Abstract
Gap junctions play an important role in the synchronized neuronal discharges. The main reason of the epileptic seizures is disruption of this synchronization. Therefore, the aim of the present study is to explore the combination valproic acid with carbenoxolone in pentylenetetrazole-kindled rats. In the first set of experiments, pentylenetetrazole (35 mg/kg intraperitoneally was administered to the rats to produce the kindling and then permanent screw electrodes to record electroencephalographic signals. The kindled rats were divided into six groups. While electroencephalographic recordings received from animals, behavioral evaluation was done by an observer. The data analysis was performed using T test and Mann-Whitney U tests. The dose of 40 mg/kg carbenoxolone was the most effective in carbenoxolone treatment groups. It prevented generalized seizures by 50%, reduced seizure stage, seizure duration and spike frequency. There was no significant difference between carbenoxolone-valproic acid combination and valproic acid on any seizure parameters. The current study is the first study which shows the interaction of carbenoxolone with valproic acid in pentylenetetrazole kindling model. As a result, carbenoxolone-valproic acid combination was not more effective than the standalone use of these drugs.
Keywords: Gap junction, carbenoxolone, valproic acid, kindling model, epilepsy
Introduction
Epilepsy is a one of the most common neurological disorder that affects 0.5%-2% of mankind [1,2]. Regardless of the availability of numerous new antiepileptic drugs, 30% of the epilepsy patients still have unrestrained seizures [3]. For this reason, numerous experimental seizure models have been developed in order to realize the essential mechanisms of epileptic disorders and to find out new drugs for seizure management [4,5].
One of them is chemical kindling that may be induced by repeated administration of an initially subconvulsive chemical stimulus that leads progressive increase in vulnerability to seizures [6-8]. Pentylenetetrazole is the most preferred drug to develop chemical kindling and it is reported that PTZ-kindled seizures mimic primary generalized epilepsy in aspect of ILEA classification [9]. It is thought that the action mechanisms of PTZ include blocking the GABA-mediated Cl- influx associated with the GABA-A receptor blockage [10]. This blockage induces neuronal membrane depolarization that causes the propagation of seizure activity. [8,11,12].
There is growing evidence that gap junction blockers (GJBs) may have preventing effects against seizures in experimental epilepsy models [13-15]. However, proconvulsant effects have also been reported [16,17]. Carbenoxolone (CBX) is one of the most widely used GJBs in these models. In our previous study we found that CBX (at a dose of 40 mg/kg) had antiseizure effect in PTZ-kindled rats [7].
Valproic acid (VPA) is one of the most commonly used antiepileptic drugs owing to its wide antiseizure spectrum of epileptic disorders. Although its mechanism of antiepileptic action is not completely clear, it is thought that GABA-A receptors are responsible for the main antiepileptic effect. Moreover, VPA enhances GABA production, reduces GABA transaminase, and inhibits excitatory neurotransmission [18-20]. It has been suggested that VPA successfully restrain absence, myoclonic, and tonic-clonic seizures of the primary generalized epilepsies [21]. It is found that VPA have anticonvulsive effect on PTZ-kindled model [22-24]. Moreover, it is suggested that CBX increase the efficacy of VPA in audiogenic epileptic mice [25]. However, to our knowledge, there is no study investigating the effect of CBX on the efficacy of VPA in PTZ-kindled model. Therefore, the aim of the present study is to explore the combination VPA with CBX in PTZ-kindled rats.
Materials and the methods
Research animals and the induction of the PTZ kindling model
In this study, 12-16 week-old female albino rats of Wistar breed with body weights of 200 ± 50 have been used. The animals have been acquired from Ondokuz Mayıs University Surgical Research Center. Before starting the experiments, a permission was granted upon our request (OMÜ HAYDEK/2008-48) by Ondokuz Mayıs University Research Animals Ethics Committee, and the ethics code has been observed throughout the experiment.
In order to induce kindling, three days of the week (Mondays-Wednesdays-Fridays) 35 mg/kg pentylenetetrazole was administered intraperitoneally until the animals kindled. Following the injection the animals were observed for thirty minutes within their glass observation cages (35×35×35 cm). The ictal behaviours of the animals were evaluated according to the Racine score [26]. If the seizure behavior was stage 4 or more severe, it was confirmed as a generalized seizure. Kindling was considered to be completed when an animal had experienced a total of five generalized seizures. Permanent screw electrodes were placed on the skulls of each of those kindled animals.
Surgical operation
The completely kindled animals were taken into surgery. In order to be able to record the brain activities of the awake animals, permanent screw electrodes were implanted to their skulls. For this reason, the animals were kept hungry for 12 hours before the operation in order to prepare them for the operation conditions. The next day anaesthesia was induced to the animals with a combination of 100 mg/kg Ketamine HCl-10 mg/kg-Xylazine. Each animal’s head has been fixed to the stereotaxic device. A 3 cm long incision was performed on the scalp along the rostro-caudal axis. After the incision, any scalp that covers the frontal and occipital cortex was removed from its fascia and tendon extensions.
Afterwards, with the aid of a drill 4 holes that were 1 mm in diameter and 1 mm in depth were drilled. The stainless steel screw electrodes were placed in these holes.
The screws have been fixed in accordance with the coordinates obtained from the rat brain atlas [27]: 1. At 4 mm anterior of Bregma point, 3mm left lateral from the midline, (positive electrode, over the primary motor region); 2. At 4 mm posterior of Bregma point 3mm left lateral of the midline, (negative electrode, over the medial parietal association cortex); 3. At 4 mm posterior of Bregma point 3 mm right lateral form the midline, (ground electrode); 4. At 4 mm anterior of Bregma point, 3 mm right lateral from the midline (in order for the acrylic to hold the electrode in a more secure manner a loose screw was placed in this hole).
Electrophysiologic recording process
The electrodes that were screwed in, to be able to obtain an EEG record, were connected to a small (3-prong) plug with thin cables. The screws and the plug were fastened to the skull through the use of dental acrylic. Following the operation, each animal was placed in a separate cage and were given at least five days’ time to recover.
At the end of the recovery period, the animals were connected to the PowerLab (AD Instruments) data acquisition system with the aid of a small plug placed on their heads. Thus, the cerebral electrical activity was transmitted to a computer environment via the Chart 5.1.1 program. Due to the macro properties of this software, it has been split into one-second slices. The number of spikes per minute have been calculated automatically due to this software’s such properties.
Drugs and routes
PTZ, CBX and VPA were obtained from Sigma-Aldrich Co. PTZ and CBX were dissolved in physiologic saline. VPA dissolved in distilled water. The entire chemical was given intraperitoneally (i.p.). VPA was injected 30 min prior to PTZ. But CBX was given 60 min before PTZ administration.
Experimental groups
Only kindled-rats were enrolled in groups. After kindling, the animals were divided into six groups. Number of PTZ injection which was used to complete the kindling for each group, was similar. Seizure score and EEG recordings were assessed simultaneously for 30 min in all groups.
1. PTZ (n = 6): 35 mg/kg PTZ
2: CBX-10 (n = 6): 10 mg/kg CBX
3. CBX-20 (n = 6): 20 mg/kg CBX
4. CBX 40 (n = 6): 40 mg/kg CBX
5 VPA (n = 6): 300 mg/kg VPA
6. CBX-VPA (n = 6): 40 mg/kg CBX + 300 mg/kg VPA
Statistical analysis
The data obtained were analyzed using the SPSS 16.0 package program for Windows. Data are expressed as means ± SEM. Statistical significance was determined as P values less than 0.05. Normal distribution was assessed in accordance with the Shapiro Wilk’s test. T test was used for comparisons of two groups, and Mann-Whitney U test was performed if variables did not show a normal distribution.
Results
In CBX-10 group and CBX-20 groups spike frequency increased at 10th minute of PTZ injection in comparison with PTZ group (P<0.05) (Figure 1). However, in CBX-10 group there was no significant change at other minutes. CBX 20 mg/kg significantly reduced the spike frequency after 25th minute of PTZ injection compared to PTZ group (Figure 1).
Figure 1.
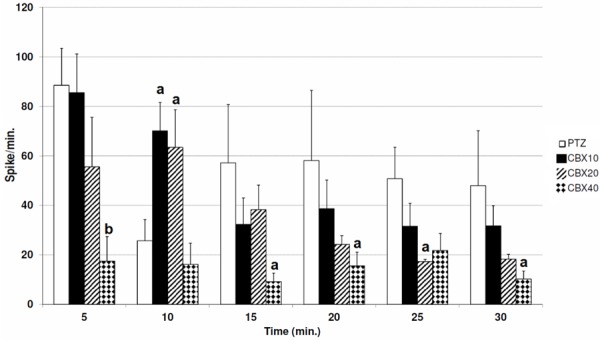
Comparing the spike frequency between CBX groups (in comparison with PTZ group (a: P<0.05, b: P<0.01).
At 40 mg/kg dose CBX reduced spike frequency after 5th (P<0.01), 15th (P<0.05), 20th (P<0.05) and 30 (P<0.05) minutes of PTZ injection compared with PTZ group (Figure 1).
CBX also reduced the seizure severity score at 40 mg/kg dose in comparison to PTZ group (P<0.05). Moreover, in CBX-40 group generalized seizures prevented in 3 of 6 animals, while in CBX-10, CBX 20 and PTZ group generalized seizures were observed in entire rats (Figure 2).
Figure 2.
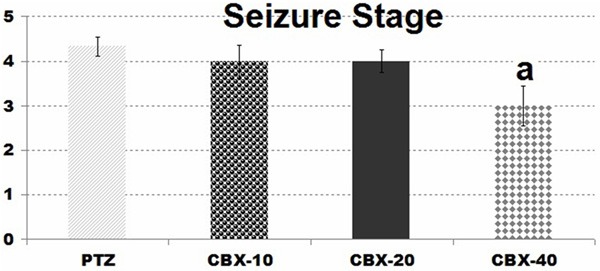
Comparison of seizure stage in CBX groups: (in comparison with PTZ group (a: P<0.05).
However in CBX 40 group onset of myoclonic seizures were prolonged significantly (P<0.05), 10 and 20 mg/kg doses of CBX have been unable to increase the onset of myoclonic seizures (Figure 3).
Figure 3.
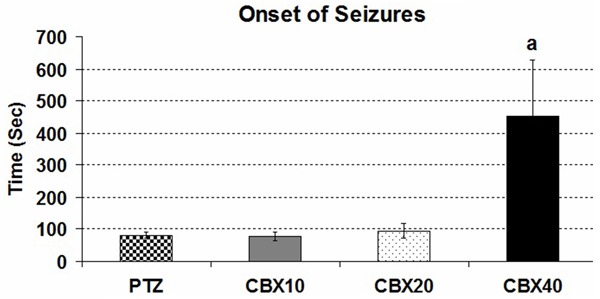
Comparison of the seizure onset in CBX groups: (in comparison with PTZ group (a: P<0.05) (Onset of seizure = The latency to the first myoclonic activity).
In CBX 20 and CBX 40 group duration of generalized seizures were reduced in comparison with PTZ group (P<0.05) (Figure 4).
Figure 4.
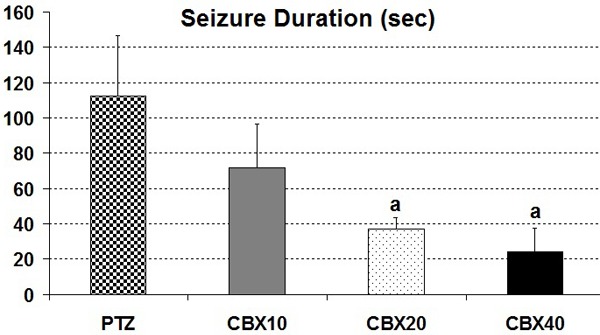
Comparison of the seizure duration in CBX groups: (in comparison with PTZ group (a: P<0.05).
In VPA group, myoclonic jerks were seen, in only 2 of 6 animals. Similarly, in CBX-VPA group 2 of 6 animals had myoclonic jerks. Therefore, the standard error values of myoclonic jerk latencies of VPA and CBX-VPA groups were not been calculated. Generalized seizures also were not observed in any animals of VPA and CBX-VPA groups.
VPA decreased the spike frequency at 5th, 25th (P<0.001); 15th (P<0.01); 10th, 20th and 30th (P<0.05) after PTZ application in comparison with PTZ group (Figure 5). VPA was also more effective at 5th, 25th and 30th min. after PTZ injection when compared to CBX group accord with spike frequency. Similarly CBX-VPA combination reduced the spike frequency at 5th (P<0.001); 10th, 15th, 20th, 25th and 30th (P<0.05) min. in comparison with PTZ group. When compared with CBX group at 5th min CBX-VPA combination is more effective according to spike frequency. However, there were no differences between VPA and CBX-VPA groups in terms of spike frequency and seizure stage (Figure 6).
Figure 5.
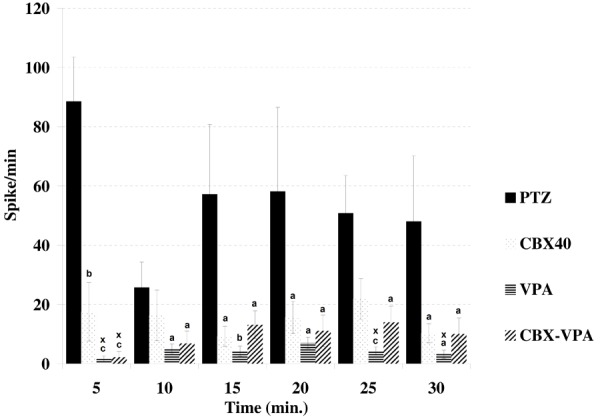
Comparison of the spike frequency between groups (in comparison with PTZ group; a: P<0.05, b: P<0.01, c: P<0.001; in comparison with CBX 40x: P<0.05).
Figure 6.
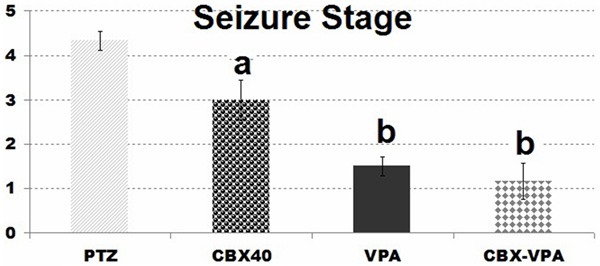
Comparison of seizure stage in groups: (in comparison with PTZ group (a: P<0.05, b: P<0.01,).
Discussion
This is the first study which shows the interaction of CBX, a gap junction blocker, with VPA in PTZ kindled rats. With this study, the effects of CBX and VPA in PTZ kindled awaken rats and CBX’s interaction with VPA in this model was evaluated with both electrophysiological and behavioral parameters.
In order to prevent audiogenic seizures in DBA/2 mice, Gareri et.al injected carbenoxolone i.p. 60 minutes before the audiogenic stimulation in 10, 15, 20 and 30 mg/kg, i.p doses and showed that audiogenic seizures restrained tonic-clonic phase in a dose-dependent manner, and likewise 40 mg/kg i.p. CBX which is injected 60 and 180 minutes before the audiogenic stimulation shortened the wild running phase of the audiogenic seizures [25].
Lan L et.al (2007a and 2007b) watched CBX’s effects on PTZ kindling model in two studies. In one of these studies, they injected carbenoxolone to kindled animals in 10 mg/kg ip dose for 3 days and showed that CBX reduced spontaneous seizures. And then they showed that GFAP-Li, Fos-Li and NMDAR-Li neurons increased in immunohistochemical terms in animals [28]. In the other study, they showed that 10 mg/kg ip CBX reduced rhythmic spikes and wave complexes. However, 10 mg/kg carbenoxolone was found ineffective in our study. This may be because Li et.al used carbenoxolone for three days. Moreover, Lan L et.al injected PTZ every day [29]. Yet, we created the kindling model by injecting 35 mg/kg PTZ only three days a week. Ding et.al injected carbenoxolone in 10 and 20 mg/kg doses before creating PTZ kindling model and showed that the seizure latency were lengthened in a dose-dependent manner. Twenty mg/kg carbenoxolone also reduced the seizure score. Besides, 10 and 20 mg/kg doses hippocampus led to increase in Cx32 expression in the study by Ding et.al [30].
In the current study, carbenoxolone was injected in 10, 20 and 40 mg/kg i.p. doses 60 minutes before PTZ. 20 and 40 mg/kg carbenoxolone reduced the spike frequency. 10 and 20 mg/kg carbenoxolone did not prevent generalized seizures and did not lead to a significant change in jerk latencies. Moreover, 40 mg/kg carbenoxolone significantly lengthened myoclonic jerk latency. Similarly, 10 and 20 mg/kg carbenoxolone prolonged the seizure latency in the study by Ding et.al [30].
In a study by Hosseinzadeh and Asl, CBX was used as 100, 200 and 300 mg/kg ip in the acute PTZ model in mice and 300 mg/kg was found to be the most effective. However, they also saw that CBX in such doses reduced the motor activity [31]. CBX can lead to side effects such as ataxia, tachypnea, tachycardia and vomiting when it is applied systematically [32]. It was observed that CBX reduced stereotypical movements when systematically applied to the rats in 7-35 mg/kg dose interval [32]. In addition, 100 mg/kg and higher doses of carbenoxolone lead to reduction in coordination, sedation and muscle relaxant effect in mice [31]. We avoided using higher doses of carbenoxolone due to these side effects.
VPA is an effective antiepileptic drug in the kindling model. It shortens afterdischarge duration and reduces the severity of the seizures. VPA prevents kindled in chronic application, too [22-24]. Seizures with light severity were seen in half of the animals when 200 mg/kg i.p VPA 40 mg/kg PTZ were simultaneously applied [33]. However, 300 mg/kg VPA generalized seizures were completely prevented in our study. It also reduced spike frequency. The difference between them may be because they injected VPA in less dose and PTZ simultaneously. In the literature scanning, there were no studies which showed VPA’s effect on the spike frequency in the rats which were kindled with PTZ. Silver et.al, on the other hand, showed that 100, 200 and 400 mg/kg VPA in rats reduced the seizure score in amygdale kindling model in a dose-dependent manner and shortened afterdischarge duration [34]. Likewise, [35] showed that 200 and 300 mg/kg i.p. VPA which was injected 30 minutes before electrical stimulation in guinea pigs shortened afterdischarge duration of amygdale kindling seizures in a dose-dependent manner and reduced the frequency of the seizure score. In the study which we did in the light of this information, we injected VPA in 300 mg/kg dose i.p. 30 minutes before PTZ.
In the present study, in VPA group generalized seizures were completely restrained. It also significantly reduced the seizure score and spike frequency. These results show that VPA is effective towards PTZ kindling seizures. Li et.al showed that VPA reduced glutamate and aspartate release in rats which were kindled with PTZ. The disruption of the balance between the excitatory and inhibitor neurotransmitters plays an important role in epilepsy [33,36].
Gareri et.al showed that 40 mg/kg CBX shortened the wild running phase of the seizures in DBA/2 audiogenic mice. Furthermore, in this study, CBX significantly increased the antiepileptic effectiveness of VPA in audiogenic seizures [25]. In our study, on the other hand, carbenoxolone did not cause any increase in the effectiveness of VPA. In our study, unlike the study by Gareri et.al, carbenoxolone did not contribute to VPA’s antiepileptic effect on potentialization level when all parameters were evaluated. This may be because different parts of the brain can be affected in audiogenic seizures and PTZ kindled seizures.
In conclusion, the current study is the first study which shows the interaction of CBX with VPA in PTZ kindling model. As a result, CBX-VPA combination was not more effective than the standalone use of these drugs.
Acknowledgements
This project was supported by the Ondokuz Mayis University Research Foundation (PYO.TIP.1904.09.010).
Disclosure of conflict of interest
None.
References
- 1.Hauser WA, Hesdorffer DC. Demos, New York: 1990. Epilepsy: Frequency, causes and consequences. [Google Scholar]
- 2.Li D, Li P, He Z, Cen D, Meng Z, Liang L, Luo X. Human intravenous immunoglobulins suppress seizure activities and inhibit the activation of GFAP-positive astrocytes in the hippocampus of picrotoxin-kindled rats. Int J Neurosci. 2012;122:200–8. doi: 10.3109/00207454.2011.639470. [DOI] [PubMed] [Google Scholar]
- 3.Saad K, Hammad E, Hassan AF, Badry R. Trace element, oxidant, and antioxidant enzyme values in blood of children with refractory epilepsy. Int J Neurosci. 2014;124:181–6. doi: 10.3109/00207454.2013.831851. [DOI] [PubMed] [Google Scholar]
- 4.Sander JW. The epidemiology of epilepsy revisited. Curr Opin Neurol. 2003;16:165–70. doi: 10.1097/01.wco.0000063766.15877.8e. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs MP, Fischbach GD, Davis MR, Dichter MA, Dingledine R, Lowenstein DH, Morrell MJ, Noebels JL, Rogawski MA, Spencer SS, Theodore WH. Future directions for epilepsy research. Neurology. 2001;57:1536–42. doi: 10.1212/wnl.57.9.1536. [DOI] [PubMed] [Google Scholar]
- 6.Lukasiuk K, Wilczynski GM, Kaczmarek L. Extracellular proteases in epilepsy. Epilepsy Res. 2011;96:191–206. doi: 10.1016/j.eplepsyres.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Sefi l F, Bagirici F, Acar MD, Marangoz C. Influence of carbenoxolone on the anticonvulsant efficacy of phenytoin in pentylenetetrazole kindled rats. Acta Neurobiol Exp (Wars) 2012;72:177–84. doi: 10.55782/ane-2012-1890. [DOI] [PubMed] [Google Scholar]
- 8.Malhi SM, Jawed H, Hanif F, Ashraf N, Zubair F, Siddiqui BS, Begum S, Kabir N, Simjee SU. Modulation of c-Fos and BDNF protein expression in pentylenetetrazole-kindled mice following the treatment with novel antiepileptic compound HHL-6. Biomed Res Int. 2014;2014:876712. doi: 10.1155/2014/876712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbert ME, Goodman JH. Chemical Kindling. In: Pitkänen A, Philip A, Solomon LM, editors. Models of Seizures and Epilepsy. vol. Chapter 31. Elsevier Academic Press; 2006. pp. 379–391. [Google Scholar]
- 10.Bahcekapili N, Akgun-Dar K, Albeniz I, Kapucu A, Kandil A, Yagiz O, Uzum G. Erythropoietin pretreatment suppresses seizures and prevents the increase in inflammatory mediators during pentylenetetrazole-induced generalized seizures. Int J Neurosci. 2014;124:762–70. doi: 10.3109/00207454.2013.878935. [DOI] [PubMed] [Google Scholar]
- 11.Sejima H, Ito M, Kishi K, Tsuda H, H Shiraishi. Regional excitatory and inhibitory amino acid concentrations in pentylenetetrazol kindling and kindled rat brain. Brain Dev. 1997;19:171–5. doi: 10.1016/s0387-7604(96)00492-5. [DOI] [PubMed] [Google Scholar]
- 12.Hansen SL, Sperling BB, Sanchez C. Anticonvulsant and antiepileptogenic effects of GABAA receptor ligands in pentylenetetrazole-kindled mice. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:105–13. doi: 10.1016/j.pnpbp.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 13.Gajda Z, Szupera Z, Blazso G, Szente M. Quinine, a blocker of neuronal cx36 channels, suppresses seizure activity in rat neocortex in vivo. Epilepsia. 2005;46:1581–91. doi: 10.1111/j.1528-1167.2005.00254.x. [DOI] [PubMed] [Google Scholar]
- 14.Sayyah M, Rezaie M, Haghighi S, Amanzadeh A. Intra-amygdala all-trans retinoic acid inhibits amygdala-kindled seizures in rats. Epilepsy Res. 2007;75:97–103. doi: 10.1016/j.eplepsyres.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 15.Bostanci MO, Bagirici F. Anticonvulsive effects of quinine on penicillin-induced epileptiform activity: an in vivo study. Seizure. 2007;16:166–72. doi: 10.1016/j.seizure.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Voss LJ, Jacobson G, Sleigh JW, Steyn-Ross A, Steyn-Ross M. Excitatory effects of gap junction blockers on cerebral cortex seizure-like activity in rats and mice. Epilepsia. 2009;50:1971–8. doi: 10.1111/j.1528-1167.2009.02087.x. [DOI] [PubMed] [Google Scholar]
- 17.Voss LJ, Mutsaerts N, Sleigh JW. Connexin36 gap junction blockade is ineffective at reducing seizure-like event activity in neocortical mouse slices. Epilepsy Res Treat. 2010;2010:310753. doi: 10.1155/2010/310753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johannessen CU, Johannessen SI. Valproate: past, present, and future. CNS Drug Rev. 2003;9:199–216. doi: 10.1111/j.1527-3458.2003.tb00249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loscher W. Valproate: a reappraisal of its pharmacodynamic properties and mechanisms of action. Prog Neurobiol. 1999;58:31–59. doi: 10.1016/s0301-0082(98)00075-6. [DOI] [PubMed] [Google Scholar]
- 20.Loscher W. Basic pharmacology of valproate: a review after 35 years of clinical use for the treatment of epilepsy. CNS Drugs. 2002;16:669–94. doi: 10.2165/00023210-200216100-00003. [DOI] [PubMed] [Google Scholar]
- 21.Rogawski MA, Loscher W. The neurobiology of antiepileptic drugs. Nat Rev Neurosci. 2004;5:553–64. doi: 10.1038/nrn1430. [DOI] [PubMed] [Google Scholar]
- 22.Dziki M, Honack D, Loscher W. Kindled rats are more sensitive than non-kindled rats to the behavioural effects of combined treatment with MK-801 and valproate. Eur J Pharmacol. 1992;222:273–8. doi: 10.1016/0014-2999(92)90866-3. [DOI] [PubMed] [Google Scholar]
- 23.Loscher W, Honack D. Effects of the competitive NMDA receptor antagonist, CGP 37849, on anticonvulsant activity and adverse effects of valproate in amygdala-kindled rats. Eur J Pharmacol. 1993;234:237–45. doi: 10.1016/0014-2999(93)90959-l. [DOI] [PubMed] [Google Scholar]
- 24.Mori N, Ohta S. Comparison of anticonvulsant effects of valproic acid entrapped in positively and negatively charged liposomes in amygdaloid-kindled rats. Brain Res. 1992;593:329–31. doi: 10.1016/0006-8993(92)91330-h. [DOI] [PubMed] [Google Scholar]
- 25.Gareri P, Condorelli D, Belluardo N, Gratteri S, Ferreri G, Donato Di Paola E, De Sarro A, De Sarro G. Influence of carbenoxolone on the anticonvulsant efficacy of conventional antiepileptic drugs against audiogenic seizures in DBA/2 mice. Eur J Pharmacol. 2004;484:49–56. doi: 10.1016/j.ejphar.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 26.Racine RJ. Modification of seizure activity by electrical stimulation. I. After-discharge threshold. Electroencephalogr Clin Neurophysiol. 1972;32:269–79. doi: 10.1016/0013-4694(72)90176-9. [DOI] [PubMed] [Google Scholar]
- 27.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th edition. Amsterdam: Elsevier; 2007. [Google Scholar]
- 28.Lan L, Zhao X, Lan Y, Duan L, Cao R, Liu Y, Rao Z. [Effect of carbenoxolone on expression of Fos, NMDAR2 and GFAP in the hippocampus of pentylenetetrazo-kindled epileptic rats] . Zhongguo Dang Dai Er Ke Za Zhi. 2007;5:465–8. [PubMed] [Google Scholar]
- 29.Lan L, Zhao X, Lan Y, Duan L, Cao R, Liu Y, Rao Z. Effect of carbenoxolone on behavior and electroencephalogram of PTZ-kindled epileptic rats. Journal of Xi’an Jiaotong University (Medical Sciences) 2007;28:627–630. [Google Scholar]
- 30.Ding PP, Xu LL, Bao YY. Effects of carbenoxolone on upregulation of connexin 32 in hippocampus of pentylenetetrazole kindled rats. Chinese Pharmaceutical Journal. 2006;41:182–185. [Google Scholar]
- 31.Hosseinzadeh H, Nassiri Asl M. Anticonvulsant, sedative and muscle relaxant effects of carbenoxolone in mice. BMC Pharmacol. 2003;3:3. doi: 10.1186/1471-2210-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Juszczak GR, Swiergiel AH. Properties of gap junction blockers and their behavioural, cognitive and electrophysiological effects: animal and human studies. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:181–98. doi: 10.1016/j.pnpbp.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 33.Li ZP, Zhang XY, X Lu, Zhong MK, Ji YH. Dynamic release of amino acid transmitters induced by valproate in PTZ-kindled epileptic rat hippocampus. Neurochem Int. 2004;44:263–70. doi: 10.1016/s0197-0186(03)00148-7. [DOI] [PubMed] [Google Scholar]
- 34.Silver JM, Shin C, McNamara JO. Antiepileptogenic effects of conventional anticonvulsants in the kindling model of epilespy. Ann Neurol. 1991;29:356–63. doi: 10.1002/ana.410290404. [DOI] [PubMed] [Google Scholar]
- 35.Gilbert TH, Corley SM, Teskey GC. Conventional anticonvulsant drugs in the guinea pig kindling model of partial seizures: effects of acute phenobarbital, valproate, and ethosuximide. Exp Brain Res. 2002;146:336–44. doi: 10.1007/s00221-002-1183-9. [DOI] [PubMed] [Google Scholar]
- 36.Long L, Xiao B, Feng L, Yi F, Li G, Li S, Mutasem MA, Chen S, Bi F, Li Y. Selective loss and axonal sprouting of GABAergic interneurons in the sclerotic hippocampus induced by LiCl-pilocarpine. Int J Neurosci. 2011;121:69–85. doi: 10.3109/00207454.2010.530007. [DOI] [PubMed] [Google Scholar]


