Abstract
Background: A number of studies have been conducted to explore the association of XRCC1 polymorphisms with Breast cancer (BC) risk in Asians, but the results have been inconsistent. We therefore performed the present meta-analysis to explore the relationship in detail. Materials and Methods: Reported studies were searched from 1990 to October 15, 2014 in PubMed and Wan fang Med Online. We performed a meta-analysis of 13 published case-control studies fitting our eligibility criteria. These studies involved XRCC1 Arg399Gln polymorphisms in 4984 BC cases and 5744 controls in dominant (ArgArg vs. GlnGln+ArgGln), recessive (ArgGln+ArgArg vs. GlnGln), and co-dominant (ArgArg vs. GlnGln) inheritance models. The total odds Ratio (OR) and 95% CI were calculated and analyzed by Review Manager 5.2 and STATE 12. Results: Overall, significantly increased BC risk was observed in any genetic model (dominant model: odds ration [OR] = 1.31, 95% confidence interval [CI] = [1.08, 1.58]; recessive model: OR = 0.63, 95% CI = [0.50, 0.81]; codominant model: OR = 2.52, 95% CI: [1.38, 4.60]) when all eligible studies were pooled into the meta-analysis. In further stratified analyses, no association was found between Arg399Gln polymorphism and BC risk in Chinese fewer than three hereditary models. Conclusions: Our results suggest that the XRCC1 Arg399Gln polymorphism may be associated with increased Breast cancer risk among Asians, except Chinese population.
Keywords: XRCC1 gene, polymorphism, Arg399Gln, breast cancer
Introduction
Breast cancer is the most common invasive cancer in women, and like other forms of cancer it results from multiple hereditary and environmental modulators, possibly in an interactive manner. Many risk factors such as ionizing radiation and alcohol consumption have been established to account for approximately 30% of breast cancer patients [1]. Base-excision repair (BER) is an important DNA repair pathway that is responsible for the repair of base damage resulting from exposure to X-rays, oxygen radicals, and alkylating agents [2-4]. The X-ray repair cross-complementing group 1 gene (XRCC1) is one of these DNA repair genes in the pathway. XRCC1 acts as a central scaffolding protein by binding to DNA ligase III, DNA polymerase β, and poly (ADP-ribose) polymerase in BER at the site of damaged DNA [5] Masson et al.
Previous epidemiology studies on the association between genetic polymorphisms of XRCC1 and BC have given inconsistent results in Asians. In hospital-based case-control studies conducted among Korean women, Kim et al. and Seoul et al. found the Gln allele was associated with an increased risk of breast cancer [6,7]. Several studies [8-11] demonstrated that Arg399Gln polymorphism might not play a significant role in the risk of breast cancer among Chinese women. While in other studies, significant association was found between Arg399Gln polymorphism and BC risk in Chinese [12,13]. For Indians, three studies showed consistent association of Arg399Gln polymorphism and BC risks [14-16].
Based on previously published studies, several meta-analysis have been conducted on the Arg399Gln and breast cancer risk [17-22], but not special in the Asian population or Chinese. Maybe due to heterogeneity across different countries and relative few studies in Asians, no conclusion has been drawn yet in the Asian countries. Therefore, we have performed such a meta-analysis on XRCC1 Arg399Gln polymorphism and BC.
Materials and methods
Literature inclusion criteria
(1) The subjects of literature must be Chinese and Indian; (2) Case-control study of the Arg399Gln polymorphism and breast cancer risk; (3) The papers must provide the sample size, the OR information such as genotype frequency that can calculate OR and 95% CI; (4) When more than one paper used the same study population, we included a recent literature.
Literature exclusion criteria
(1) There was no controls; (2) Duplicated data; (3) The articles were reviews; (4) Controls were with other malignancies. PubMed and Wanfang Med Online were searched by using key words: “Arg399Gln”; “rs25487”; “X-ray repair cross-complementing group 1”; “XRCC1”; “breast neoplasms”; “breast cancer”; “polymorphism”. The date of the search interval was from 1990 to October 15, 2014 and the scope of the search was all papers consisted of journals and dissertations.
Study selection and data extraction
According to pre-established criteria of inclusion and exclusion, a double-check procedure was carried out to make sure the accuracy of the data entry. The following year, publication language, country, the data of total and exposure number in cases and control groups. A standardized procedure was performed to estimate Odds Ratio of cases and controls. Characteristics of studies were summarized.
Statistical analysis methods
Hardy-Weinberg equilibrium was tested by the chi-square test based on an Excel program. In meta-analysis, we examined the association between allele Arg of Arg399Gln and the risk of breast cancer compare to that of allele Gln, as well as using codominant (Arg/Arg versus Gln/Gln), recessive [(Arg/Gln+Arg/Arg) versus Gln/Gln] and dominant [Arg/Arg versus (Gln/Gln+Arg/Gln)] genetic models. Statistical analysis was done by using Review Manager 5.2 and STATA 12. Adjusted OR value and 95% CI were calculated for each study, and crude OR value should be calculated if adjusted OR value was not available. The Cochrane Q statistics test and I2 were performed for heterogeneity in this meta-analysis with a P > 0.10 and I2 < 50%, simultaneously, while a random effects model was selected when P < 0.10 or I2 > 50%. The funnel plot was drawn to evaluate publication bias. Egger’s test and Begg’s test were also done to check the publication bias. All the tests were two-sided, a P value of 0.05 for any test or model.
Results
Overview of included studies
According to the search strategy, 13 papers were selected in Figure 1. A total of 13 publications with 13 case-control studies of 4984 BC cases and 5744 controls were finally included in this meta-analysis. We had read all the 13 papers. All these 13 studies were conducted in Asian population, among them, 7 studies were dealing with Chinese people including 3250 BC cases and 4083 controls. The characteristics of individual studies are summarized in Table 1.
Figure 1.
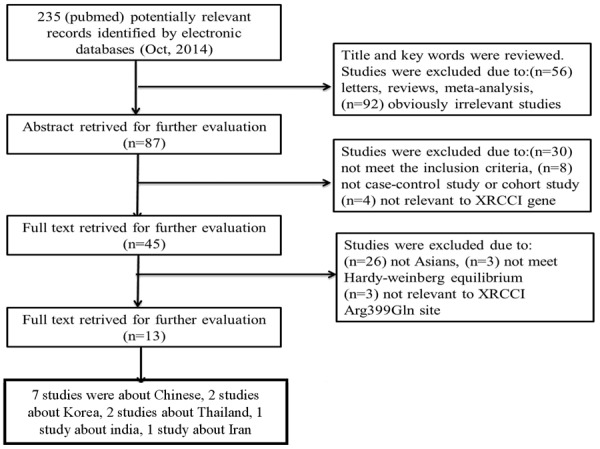
Flow chart of literature search and selection in the meta-analysis.
Table 1.
General Characteristics of Studies Included in the Meta-analysis
| Author | Country | publication language | Year | Genotype distribution | OR (95% CI) | OR (95% CI) | Control X2 HWE | P value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| case | control | ||||||||||||
|
| |||||||||||||
| ArgArg | ArgGln | GlnGln | ArgArg | ArgGln | GlnGln | ArgArg/(GlnGln+ArgGln) | (ArgGln+ArgArg)/GlnGln | ||||||
| Shu | China | English | 2003 | 561 | 442 | 85 | 610 | 498 | 74 | 1.00 (0.85-1.18) | 0.79 (0.57-1.09) | 4.369a | 0.037 |
| Jin MJ | China | Chinese | 2006 | 48 | 27 | 8 | 127 | 97 | 27 | 0.88 (0.39-2.03) | 1.13 (0.49-2.59) | 1.658 | 0.198 |
| Qian Y | China | Chinese | 2010 | 349 | 255 | 62 | 412 | 304 | 73 | 1.01 (0.71-1.44) | 0.99 (0.70-1.42) | 2.382 | 0.123 |
| Li Liu | China | English | 2011 | 518 | 402 | 84 | 547 | 367 | 81 | 1.03 (0.75-1.42) | 0.97 (0.71-1.33) | 3.014 | 0.083 |
| Peijian Ding | China | English | 2014 | 318 | 209 | 79 | 347 | 254 | 32 | 2.82 (1.84-4.32) | 0.36 (0.23-0.54) | 2.814 | 0.093 |
| Hao Luo | China | English | 2014 | 83 | 90 | 21 | 137 | 91 | 17 | 1.63 (0.83-3.18) | 0.61 (0.31-1.20) | 0.126 | 0.723 |
| Zhai XJ | China | English | 2006 | 173 | 101 | 28 | 347 | 240 | 52 | 1.15 (0.71-1.87) | 0.87 (0.54-1.40) | 1.315 | 0.251 |
| Hsu | China Taiwan | English | 2010 | 198 | 149 | 48 | 276 | 202 | 53 | 1.25 (0.82-1.89) | 0.80 (0.53-1.21) | 3.087 | 0.079 |
| Sangrajrang | Thailand | English | 2008 | 268 | 201 | 38 | 246 | 158 | 20 | 1.64 (0.94-2.86) | 0.61 (0.35-1.07) | 0.715 | 0.398 |
| IARC-Thai | Thailand | English | 2006 | 241 | 188 | 31 | 228 | 141 | 19 | 1.40 (0.78-2.53) | 0.71 (0.40-1.28) | 0.221 | 0.638 |
| Kim | Korea | English | 2002 | 92 | 79 | 34 | 90 | 101 | 14 | 1.04 (0.70-1.54) | 0.37 (0.19-0.71) | 4.156a | 0.041 |
| Seoul | Korea | English | 2006 | 148 | 119 | 41 | 149 | 144 | 21 | 0.98 (0.71-1.34) | 0.47 (0.27-0.81) | 3.139 | 0.076 |
| Chacko | India | English | 2005 | 56 | 50 | 17 | 79 | 35 | 9 | 2.15 (1.29-3.58) | 0.49 (0.21-1.15) | 3.081 | 0.079 |
| Saadat | Iran | English | 2008 | 83 | 70 | 33 | 81 | 90 | 16 | 0.95 (0.63-1.43) | 0.43 (0.23-0.82) | 1.683 | 0.195 |
| Mitra | India | English | 2008 | 44 | 52 | 54 | 83 | 107 | 35 | 1.41 (0.90-2.19) | 0.33 (0.20-0.54) | 0.003 | 0.958 |
| Syamala | India | English | 2009 | 147 | 154 | 58 | 193 | 126 | 48 | 0.63 (0.47-0.84) | 0.78 (0.52-1.18) | 12.743a | 0.0004 |
The studies are not in HWE.
Meta-analysis results
A summary of our results is shown in Table 2. For each study, we investigated the association based on the assumption of different inheritance models of the Arg399Gln allele. In all the three inheritance models of Arg399Gln, there was between-study heterogeneity in the individual studies (all P < 0.01 and I2 > 25%, Table 2), so we analyzed the data using the random-effect model.
Table 2.
Summary OR and 95% CI of XRCC1 Arg399Gln polymorphism and breast cancer risk
| Contrast | N of studies | Population | OR | 95% CI | test for overall effect | test for heterogeneity | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Z | P | I2 | P | |||||
| ArgArg vs. GlnGln+ArgGln (dominant model) | 13 | Total | 1.31 | (1.08-1.58) | 2.74 | 0.006 | 57% | 0.006 |
| 7 | Chinese | 0.77 | (0.57-1.04) | 1.71 | 0.005 | 68% | 0.09 | |
| ArgArg+ArgGln vs. GlnGln (recessive model) | 13 | Overall | 0.63 | (0.50-0.81) | 3.73 | 7E-04 | 65% | 0.0002 |
| 7 | Chinese | 1.22 | (0.87-1.70) | 1.16 | 0.001 | 73% | 0.25 | |
| ArgArg vs. GlnGln (codominant model) | 13 | Overall | 0.63 | (0.50-0.80) | 3.9 | 0.004 | 59% | <0.0001 |
| 7 | Chinese | 0.76 | (0.56-1.03) | 1.78 | 0.009 | 65% | 0.07 | |
We found that Arg399Gln had a weak correlation with the risk of BC in Asians (OR = 1.31, 95% CI: [1.08, 1.58] in the dominant model; and OR = 0.63, 95% CI: [0.50, 0.81] in the recessive model, Table 2; Figures 2, 3). Arg399Gln had a strong correlation with the risk of BC in the co-dominant model (OR = 0.63, 95% CI: [0.50, 0.80], Table 2; Figure 4). However, seven studies dealing with Chinese suggested no associations with BC risk in any hereditary model (OR = 0.77, 95% CI: [0.57, 1.04] in the dominant model (Figure 6), OR = 1.22, 95% CI: [0.87, 1.70] in the recessive model (Figure 5); and OR = 0.76, 95% CI: [0.56, 1.03] in the codominant model) (Figure 7).
Figure 2.
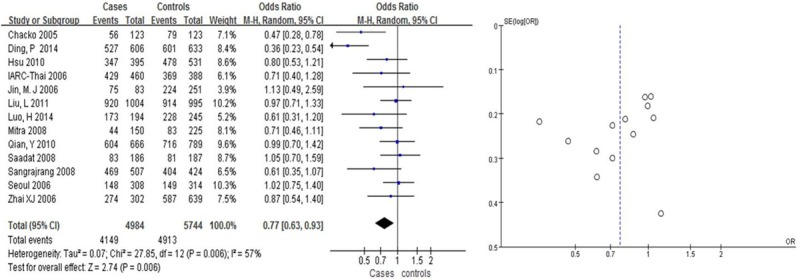
Pooled gene effect for Arg399Gln in relation to breast cancer via a dominant model.
Figure 3.
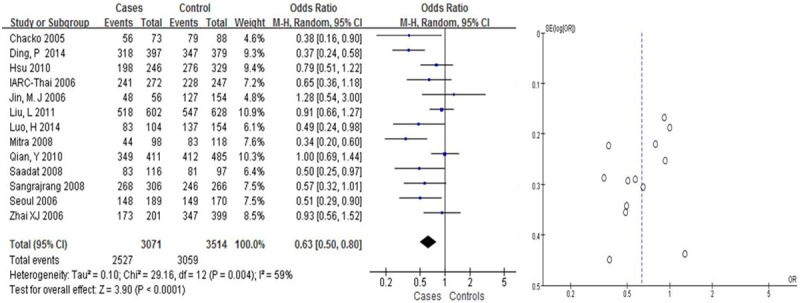
Pooled gene effect for Arg399Gln in relation to breast cancer via a codominant model.
Figure 4.
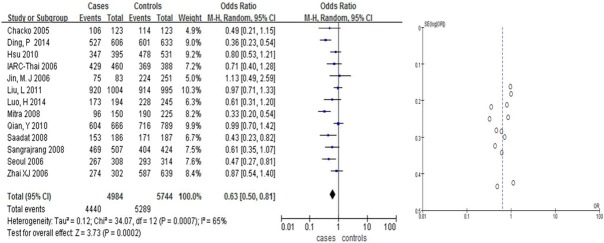
Pooled gene effect for Arg399Gln in relation to breast cancer via a recessive model.
Figure 6.
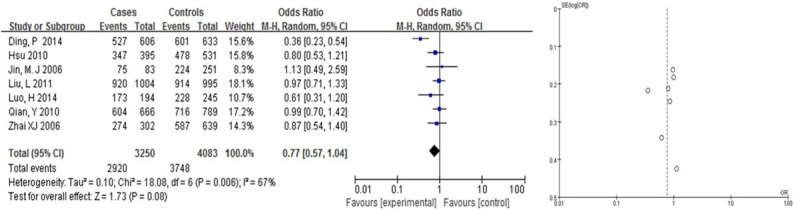
Pooled gene effect for Arg399Gln in relation to breast cancer in Chinese via a dominant model.
Figure 5.
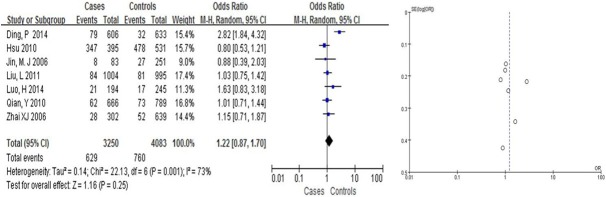
Pooled gene effect for Arg399Gln in relation to breast cancer in Chinese via a recessive model.
Figure 7.
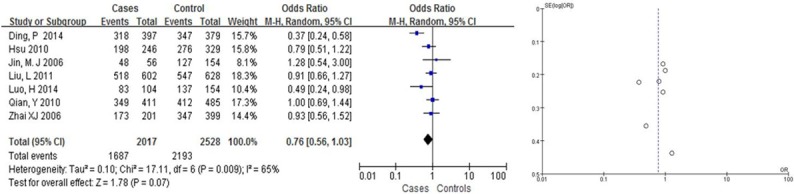
Pooled gene effect for Arg399Gln in relation to breast cancer in Chinese via a codominant model.
Test of heterogeneity
Q test and I2 were calculated to test the heterogeneity in Table 2. P value was less than 0.10, so we analyzed the pooled ORs with random effects model. Many factors might lead to heterogeneity. One is the small sample sizes of cases and controls.
Publication bias
Funnel plots was performed to assess the publication bias in Figures 2, 3 and 4. In addition, the Egger’s test and Begg’s test were also selected to test publication bias in Table 3. For the comparison of 399Arg versus 399Gln, the P value of Begg’s test of studies dealing with Chinese was 0.175 > 0.05, so it indicated that there was no publication bias. Sensitivity analysis was performed by sequential omission of individual studies, and the results also indicated that the pooled result was robust.
Table 3.
Publication bias for all analysis
| Population | P value | |
|---|---|---|
|
| ||
| Egger’s test | Begg’s Test | |
| Chinese | 0.415 | 0.175 |
| Asians | 0.161 | 0.502 |
Discussion
Meta-analysis is a powerful tool for summarizing the different studies. It can not only overcome the problem of small size and inadequate statistical power of genetic studies of complex traits, but also can provide more reliable results than a single case-control study. In addition, with the much larger sample size from the combined reports, the impact from ethnicity and other factors can be better elucidated. This meta-analysis incorporated 13 studies of 4984 breast cancer cases and 5744 controls, has further increased the sample size and enlarged the statistical power to reflect the precision effect of the Arg399Gln in breast cancer in the Asian population.
Based on our results, individuals who had the Gln allele were more likely to have BC (recessive model: OR = 0.63, 95% CI: [0.50, 0.81]; codominant model: OR = 0.63, 95% CI: [0.50, 0.80]). This is consistent with previous meta-analysis. Huang et al. (Huang, Li, & Yu, 2009) also demonstrated that Arg399Gln polymorphism increased breast cancer risk in Asians [odds ratio (OR) = 1.26, 95% confidence interval (CI): 0.96-1.64]. DNA is continuously damaged by endogenous and exogenous mutagens and carcinogens. The damages are fixed by multiple DNA repair pathways including BER, nucleotide excision repair, mismatch repair, and double-strand break repair [2]. Cells with unrepaired DNA damage undergo either apoptosis or unregulated growth to malignancy. As a DNA repair gene, XRCC1 X-ray Polymorphisms in XRCC1 gene, involving an amino acid change at evolutionarily conserved regions, could alter the XRCC1 function. Codon 399 is located in the poly (adenosine diphosphate-ribose) polymerase-binding domain and within an identified BRCA1 COOH terminus domain [23]. In view of its functional significance, it is biologically possible that the Arg399Gln polymorphism may modulate the risk of breast cancer.
The Arg399Gln variant presented no association with BC risk in Chinese population. Further sensitivity analysis suggested the stability of the current results, by showing similar ORs before and after sequential removal of single study. This result was conflict with the overall result. The power of the test was enough because of 3250 BC cases and 4083 controls. The inconsistence might be due to two main reasons. Firstly, the environment and cultural were different across different countries. Different countries have different life styles and diet habits. Besides the environmental and cultural divergences, it cannot be totally ruled out that the evolutionary history of linkage disequilibrium patterns will vary significantly across different ethnic populations. Generally, a locus is in close linkage with another nearly causal locus in one ethnic group but not in another [24]. As a consequence, there is a need to construct a database of breast cancer-susceptibility genes or polymorphisms in each racial/ethnic group.
In conclusion, our meta-analyses, under both recessive and dominant models, indicate that the Arg399Gln polymorphism associates with an increased risk of breast cancer in the Asian population, except Chinese population. With the large population size for our analyses, we feel that the results are reliable. However, more comparative studies are needed to evaluate associations in other countries. Furthermore, mechanistic studies need to be conducted to evaluate the underlying reasons for the association. Thirdly, we cannot take environment factors, such as smoking, estrogen level, and other clinic characteristics into account, to analyze the role of gene-environment, which prevented further adjustment in risk estimates and may have overestimated the true effect size.
Acknowledgements
This study was supported by The First Affiliated Hospital of Zhengzhou University.
Disclosure of conflict of interest
None.
References
- 1.Madigan MP, Ziegler RG, Benichou J, Byrne C, Hoover RN. Proportion of breast cancer cases in the United States explained by well-established risk factors. J Natl Cancer Inst. 1995;87:1681–1685. doi: 10.1093/jnci/87.22.1681. [DOI] [PubMed] [Google Scholar]
- 2.Goode EL, Ulrich CM, Potter JD. Polymorphisms in DNA repair genes and associations with cancer risk. Cancer Epidemiol Biomarkers Prev. 2002;11:1513–1530. [PubMed] [Google Scholar]
- 3.Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–374. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 4.Wood RD, Mitchell M, Sgouros J, Lindahl T. Human DNA repair genes. Science. 2001;291:1284–1289. doi: 10.1126/science.1056154. [DOI] [PubMed] [Google Scholar]
- 5.Cappelli E, Taylor R, Cevasco M, Abbondandolo A, Caldecott K, Frosina G. Involvement of XRCC1 and DNA ligase III gene products in DNA base excision repair. J Biol Chem. 1997;272:23970–23975. doi: 10.1074/jbc.272.38.23970. [DOI] [PubMed] [Google Scholar]
- 6.Kim SU, Park SK, Yoo KY, Yoon KS, Choi JY, Seo JS, Park WY, Kim JH, Noh DY, Ahn SH, Choe KJ, Strickland PT, Hirvonen A, Kang D. XRCC1 genetic polymorphism and breast cancer risk. Pharmacogenetics. 2002;12:335–338. doi: 10.1097/00008571-200206000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Breast Cancer Association Consortium. Commonly studied single-nucleotide polymorphisms and breast cancer: results from the Breast Cancer Association Consortium. J Natl Cancer Inst. 2006;98:1382–1396. doi: 10.1093/jnci/djj374. [DOI] [PubMed] [Google Scholar]
- 8.Shu XO, Cai Q, Gao YT, Wen W, Jin F, Zheng W. A population-based case-control study of the Arg399Gln polymorphism in DNA repair gene XRCC1 and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:1462–1467. [PubMed] [Google Scholar]
- 9.Qian Y, Zhang JP, Dong J, Wang FR, Lin YD, Xu M, Wu LL, Shi P, Shen HB. [Relationship between polymorphisms of X-ray repair cross-complementing group 1 gene Arg194Trp, Arg399Gln and susceptibility of breast cancer] . Zhonghua Yu Fang Yi Xue Za Zhi. 2010;44:242–246. [PubMed] [Google Scholar]
- 10.Ming-Shiean H, Yu JC, Wang HW, Chen ST, Hsiung CN, Ding SL, Wu PE, Shen CY, Cheng CW. Synergistic effects of polymorphisms in DNA repair genes and endogenous estrogen exposure on female breast cancer risk. Ann Surg Oncol. 2010;17:760–771. doi: 10.1245/s10434-009-0802-0. [DOI] [PubMed] [Google Scholar]
- 11.Jin MJ, Chen K, Zhang SS, Zhang YJ, Ren YJ, Xu H, Yao KY, Li QL, Ma XY. [Association of single nucleotide polymorphisms and haplotypes in DNA repair gene XRCC1 with susceptibility of breast cancer] . Zhejiang Da Xue Xue Bao Yi Xue Ban. 2006;35:370–376. doi: 10.3785/j.issn.1008-9292.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Luo H, Li Z, Qing Y, Zhang SH, Peng Y, Li Q, Wang D. Single nucleotide polymorphisms of DNA base-excision repair genes (APE1, OGG1 and XRCC1) associated with breast cancer risk in a Chinese population. Asian Pac J Cancer Prev. 2014;15:1133–1140. doi: 10.7314/apjcp.2014.15.3.1133. [DOI] [PubMed] [Google Scholar]
- 13.Ding P, Yang Y, Cheng L, Zhang X, Cheng L, Li C, Cai J. The relationship between seven common polymorphisms from five DNA repair genes and the risk for breast cancer in northern Chinese women. PLoS One. 2014;9:e92083. doi: 10.1371/journal.pone.0092083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chacko P, Rajan B, Joseph T, Mathew BS, Pillai MR. Polymorphisms in DNA repair gene XRCC1 and increased genetic susceptibility to breast cancer. Breast Cancer Res Treat. 2005;89:15–21. doi: 10.1007/s10549-004-1004-x. [DOI] [PubMed] [Google Scholar]
- 15.Mitra AK, Singh N, Singh A, Garg VK, Agarwal A, Sharma M, Chaturvedi R, Rath SK. Association of polymorphisms in base excision repair genes with the risk of breast cancer: a case-control study in North Indian women. Oncol Res. 2008;17:127–135. doi: 10.3727/096504008785055567. [DOI] [PubMed] [Google Scholar]
- 16.Syamala VS, Syamala V, Sreedharan H, Raveendran PB, Kuttan R, Ankathil R. Contribution of XPD (Lys751Gln) and XRCC1 (Arg399Gln) polymorphisms in familial and sporadic breast cancer predisposition and survival: an Indian report. Pathol Oncol Res. 2009;15:389–397. doi: 10.1007/s12253-008-9135-8. [DOI] [PubMed] [Google Scholar]
- 17.Huang Y, Li L, Yu L. XRCC1 Arg399Gln, Arg194Trp and Arg280His polymorphisms in breast cancer risk: a meta-analysis. Mutagenesis. 2009;24:331–339. doi: 10.1093/mutage/gep013. [DOI] [PubMed] [Google Scholar]
- 18.Li H, Ha TC, Tai BC. XRCC1 gene polymorphisms and breast cancer risk in different populations: a meta-analysis. Breast. 2009;18:183–191. doi: 10.1016/j.breast.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Saadat M, Ansari-Lari M. Polymorphism of XRCC1 (at codon 399) and susceptibility to breast cancer, a meta-analysis of the literatures. Breast Cancer Res Treat. 2009;115:137–144. doi: 10.1007/s10549-008-0051-0. [DOI] [PubMed] [Google Scholar]
- 20.Wu K, Su D, Lin K, Luo J, Au WW. XRCC1 Arg399Gln gene polymorphism and breast cancer risk: a meta-analysis based on case-control studies. Asian Pac J Cancer Prev. 2011;12:2237–2243. [PubMed] [Google Scholar]
- 21.Bu T, Liu L, Sun Y, Zhao L, Peng Y, Zhou S, Li L, Chen S, Gao Y. XRCC1 Arg399Gln polymorphism confers risk of breast cancer in American population: a meta-analysis of 10846 cases and 11723 controls. PLoS One. 2014;9:e86086. doi: 10.1371/journal.pone.0086086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yi L, Xiao-Feng H, Yun-Tao L, Hao L, Ye S, Song-Tao Q. Association between the XRCC1 Arg399Gln polymorphism and risk of cancer: evidence from 297 case-control studies. PLoS One. 2013;8:e78071. doi: 10.1371/journal.pone.0078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen MR, Jones IM, Mohrenweiser H. Nonconservative amino acid substitution variants exist at polymorphic frequency in DNA repair genes in healthy humans. Cancer Res. 1998;58:604–608. [PubMed] [Google Scholar]
- 24.Niu W, Qi Y, Wu Z, Liu Y, Zhu D, Jin W. A meta-analysis of receptor for advanced glycation end products gene: four well-evaluated polymorphisms with diabetes mellitus. Mol Cell Endocrinol. 2012;358:9–17. doi: 10.1016/j.mce.2012.02.010. [DOI] [PubMed] [Google Scholar]


