Abstract
Objective: The interstitial cells of Cajal (ICCs) interact morphologically and functionally with the elements of the enteric nervous system in the digestive tract. However, direct evidence that ICCs participate in the differentiation of the enteric nervous system is lacking. In this work, we examined in co-culture experiments whether ICCs could stimulate the differentiation of neuroepithelial stem cells (NESCs) to neurons. Methods: NESCs were harvested from the neural tube of embryonic (E11.5) rats, and ICCs were isolated from the colons of newborn rats. Various cell types were identified immunohistochemically. Results: NESCs reacted with antibodies to the stem-cell marker nest in; ICCs reacted with c-kit antibodies. NESCs, when differentiated into astrocytes, were identified with a marker GFAP, and neurons with marker MAP2. NESCs co-cultured with ICCs, compared with NESCs cultured alone, yielded a significantly greater number of cells positive for the neuronal markers PGP9.5 and nNOS. The co-cultured NESCs also produced more PGP9.5 and nNOS proteins, as measured by Western blotting. In addition, co-cultured ICCs connected morphologically with differentiated NESCs. Conclusion: These in vitro findings demonstrated that ICCs could induce the neuronal differentiation of NESCs, which connected with differentiated neurons into a network morphologically. The findings provide an experimental basis for in vivo application of the simultaneous transplantation of NESCs and ICCs.
Keywords: Enteric nervous system, neuroepithelial stem cells, interstitial cells of cajal, co-culture, differentiation
Introduction
The neural tube is the primordium of the central nervous system. Neuroepithelial cells that constitute the neural tube wall, also known as neuroepithelial stem cells (NESCs) [1], are the most primitive neural stem cells. NESCs can be differentiated into nerve cells and neurons, and they have been successfully used for the treatment of the diseases of the enteric nervous system due to deficiency of ganglion cells [2,3]. Interstitial cells of Cajal (ICCs) are a kind of specialized cells located between the autonomic nerve endings and smooth muscle cells of the digestive tract, where they serve as pacemaker cells producing and spreading slow waves. ICCs can connect with many synapses, forming a network, and they are closely related to intestinal neurons, thus participating in signal transmission from the enteric nervous system tosmooth muscle cells [4,5].
Thus far, most research has focused on the differentiation and survival of NESCs and ICCs individually. Whether the ICCs can affect the differentiation of NESCs is unknown. In this work, we co-cultured ICCs with NESCs in vitro in order to determine whether neural differentiation of NESCs resulted.
Materials and methods
Cell isolation and culture of NESCs
Staged-pregnant female Wistar rats at embryonic day 11.5 were used for the isolation of NESCs. The Guide for the Care and Use of Laboratory Animals published by the National Institute of Health (NIH publication No 85-23, revised 1985) was followed for all experiments. The rats were deeply anesthetized with sodium pentobarbital (45 mg/kg, intraperitoneally) for isolation of NESCs. Trunk segments of embryos were isolated in a dish containing cold Hank’s buffered salt solution (0.4 KCl, 0.06 KH2PO4, 8 NaCl, 0.35 NaHCO3, 0.06 Na2HPO4 g/L, pH 7.4). Neural tubes were separated from somites by the use of gentle trituration. The tubes were dissociated with a 0.05% trypsin/ethylenediaminetetraacetic acid (EDTA, Sigma, USA) solution for 5 min at 37°C. After digestion, a cell suspension was obtained and resuspended in neurobasal medium containing B27 (Invitrogen, USA), plus 20 ng/ml b fibroblast growth factor (bFGF) (Hyclone, USA). The cells were inoculated into T25 cell-culture bottles (Falcon, USA), containing serum-free medium DMEM/F12 (Gibco, USA) and bFGF, at 37°C with 5% CO2.
Identification of NESCs
NESCs were prepared for immunofluorescence labeling by fixation in 4% paraformaldehyde (room temperature for 15 min). After fixation, the cells were incubated in 10% normal goat serum (room temperature for 20 min) and then incubated at 4°C overnight with polycolonal antibody against the stem-cell marker nestin (1:200, Abcam, USA). Immunoreactivity was detected by incubation with fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG (1:100, KPL, USA) at room temperature for 1 h. A fluorescence microscope (Olympus, Tokyo, Japan) was used to observe immunostaining of cells.
After 7~10 days, differentiated NESCs were prepared for immunofluorescence labeling by fixation in 4% paraformaldehyde (room temperature for 15 min). After fixation, the cells were incubated in 10% normal goat serum (room temperature for 20 min) and then incubated at 4°C overnight with polycolonal antibody against glial fibrillary acidic protein (GFAP) (1:200, Sigma, USA) or microtubule associated protein 2 (MAP2) (1:100, Chemicon, USA). Immunoreactivity was detected by incubation with FITC-conjugated goat anti-mouse IgG (1:100, KPL, USA) or goat anti-rabbit IgG (1:100, KPL, USA) at room temperature for 1 h. A fluorescence microscope (Olympus, Tokyo, Japan) was used to observe immunostaining of cells.
Isolation and cell culture of ICCs
Isolation and cell culture of ICCs were done as previously describe [6,7]. Twenty-day-old Wistar rats were deprived of food but not water for 24 hours. The animals were killed with a pentobarbital overdose (30 mg/kg) followed by cervical dislocation. All procedures were conducted in accordance with the international standards cited above. Briefly, segments of colon were flushed with D-Hank’ ssolution, and the mucosa and submucosa layers were stripped sharply. Muscle pieces were dissociated with type II collagenase (Sigma, USA) at 37°C for 30 min, and the cells were resuspended with M199 medium (Hyclone, USA). The resuspended cells were added into Ficoll 400 density-gradient centrifugal liquid and centrifuged at 30 g for 10 min. Finally, the cells were incubated in M199 medium containing 10% fetal bovine serum (FBS), 1% antimycotic (Sigma, USA), Stem cell factor (SCF) (5 ng/ml, Sigma, USA) and bFGF (20 μg/L, Hyclone, USA).
Identification of ICCs
After 7 days, ICCs were prepared for immunofluorescence labeling by fixation in 4% paraformaldehyde (room temperature for 15 min). After fixation, cells were incubated in 10% normal goat serum (room temperature for 20 min) and then incubated at 4°C overnight with polycolonal antibody against c-kit 100 (1:200, Bioscience, USA) or c-kit 400 (1:200, Bioscience, USA). Immunoreactivity was detected by incubation with FITC-conjugated goat anti-rabbit IgG (1:100, KPL, USA) at room temperature for 1 h. A fluorescence microscope (Olympus, Tokyo, Japan) was used to observe immunostaining of cells.
Co-culture of NESCs with ICCs
Third-generation NESCs wereinoculated into the ICCs at a ratio of NESCs: ICCs of about 1:10. The cell concentration was adjusted to 1 × 105/mL. The culture medium was DMEM/F12 with 10% FBS, and the culture conditions 37°C and 5% CO2. Control cells were single-culture NESCs cultivated under the same conditions. The culture medium was changed every three days. After 7 days of culture, the co-cultured NESCs and single-cultured NESCs were ready for immunofluorescence staining.
NESCs immunocytochemical stain
Differentiated NESCs were prepared for immunofluorescence labeling by fixation in 4% paraformaldehyde (room temperature for 15 min). After fixation, the cells were incubated in 10% normal goat serum(room temperature for 20 min) and then incubated at 4°C overnight with polycolonal antibody PGP9.5 (1:500; Abcam, USA) or with polyclonal antibody nNOS (1:200; Cell Signaling, USA). Immunoreactivity was detected by incubation with FITC-conjugated goat anti-rabbit IgG (1:100; KPL, USA) at room temperature for 1 h. A fluorescence microscope (Olympus, Tokyo, Japan) was used for observing immunostaining of cells.
Double-labeledimmunocytochemical stain of ICCs and NESCs
Co-cultured cells were prepared in a similar manner, but the primary antibodies were rabbit monoclonal antibody to c-Kit (1:100; Biolegend, San Diego, USA) for ICCs and monoclonal antibody to MAP2 (1:100; Chemicon, Temerula, CA, USA) for differentiated NESCs. The secondary antibodies were FITC-conjugated goat anti-rabbit IgG (1:100; KPL, USA) and TRITC (rhodamine)-conjugated goat antimouse IgG (1:200; KPL, USA). The cells were observed with a fluorescence microscope (Olympus, Tokyo, Japan).
Western blotting
Total protein from co-culturedNESCs and single cultured-NESCs was extracted by use of RIPA lysis buffer followed by centrifugation at 4°C at 12000 rpm for 5 min. The supernatant (protein extract) was obtained, and itsprotein concentration was measured with a BCA protein assay kit (Pierce, USA). Ten micrograms of protein for each sample was resolved on 10% SDS-PAGE gels and transferred to PVDF membranes. Nonspecific binding to the membranes was blocked with 5% non-fat milk for 2 h at room temperature. Antibodies to PGP9.5 (1:500, Abcam, USA), nNOS (1:1000, Cell Signaling, USA), and GAPDH (1:2000, Santa Cruz, USA) were applied at 4°C overnight. The membraneswere then washed four times with TBST. After incubation with HRP-conjugated secondary antibody for 2 h at room temperature, the membraneswere washed again as described above. Protein bands were visualized with a chemiluminescent substrate for FITC (KPL, USA) and captured on X-ray film. Results were scanned and quantified by use Quantity One analysis software (Bio-Rad, USA).
Statistical analysis
The data obtained were expressed as mean ± SEM. Student t-test was used for comparison of the results ofsingle-culturedand co-culturedcells, with P < 0.05 taken as significant difference.
Results
Identification of NESCs and ICCs
Cultured NESCs expressed the stem-cell marker nestin (Figure 1). NESCs cultured in serum medium for seven days differentiated and expressed the astrocyte marker GFAP and the neuron marker MAP2 (Figure 2). Cultured ICCs expressed c-kit-100 and c-kit-400 (Figure 3A and 3B).
Figure 1.
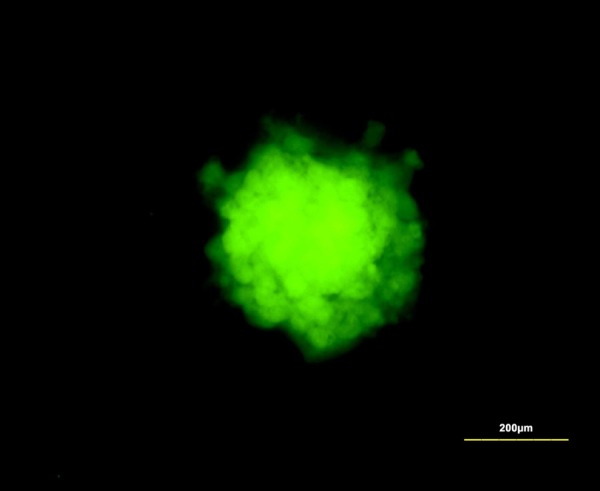
Cultured NESCs expressed stem-cell marker nestin. Scale bar is 200 µm.
Figure 2.
Cultured NESCs differentiated and expressed astrocytes marker GFAP (green) and neuron marker MAP2 (red). Scale bar is 200 µm.
Figure 3.
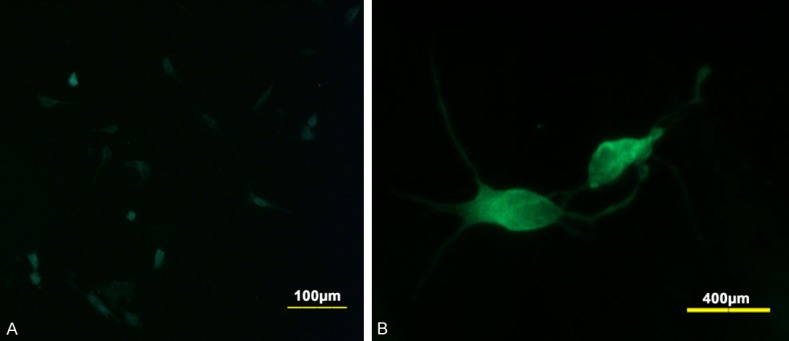
Culture ICC expressed c-kit 100 (A) and c-kit 400 (B). Scale bar of (A) is 100 µm. Scale bar of (B) is 400 µm.
Neural differentiation of NESCs
After 7-day co-culture, NESCs had differentiated into neurons, as indicated by their expression of the enteric neuronal markers PGP9.5 (Figure 4) and nNOS (Figure 5). The protein expression of PGP9.5 and nNOS by co-cultured NESCs, determined with Western blotting, was significantly higher than by single-cultured NESCs (Figure 6).
Figure 4.
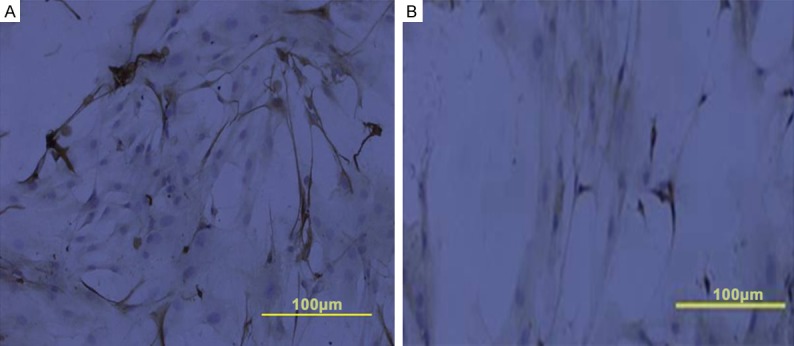
Cultured NESCs differentiated and expressed the enteric neuronal marker PGP9.5. Immunocytochemical staining of co-culturedcells (A) and single-culture cells (B). Scale bar is 100 µm in panels (A and B).
Figure 5.
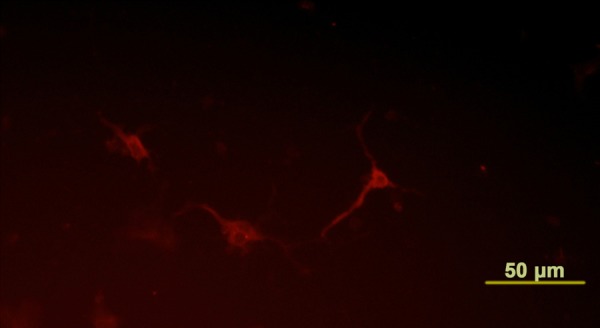
Neural differentiation of NESCs. After 7-day co-culture, NESCs were observed to differentiate into neurons, which were identified by staining with an antibody against nNOS (red). Scale bar is 50 µm.
Figure 6.
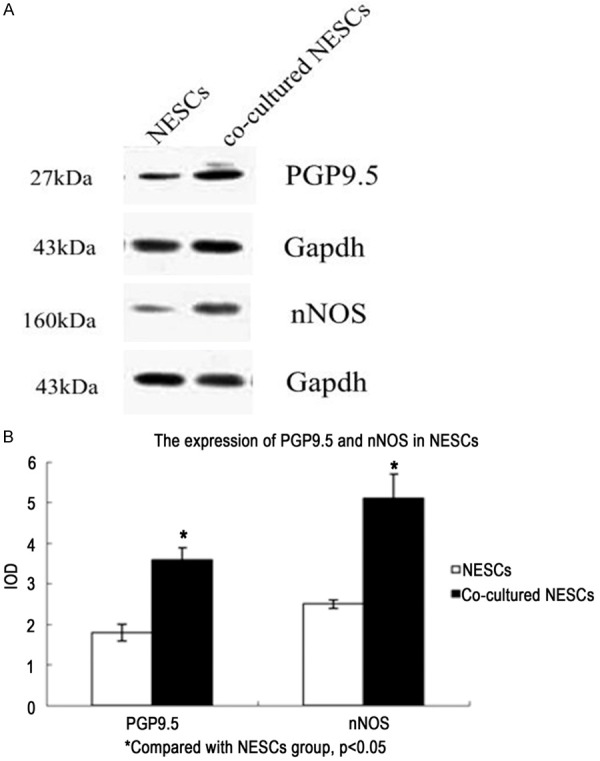
Protein expression of PGP9.5 and nNOS as determined in Western blotting. Representative image of protein expression of PGP9.5 and nNOS (A). Statistical image of protein expression of PGP9.5 and nNOS (B). Data are shown as mean ± SEM. P < 0.05 vs. NESCs group.
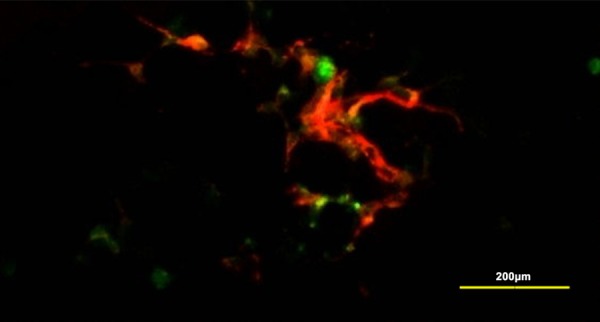
Interrelation of NESCs and ICCs
The co-cultured ICCs survived well and connected with differentiated NESCs morphologically (Figure 7A). Double immunofluorescence staining for c-kit and MAP2 showed that ICCs interconnected with neurons which had differentiated from NESCs (Figure 7B).
Figure 7.
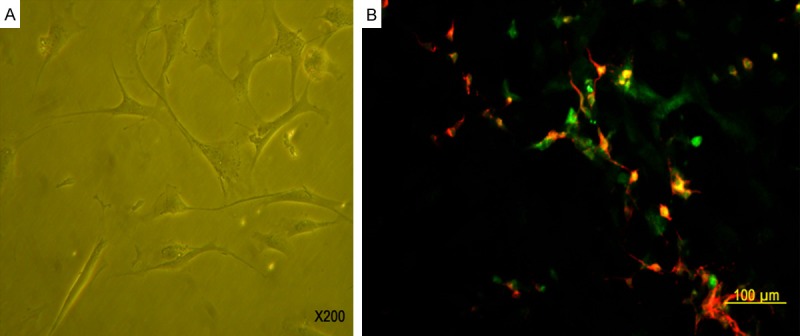
Interrelation of NESCs and ICCs. In co-cultured medium, NESCs differentiated into neurons. The co-cultured ICCs survived well and connected with differentiated NESCs morphologically (A, 200 ×). Double immunofluorescence staining for c-kit (green) and MAP2 (red) showed that ICCs (green) interconnected with neurons which had differentiated from NESCs (red). Scale bar is 100 µm in panel B.
Discussion
In this study, we aimed to determine if ICCs can promote the differentiation of NESCs into neurons. We found that indeed they can when co-cultured with NESCs, as evidenced by the NESCs acquiring the enteric neuronal marker PGP9.5 and positivity for nNOS, and by increased synthesis of these proteins. Moreover, the co-cultured NESCs formed interconnected network swith morphologically differentiated neurons. These findings provide evidence of a role of ICCs in the neuroregulation of the gut.
Gastrointestinal behavior reflects the integrated function of the smooth muscle, mucosal epithelium, and blood vessels. The neural regulation of gastrointestinal function is dependent on three systems: the central nervous system, the autonomic nervous system, and the enteric nervous system (ENS). The neuroregulation is very complex, and the ENS plays a major role [8-10]. The ENS is a reticular structure composed of neurons, neurotransmitters and proteins and their supporting cells in the gastrointestinal wall. The ganglia of ENS neurons inter-connect to form an independent nervous system similar to that of the brain and spinal cord. The cells integrate and process information, so as to control and regulate the smooth muscle, mucosal epithelium, and the vascular system distributed throughout the length of the gastrointestinal tract. Control of the ENS is highly independent and does not have to rely on the central nervous system; thus it is sometimes called the “miniature brain” of the gastrointestinal tract. Study of the ENS has become anew sub-discipline of study of the autonomic nervous system [11,12]. As a result of the discovery of new neural transmitters and elucidation of their mechanisms of action, the ENS has become better understood.
Neural stem cells have the potential of differentiating into neurons, astrocytes, and oligodendrocytes, and are capable of self-renewal. Neural stem cells have been isolated and cultivated from embryos and from the subventricular zone, striatum, hippocampus, spinal cord, and other neurological tissues of adult animals. In recent years, significant progress has been made in the treatment of nerve injury and neurodegenerative disease through the transplantation of neural stem cells or neural stem cells induced by specific genes [13,14]. In the gastrointestinal tract, when NESCs derived from rat embryonic neural tube were transplanted to the colon wall in a rat model of Hirschsprung’s disease, the transplanted cells differentiated into nerve cells and glial cells, and significantly improved the colonic motility [3]. NESCs have the advantage of easy separation, cultivation and amplification as well as low immunogenicity [1]. Perhaps the differentiation of NESCs to neurons by ICCs, as we have demonstrated in vitro, could lead to in vivo applications for the treatment of gastrointestinal functional disorders. This approach could avoid the potential risk involved in the use of gene vectors for this purpose.
It is attractive to think that the ability of ICCs to stimulate the differentiation of NESCs to neurons could lead to practical application. ICCs of the gastrointestinal tract come from the mesenchymal cells of the embryonic layer in the primitive digestive tract. The cells are the pacemaker cells of the slow wave movement of the intestinal wall, and they participate in the transfer of excitatory and inhibitory neurotransmission through contact with the ganglion cell axons in the intestinal wall [4,15,16]. Deficiency of ICCs can cause a variety of gastrointestinal neuromotor disorders [17]. As we showed in this work, an enzymolysis methodin combination with density-gradient centrifugation [6,7], permits separation of ICCs, which can be cultivatedin vitro so their function and cell-signaling transduction can be studied [18,19]. Whether these techniques can lead to in vivo use of ICCs in gut neuroregulationdeserves investigation.
In summary, we demonstrated in vitro that ICCs can promote the differentiation of NESCs into neurons, which interconnect with differentiated neurons into a network morphologically. The findings provide an experimental basis for in vivo application of the simultaneous transplantation of NESCs and ICCs in states of intestinal neurological dysregulation.
Acknowledgements
This work is supported by National Natural Science Foundation of China (No. 30973142).
Disclosure of conflict of interest
None.
Abbreviations
- bFGF
fibroblast growth factor
- BSA
Bovine serum albumin
- ChAT
choline acctyltransferase
- DMEM
Dulbecco’s modified eagle’s medium
- ENS
enteric nervous system
- FITC
fluorescein isothiocyanate
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GFAP
glial fibrillary acidic protein
- MAP2
microtubule associated protein 2
- nNOS
neuronal nitric oxide synthase
- PAGE
Polyacrylamide gel electrophresis
- PGP9.5
protein gene product 9.5
- SCF
stem cell factor
- TRITC
tetraethyl rhodamine isothiocyanate
References
- 1.Rao M. Stem and precursor cells in the nervous system. J Neurotrauma. 2004;21:415–27. doi: 10.1089/089771504323004566. [DOI] [PubMed] [Google Scholar]
- 2.Theocharatos S, Kenny SE. Hirschsprung’s disease: current management and prospects for transplantation of enteric nervous system progenitor cells. Early Hum Dev. 2008;84:801–4. doi: 10.1016/j.earlhumdev.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Liu W, Wu RD, Dong YL, Gao YM. Neuroepithelial stem cells differentiate into neuronal phenotypes and improve intestinal motility recovery after transplantation in the aganglionic colon of the rat. Neurogastroenterol Motil. 2007;19:1001–9. doi: 10.1111/j.1365-2982.2007.00981.x. [DOI] [PubMed] [Google Scholar]
- 4.Ward SM, Sanders KM. Involvement of intramuscular interstitial cells of Cajal in neuroeffector transmission in the gastrointestinal tract. J Physiol. 2006;576:675–82. doi: 10.1113/jphysiol.2006.117390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang XY, Paterson C, Huizinga JD. Cholinergic and nitrergic innervation of ICC-DMP and ICC-IM in the human small intestine. Neurogastroenterol Motil. 2003;15:531–43. doi: 10.1046/j.1365-2982.2003.00429.x. [DOI] [PubMed] [Google Scholar]
- 6.Okada T, Sasaki F, Honda S, Cho K, Matsuno Y, Itoh T, Kubota KC, Todo S. Disorders of interstitial cells of Cajal in a neonate with segmental dilatation of the intestine. J Pediatr Surg. 2010;45:e11–4. doi: 10.1016/j.jpedsurg.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 7.Eshraghian A, Eshraghian H. Interstitial cells of Cajal: a novel hypothesis for the pathophysiology of irritable bowel syndrome. Can J Gastroenterol. 2011;25:277–9. doi: 10.1155/2011/478370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boeckxstaens GE. Understanding and controlling the enteric nervous system. Best Pract Res Clin Gastroenterol. 2002;16:1013–23. doi: 10.1053/bega.2002.0336. [DOI] [PubMed] [Google Scholar]
- 9.Gariepy CE. Developmental disorders of the enteric nervous system: genetic and molecular bases. J Pediatr Gastroenterol Nutr. 2004;39:5–11. doi: 10.1097/00005176-200407000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Benarroch EE. Enteric nervous system: functional organization and neurologic implications. Neurology. 2007;69:1953–7. doi: 10.1212/01.wnl.0000281999.56102.b5. [DOI] [PubMed] [Google Scholar]
- 11.Gershon MD. Nerves, reflexes, and the enteric nervous system: pathogenesis of the irritable bowel syndrome. J Clin Gastroenterol. 2005;39(Suppl 3):S184–93. doi: 10.1097/01.mcg.0000156403.37240.30. [DOI] [PubMed] [Google Scholar]
- 12.Burns AJ, Roberts RR, Bornstein JC, Young HM. Development of the enteric nervous system and its role in intestinal motility during fetal and early postnatal stages. Semin Pediatr Surg. 2009;18:196–205. doi: 10.1053/j.sempedsurg.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Gershon MD. Transplanting the enteric nervous system: a step closer to treatment for aganglionosis. Gut. 2007;56:459–61. doi: 10.1136/gut.2006.107748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schafer KH, Micci MA, Pasricha PJ. Neural stem cell transplantation in the enteric nervous system: roadmaps and roadblocks. Neurogastroenterol Motil. 2009;21:103–12. doi: 10.1111/j.1365-2982.2008.01257.x. [DOI] [PubMed] [Google Scholar]
- 15.Vanderwinden JM. Role of Interstitial Cells of Cajal and their relationship with the enteric nervous system. Eur J Morphol. 1999;37:250–6. doi: 10.1076/ejom.37.4.250.4728. [DOI] [PubMed] [Google Scholar]
- 16.Negreanu LM, Assor P, Mateescu B, Cirstoiu C. Interstitial cells of Cajal in the gut--a gastroenterologist’s point of view. World J Gastroenterol. 2008;14:6285–8. doi: 10.3748/wjg.14.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burns AJ. Disorders of interstitial cells of Cajal. J Pediatr Gastroenterol Nutr. 2007;45(Suppl 2):S103–6. doi: 10.1097/MPG.0b013e31812e65e0. [DOI] [PubMed] [Google Scholar]
- 18.Rich A, Miller SM, Gibbons SJ, Malysz J, Szurszewski JH, Farrugia G. Local presentation of Steel factor increases expression of c-kit immunoreactive interstitial cells of Cajal in culture. Am J Physiol Gastrointest Liver Physiol. 2003;284:G313–20. doi: 10.1152/ajpgi.00093.2002. [DOI] [PubMed] [Google Scholar]
- 19.Li CX, Liu BH, Tong WD, Zhang LY, Jiang YP. Dissociation, culture and morphologic changes of interstitial cells of Cajal in vitro. World J Gastroenterol. 2005;11:2838–40. doi: 10.3748/wjg.v11.i18.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]


