particles in the nuclei and/or cytoplasm. For quantitative analysis of IGF-1 protein immunoreactions, testes of 5 rats from each group were selected, and 3 sections were selected per testis. Contorted seminiferous tubules with intact structures were selected from each section under a microscope (10×40), and quantitatively analyzed using the MiVnt image analysis system (Echung Electronics, Shandong, China). IGF-1 protein expression was determined from the ratio of immunoreactive product area to the total area of the field of view. Mean values were calculated per group. Western blot assay for testicular GH and GHR protein expression was performed as follows: Of the testes stored in liquid nitrogen, 100 mg tissue from each testis was homogenized and protein was extracted. Protein concentrations were quantitated with bicinchoninic acid protein kits. Protein samples (100 μg) were electrophoresed on a 4% spacer gel and a 15% separation gel, transferred to membranes at 2 mA/cm2 for 2 h, blocked in 5% defatted milk powder overnight, incubated with primary antibody (GH 1:200; GHR 1:100; β-actin 1:1000) at room temperature, followed by secondary antibodies (1:5000) for 1 h. Super ECL Plus supersensitive luminescence solution was used to develop the membranes. Following film scanning, developed bands were analyzed using the Quantity One 4.6.2 software (BIO-RAD Crop., Hercules, CA, USA), and the absorbance (A) ratio of target band to β-actin band was calculated to represent relative protein expression.
Reverse transcription-PCR for testicular GH, GHR, and IGF-1 mRNA expression
Testicular tissues (100 mg) were ground up in liquid nitrogen. Total RNA was extracted using Trizol total RNA extraction kits. RNA (5 μL) was subjected to 1% agarose gel electrophoresis, resulting in 3 intact bands (28, 18 and 5 S), indicating the integrity of the extracted RNA. The ratio of A260 to A280 was 1.9, as detected by ultraviolet spectrophotometer, indicating that the RNA was not contaminated. Total RNA (3 μg) was used as template and reverse transcribed into cDNA. The reaction conditions were as follows: 30°C for 10 minutes, 55°C for 30 minutes, 99°C for 5 minutes, and 5°C for 5 minutes. PCR was conducted at 94°C for 2 minutes, followed by cycles of 94°C for 30 seconds, 50-65°C for 30 seconds and 72°C for 1 minute. PCR primer sequences and amplification conditions are listed in Table 1. PCR products were subjected to 2% agarose gel electrophoresis (containing 0.5 mg/L Goldview), photographed using a ZF-typed ultraviolet reflection analyzer, and quantitatively analyzed using the Quantity One 4.6.2 software. The absorbance (A) ratio of the target band to the β-actin band was used to calculate the relative mRNA expression of target genes.
Table 1.
Primers and amplification conditions of PCR
| Gene | Primer sequence | Annealing temperature (°C) | Cycle | Product length (bp) |
|---|---|---|---|---|
| GH | F: 5’-TGACACCTACAAAGAGTTCGAGCG-3’ | 65 | 33 | 368 |
| R: 5’-TGTTGGCGTCAAACTTGTCATAGG-3’ | ||||
| GHR | F: 5’-CTGGGTTGAGTTCATTGAGCTGGAT-3’ | 62 | 31 | 394 |
| R: 5’-TGTAGAGGGGAGTTGGTGGGTTGAC-3’ | ||||
| IGF-1 | F: 5’-CTGGTGGACGCTCTTCAGTTCG-3’ | 59 | 31 | 280 |
| R: 5’-TCCTTCTCCTTTGCAGCTTCC-3’ | ||||
| β-actin | F: 5’-GAGGGAAATCGTGCGTGAC-3’ | 55 | 29 | 445 |
| R: 5’-CTGGAAGGTGGACAGTGAG-3’ |
Abbreviations: GH, Growth hormone; GHR, growth hormone receptor; IGF-1, insulin-like growth factor-1.
Statistical analysis
Data were expressed as mean ± SD and were analyzed using the SPSS 13.0 software. Intergroup differences were compared by one-way analysis of variance, and paired comparisons were conducted using q-tests. P<0.05 was considered statistically significant.
Results
Body weight
Body weight in T2DM group were significantly lower than that in the normal controls (P<0.01), but were significantly higher in the sericin and metformin treated groups when compared with the T2DM group (P<0.05), (Table 2). There was no significant difference in body weight between the sericin and metformin treated groups (P>0.05), indicating that sericin can significantly inhibit body weight in diabetic rats.
Table 2.
Body weight, blood glucose, serum testosterone, GH and IGF-1 levels
| Group | Body weight (g) | Blood glucose (mmol/L) | Testosterone (μg/L) | GH (μg/L) | IGF-1 (μg/L) |
|---|---|---|---|---|---|
| Normal control | 227±26a | 11.1±2.2a | 1.54±0.18a | 1.4±0.5b | 1125±186a |
| T2DM | 178±21 | 29.0±5.4 | 0.35±0.19 | 2.6±1.1 | 520±122 |
| Sericin | 205±23b,c | 14.0±4.0a,d | 1.41±0.13a,d | 1.4±0.6b,d | 981±181a,d |
| Metformin treated | 200±23b,d | 13.3±3.5a,d | 1.36±0.16a,d | 1.4±0.6a,d | 1021±198a,d |
| F | 7.55 | 43.39 | 108.46 | 6.25 | 23.62 |
| P | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
Note: Values are mean ± SD. n=10 per group.
P<0.01, vs. T2DM;
P<0.05, vs. T2DM;
P>0.05, vs. normal control;
P<0.05, vs. normal control.
Abbreviations: GH, Growth hormone; IGF-1: insulin-like growth factor-1; T2DM: type 2 diabetes mellitus.
Blood glucose levels
Blood glucose levels in T2DM group were significantly higher than that in the normal controls (P<0.01), but were significantly lower in the sericin and metformin treated groups when compared with the T2DM group (P<0.01), (Table 2). There was no significant difference in blood glucose levels between the sericin and metformin treated groups (P>0.05), indicating that sericin can significantly reduce blood glucose concentrations in diabetic rats.
Serum testosterone levels
Serum testosterone levels in the T2DM group were significantly lower than that in the normal controls (P<0.01), but significantly higher in the sericin and metformin treated groups when compared with the T2DM group (P<0.01), (Table 2). However, there was no significant difference in serum testosterone levels between the sericin and metformin treated groups (P>0.05). The findings showed that sericin significantly increases testosterone levels in diabetic rats.
Serum GH and IGF-1 levels
Serum GH levels in the T2DM group were significantly higher than that in the normal controls (P<0.05), and IGF-1 levels were significantly lower in the T2DM group when compared with the normal controls (P<0.01), (Table 2). Compared with the T2DM group, serum GH levels were significantly lower but IGF-1 was significantly higher in the sericin (P<0.05, P<0.01) and metformin treated groups (P<0.01). Moreover, there were no significant differences between the sericin and metformin treated groups in GH or IGF-1 levels (P>0.05). These findings show that sericin significantly increase serum IGF-1 levels but decrease GH levels in diabetic rats.
Testicular IGF-1 expression
IGF-1-positive products appeared as brown-yellow and dark brown particles, distributed in the cytoplasm of interstitial cells and supporting cell cytoplasm and nuclei of primary spermatocytes (Figure 1). The testicular contorted seminiferous tubules of the T2DM group were deformed or distorted (Figure 1B), and the number of spermatogenic cells and sperm and interstitial cells was lower than in the normal control group (Figure 1A). These pathological changes were significantly ameliorated in the sericin (Figure 1C) and metformin treated (Figure 1D) groups.
Figure 1.
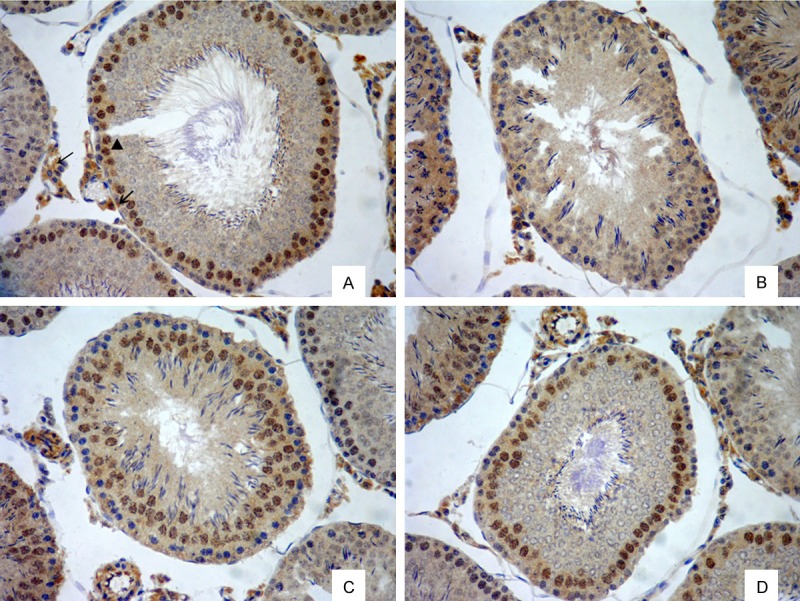
Testicular IGF-1 protein expression in rats (immunohistochemical staining, ×200). A. Normal control group. B. T2DM group. C. Sericin group. D. Metformin treated group. IGF-1 positive products appeared as brown yellow or brown particles, distributed in the cytoplasm of testicular interstitial cells (→) and supporting cell cytoplasm (↙) and nuclei of primary spermatocytes (▲).
Reverse transcription-PCR results showed a clear IGF-1 mRNA band at 280 bp and a β-actin mRNA band at 445 bp (Figure 2). Testicular IGF-1 protein and mRNA expression were significantly lower in the T2DM group than in the normal control rats (P<0.01), (Figure 3), but were significantly higher in the sericin and metformin treated groups than in the T2DM group (P<0.01). Moreover, there were no significant differences between the sericin and metformin treated groups (P>0.05). These findings show that sericin significantly upregulates testicular IGF-1 expression in diabetic rats.
Figure 2.
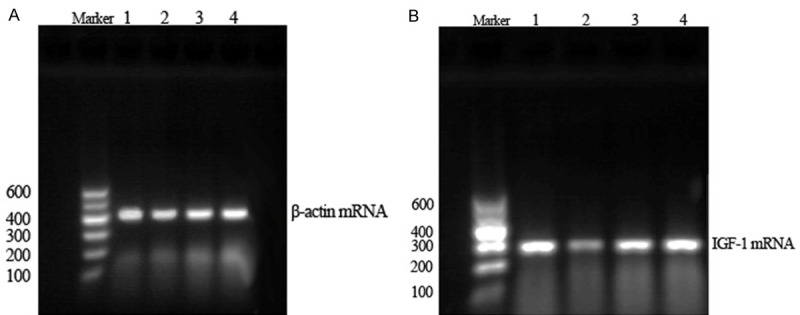
Testicular β-actin mRNA (A) and IGF-1 mRNA (B) expression in rats (RT-PCR). (1) Normal control group. (2) T2DM group. (3) Sericin group. (4) Metformin treated group. The IGF-1 mRNA expression of T2DM group decreased compared with normal control group; the IGF-1 mRNA expression of sericin group increased compared with T2DM group.
Figure 3.
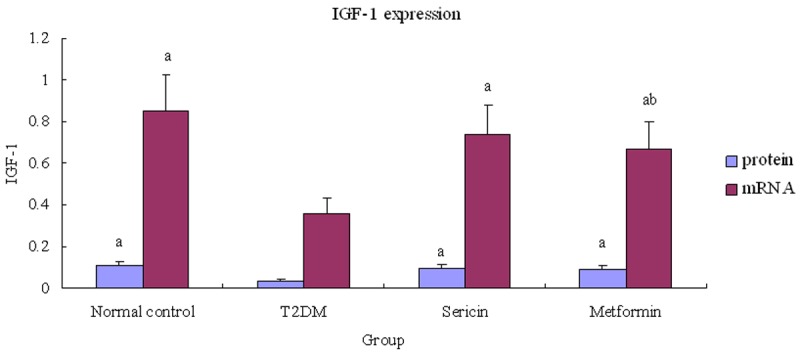
Relative testicular IGF-1 expression. Note: a P<0.01, vs. T2DM; b P<0.05, vs. normal control. Abbreviations: IGF-1, Insulin-like growth factor-1; T2DM, type 2 diabetes mellitus.
Testicular GH expression
GH protein and mRNA (Figure 4) expression were considerably higher in the T2DM group than in the normal control rats (P<0.01), but were significantly lower in the sericin and metformin treated groups than in the T2DM group (P<0.01, P<0.05), (Figure 5). Moreover, there were no significant differences between the sericin and metformin treated groups (P>0.05). These findings show that sericin significantly downregulates testicular GH expression in diabetic rats.
Figure 4.
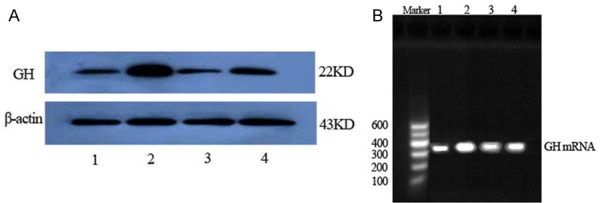
Testicular GH protein (A, western blotting) and GH mRNA (B, RT-PCR) expression in rats. (1) Normal control group. (2) T2DM group. (3) Sericin group. (4) Metformin treated group. The GH protein and mRNA expression of T2DM group increased compared with normal control group; the GH protein and mRNA expression of sericin group decreased compared with T2DM group.
Figure 5.
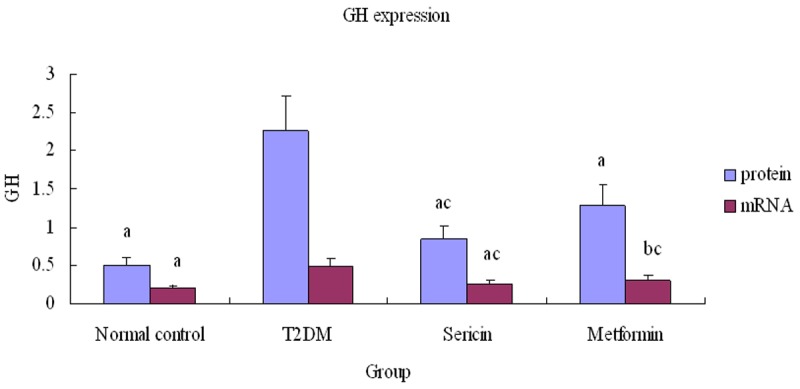
Relative testicular GH expression. Note: a P<0.01, b P<0.05, vs. T2DM; cP<0.01, vs. normal control. Abbreviations: GH, Growth hormone; T2DM, type 2 diabetes mellitus.
Testicular GHR expression
GHR protein and mRNA (Figure 6) expression were significantly lower in the T2DM group than in the normal control rats (P<0.01), but were significantly higher in the sericin and metformin treated groups than in the T2DM group (P<0.01, P<0.05), (Figure 7). Moreover, GHR protein expression was significantly higher in sericin group than in metformin treated group (P<0.01); there were no significant differences between the sericin and metformin treated groups about GHR mRNA (P>0.05). These findings show that sericin significantly upregulates testicular GHR expression in diabetic rats.
Figure 6.
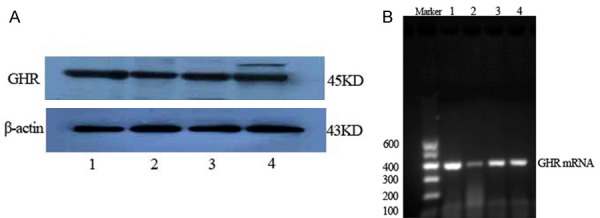
Testicular GHR protein (A, western blotting) and GHR mRNA (B, RT-PCR) expression in rats. (1) Normal control group. (2) T2DM group. (3) sericin group. (4) metformin treated group. The GHR protein and mRNA expression of T2DM group decreased compared with normal control group; the GHR protein and mRNA expression of sericin group increased compared with T2DM group.
Figure 7.
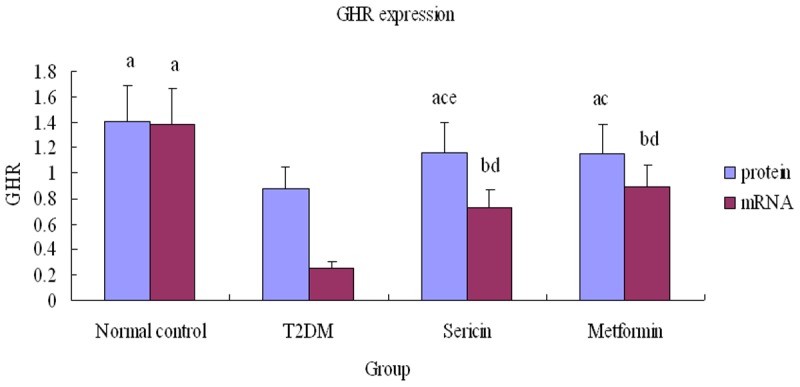
Relative testicular GHR expression. Note: a P<0.01, b P<0.05, vs. T2DM; c P<0.01, d P<0.05, vs. normal control; e P<0.01, vs. positive control. Abbreviations: GHR, Growth hormone receptor; T2DM, type 2 diabetes mellitus.
Discussion
The GH/IGF-1 axis is an important regulator of synthesis and metabolism in the human body. It interacts with the hypothalamic-pituitary-testicular axis, thereby affecting reproductive function. GH regulates reproductive function via GHR through autocrine and paracrine mechanisms, affecting the release of gonadotropin-releasing hormone, prolan B and follicle stimulating hormone, promoting testosterone synthesis, spermatic formation and activity, and affecting penile erection [15,16]. Tissue sensitivity to GH is associated with GHR and GH binding protein, and GHR gene expression is influenced by nutrition, GH, steroid hormones and diabetes mellitus [17]. Excess GH induces changes in energy assignment, resulting in excessive energy for growth but insufficient energy for the reproduction axis, causing reproductive dysfunction [18]. Moreover, excess GH inhibits GHR expression [19,20], leading to GH resistance, which directly decreases the promotive effects of GH on reproduction and reduces GH-mediated IGF-1 levels [21]. IGF-1 regulates the proliferation and differentiation of interstitial cell precursors by increasing steroidogenic enzyme expression and testosterone synthesis through autocrine and/or paracrine mechanisms. Moreover, IGF-1 interacts with various hormones and cytokines to regulate interstitial cell proliferation and differentiation, promoting testosterone synthesis and regulate reproductive function [4,22]. In the present study, serum GH levels were increased, testicular GH expression was upregulated, serum IGF-1 was decreased, and testicular GHR and IGF-1 expression were reduced in STZ diabetic rats, indicating GH/IGF-1 axis disorder and decline of reproductive function.
In the study, sericin significantly reduced blood GH levels, downregulated testicular GH expression, improved blood IGF-1 and testosterone levels and upregulated testicular GHR and IGF-1 expression. This suggested that sericin improved energy distribution by reducing GH levels, attenuating GH resistance by upregulating GHR expression, increasing GH-mediated IGF-1 levels, improving the diabetic GH/IGF-1 axis disorder and improving spermatogenic function. Moreover, the effects of sericin on reproductive function were similar to those of the metformin treated drug Metformin, which is a traditional drug used to reduce blood glucose levels. However, Metformin produces a number of adverse effects, such as anaphylactic response, gastrointestinal tract response, transaminase elevation, hypoglycemic coma, frequent micturition, megaloblastic anemia and lactic acidosis [23,24]. Lactic acidosis is the most severe adverse effect induced by Metformin, and may even result in death [25,26].
Sericin is a water soluble protein, with a variety of bioactivities. It comprises 18 amino acids, most of which, such as serine, aspartic acid, and glutamic acid, have strongly polar lateral groups (hydroxyl, carboxyl and amino groups). Results from the present study have clearly showed that sericin can improve spermatogenic dysfunction by regulating the GH/IGF-1 axis disorder in diabetes mellitus. As a natural protein, sericin also prevents chemosynthetic drug-induced toxicity and adverse effects. Some studies indicated that sericin can cause a marked elevation in serum adiponectin [8,9]. While, adiponectin can promote catabolism of glucose and fat, and improve insulin sensitivity, alleviate insulin resistance [27].
There are three major limitations in interpreting the results of the present study. First of all, there are only 40 rats in the study. The sample size of each group was small. Secondly, there has several stage of the development of type 2 diabetes mellitus, but in this experiment, we didn’t know the stage for treatment. Finally, the mechanism of sericin, which lowered blood glucose to improved reproductive dysfunction in diabetic rats, is unknown. The additional research is needed.
Conclusion
In summary, we observed that sericin can improve spermatogenic dysfunction during diabetes mellitus by regulating adiponectin level, but the exact mechanisms need to be further studied.
Acknowledgements
This trial was supported by grants from the Education Department of Hebei Province (No. 2006301), Natural Science Foundation of Hebei Province (H2012406018), and the Foundation of the Science and technology Department of Hebei Province (08276101D-19).
Disclosure of conflict of interest
None.
References
- 1.Isidro ML. Sexual dysfunction in men with type 2 diabetes. Postgrad Med J. 2012;88:152–9. doi: 10.1136/postgradmedj-2011-130069. [DOI] [PubMed] [Google Scholar]
- 2.George JT, Millar RP, Anderson RA. Hypothesis: kisspeptin mediates male hypogonadism in obesity and type 2 diabetes. Neuroendocrinology. 2010;91:302–7. doi: 10.1159/000299767. [DOI] [PubMed] [Google Scholar]
- 3.Chandrashekar V, Zaczek D, Bartke A. The consequences of altered somatotropic system on reproduction. Biol Reprod. 2004;71:17–27. doi: 10.1095/biolreprod.103.027060. [DOI] [PubMed] [Google Scholar]
- 4.Roser JF. Regulation of testicular function in the stallion: an intricate network of endocrine, paracrine and autocrine systems. Anim Reprod Sci. 2008;107:179–96. doi: 10.1016/j.anireprosci.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Kaewkorn W, Limpeanchob N, Tiyaboonchai W, Pongcharoen S, Sutheerawattananonda M. Effects of silk sericin on the proliferation and apoptosis of colon cancer cells. Biol Res. 2012;45:45–50. doi: 10.4067/S0716-97602012000100006. [DOI] [PubMed] [Google Scholar]
- 6.Isobe T, Ikebata Y, Onitsuka T, Wittayarat M, Sato Y, Taniguchi M, Otoi T. Effect of sericin on preimplantation development of bovine embryos cultured individually. Theriogenology. 2012;78:747–52. doi: 10.1016/j.theriogenology.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Nayak S, Talukdar S, Kundu SC. Potential of 2D crosslinked sericin membranes with improved biostability for skin tissue engineering. Cell Tissue Res. 2012;347:783–94. doi: 10.1007/s00441-011-1269-4. [DOI] [PubMed] [Google Scholar]
- 8.Okazaki Y, Tomotake H, Tsujimoto K, Sasaki M, Kato N. Consumption of a resistant protein, sericin, elevates fecal immunoglobulin A, mucins, and cecal organic acids in rats fed a high-fat diet. J Nutr. 2011;141:1975–81. doi: 10.3945/jn.111.144246. [DOI] [PubMed] [Google Scholar]
- 9.Okazaki Y, Kakehi S, Xu Y, Tsujimoto K, Sasaki M, Ogawa H, Kato N. Consumption of sericin reduces serum lipids, ameliorates glucose tolerance and elevates serum adiponectin in rats fed a high-fat diet. Biosci Biotechnol Biochem. 2010;74:1534–8. doi: 10.1271/bbb.100065. [DOI] [PubMed] [Google Scholar]
- 10.Fu XM, Zhong MR, Fu WL. The effects of sericin for glucose and lipid of type 2 rats. Chinese Journal of Gerontology. 2011;31:103–105. [Google Scholar]
- 11.Veerapur VP, Prabhakar KR, Kandadi MR, Srinivasan KK, Unnikrishnan MK. Antidiabetic effect of Dodonaea viscosa aerial parts in high fat diet and low dose streptozotocin-induced type 2 diabetic rats: a mechanistic approach. Pharm Biol. 2010;48:1137–48. doi: 10.3109/13880200903527736. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Qi H, Wang Y, Wu M, Cao Y, Huang W, Li L, Ji Z, Sun H. Allicin protects against myocardial apoptosis and fibrosis in streptozotocin-induced diabetic rats. Phytomedicine. 2012;19:693–8. doi: 10.1016/j.phymed.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Zhan Y, Huang C, Chen G. Hypoglycemic activity of the decoction of cocoon shell on alloxan-diabetic model mice. Canye Kexue. 2003:446–448. [Google Scholar]
- 14.Song C, Fu X, Li J, Chen Z. Effects of sericine on TGF-beta1/Smad3 signal pathway of diabetic mephropathy rats kidney. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2011;27:102–105. [PubMed] [Google Scholar]
- 15.Sirotkin AV. Control of reproductive processes by growth hormone: extra- and intracellular mechanisms. Vet J. 2005;170:307–17. doi: 10.1016/j.tvjl.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Lucy MC. Growth hormone regulation of follicular growth. Reprod Fertil Dev. 2011;24:19–28. doi: 10.1071/RD11903. [DOI] [PubMed] [Google Scholar]
- 17.Schwartzbauer G, Menon RK. Regulation of growth hormone receptor gene expression. Mol Genet Metab. 1998;63:243–53. doi: 10.1006/mgme.1998.2685. [DOI] [PubMed] [Google Scholar]
- 18.Bartke A, Chandrashekar V, Turyn D, Steger RW, Debeljuk L, Winters TA, Mattison JA, Danilovich NA, Croson W, Wernsing DR, Kopchick JJ. Effects of growth hormone overexpression and growth hormone resistance on neuroendocrine and reproductive functions in transgenic and knock-out mice. Proc Soc Exp Biol Med. 1999;222:113–23. doi: 10.1046/j.1525-1373.1999.d01-121.x. [DOI] [PubMed] [Google Scholar]
- 19.de Azevedo Figueiredo M, Lanes CF, Almeida DV, Proietti MC, Marins LF. The effect of GH overexpression on GHR and IGF-I gene regulation in different genotypes of GH-transgenic zebrafish. Comp Biochem Physiol Part D Genomics Proteomics. 2007;2:228–33. doi: 10.1016/j.cbd.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Meinhardt U, Eble A, Besson A, Strasburger CJ, Sraer JD, Mullis PE. Regulation of growth-hormone-receptor gene expression by growth hormone and pegvisomant in human mesangial cells. Kidney Int. 2003;64:421–30. doi: 10.1046/j.1523-1755.2003.00117.x. [DOI] [PubMed] [Google Scholar]
- 21.Chandrashekar V, Dawson CR, Martin ER, Rocha JS, Bartke A, Kopchick JJ. Age-related alterations in pituitary and testicular functions in long-lived growth hormone receptor gene-disrupted mice. Endocrinology. 2007;148:6019–25. doi: 10.1210/en.2007-0837. [DOI] [PubMed] [Google Scholar]
- 22.Li L, Fu YC, Xu JJ, Chen XC, Lin XH, Luo LL. Caloric restriction promotes the reproductive capacity of female rats via modulating the level of insulin-like growth factor-1 (IGF-1) Gen Comp Endocrinol. 2011;174:232–7. doi: 10.1016/j.ygcen.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Correia S, Carvalho C, Santos MS, Seiça R, Oliveira CR, Moreira PI. Mechanisms of action of metformin in type 2 diabetes and associated complications: an overview. Mini Rev Med Chem. 2008;8:1343–54. doi: 10.2174/138955708786369546. [DOI] [PubMed] [Google Scholar]
- 24.McAlister FA, Eurich DT, Majumdar SR, Johnson JA. The risk of heart failure in patients with type 2 diabetes treated with oral agent monotherapy. Eur J Heart Fail. 2008;10:703–8. doi: 10.1016/j.ejheart.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Gambaro V, Dell’acqua L, Fare F, Fidani M, Froldi R, Saligari E. A case of fatal intoxication from metformin. J Forensic Sci. 2007;52:988–91. doi: 10.1111/j.1556-4029.2007.00461.x. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen HL, Concepcion L. Metformin intoxication requiring dialysis. Hemodial Int. 2011;15(Suppl 1):S68–71. doi: 10.1111/j.1542-4758.2011.00605.x. [DOI] [PubMed] [Google Scholar]
- 27.Saxena M, Srivastava N, Banerjee M. Genetic association of adiponectin gene polymorphisms (+45T/G and +10211T/G) with type 2 diabetes in North Indians. Diabetes Metab Syndr. 2012;6:65–9. doi: 10.1016/j.dsx.2012.08.008. [DOI] [PubMed] [Google Scholar]


