Abstract
Background: The prognostic significance of intratumoral and peripheral interleukin-17 (IL-17) in tumors has been studied worldwide during these years, providing un-uniformed conclusions. Methods: We conducted a meta-analysis of published literatures that evaluated the correlation between IL-17 and clinical staging, overall survival (OS) and/or disease free survival (DFS). Results: A total of 28 studies enrolling 2902 patients were included. For the overall population, a high expression of IL-17 was found significantly correlated with worse DFS (HR = 1.59, 95% CI: 1.24-2.03) in patients with solid tumors. For gastrointestinal tumors, patients with IL-17 high seemed to have worse OS (HR = 1.85, 95% CI: 1.24-2.75) and DFS (HR = 2.41, 95% CI: 1.98-2.92). Sub-group meta-analysis revealed that IL-17 indicated late clinical staging in non-small cell lung cancer (NSCLC) patients (HR = 2.33, 95% CI: 1.25-4.32), on the other hand, early clinical staging in patients with esophageal squamous carcinoma (HR = 0.63, 95% CI: 0.42-0.94). Negative impacts of IL-17 on OS were shown in patients with hepatocellular carcinoma (HCC) (HR = 1.87, 95% CI: 1.23-2.84) or NSCLC (HR = 1.55, 95% CI: 1.02-2.35). However, positive impacts on OS were provided in patients with esophageal squamous carcinoma (HR = 0.65, 95% CI: 0.50-0.84). Besides, a high expression of IL-17 predicted better DFS in ovarian cancer patients (HR = 0.33, 95% CI: 0.11-1.00). Conclusions: Our meta-analysis revealed that IL-17 might correlate with poor OS and DFS in gastrointestinal tumors. Specifically, IL-17 was a detrimental factor for HCC and NSCLC patients, whereas a beneficial factor for patients with esophageal squamous carcinoma and ovarian cancer.
Keywords: Interleukin-17, solid tumor, prognosis, meta-analysis
Introduction
Interleukin-17 (IL-17) is a pro-inflammatory cytokine that was first described in 1993 [1]. The IL-17 gene was unexpected isolated from a murine cytotoxic lymphocyte hybridoma cDNA library and first named cytotoxic T lymphocyte (CTL) antigen-8 (CTLA-8). Later on, the human IL-17, IL-17 receptor (IL-17R) and more cytokines resembling it were discovered, which finally contributed to form a new cytokine family consisting of six cytokines (IL-17A, IL-17B, IL-17C, IL-17D, IL-17E, IL-17F) and five receptors (IL-17RA, IL-17RB, IL-17RC, IL-17RD, IL-17RE). IL-17A was commonly called IL-17 in some studies, for it was the founding cytokine of the IL-17 family [2-5]. IL-17 is mainly secreted by T helper 17 (Th17) cells while other cells such as NKT cells, CD8+ T cells, γδ-T cells, neutrophils and Paneth cells are found producing some as well [6,7]. Th17 cells produce large quantities of IL-17 as a result that most Th17-mediated effects are attributed to this cytokine. In the past two decades, IL-17 has been under intensive investigation because of its potential role in allergy, autoimmune diseases, host defense against infections, tumorigenesis and allograft transplantation [8-23].
To date, a plenty of studies have focused on the features and functions of IL-17 in solid tumors, but the correlation of IL-17 with tumor growth and progression has not been completely elucidated. On one hand, IL-17 can promote tumor growth by antiapoptotic and angiogenic activities [19,24,25]. On the other hand, it can promote effector cytotoxic T lymphocytes (CTLs) generation and enhance their anti-tumor response [26-28]. More effort is needed to shed light on the mechanism of how IL-17 influences the growth and progression of tumor.
Meanwhile, a plenty of studies have evaluated the prognostic value of IL-17 in solid tumors, and most studies suggested a highly expressed IL-17 was beneficial for tumor growth and associated with poor prognosis [29-43]. However, there were some exceptions that a high level of IL-17 in tumor tissue or peripheral blood indicated a prolonged overall survival or disease free survival in different tumors [44-48]. In some other studies, the relationship between IL-17 and prognosis turned out to be indeterminate [49-56]. Contradictory conclusions were drawn even in one tumor by different researchers [35-37,41-43,46,56]. This meta-analysis was designed to clarify the impacts of IL-17 on clinical outcomes in patients with solid tumors.
Materials and methods
Identification and eligibility of relevant studies
A prespecified protocol was designed before we performed the meta-analysis. We conducted a computer-aided literature search of PubMed/MEDLINE and EMBASE with the search strategy based on a combination of Medical Subject Headings (MeSH) and text words relating to “interleukin-17”, “IL-17”, “tumor”, “cancer”, “neoplasm”, “carcinoma” and “oncology”. We retrieved literature from the database inception to October 13, 2013. Reference list of primary identified studies and relevant reviews were also screened to prevent missing any eligible studies by the electronic search strategies.
All of the eligible studies included in our meta-analysis met the inclusion criteria as follows: 1. Patients with pathological diagnosis of solid tumors; 2. Studies focused on the correlation of IL-17 with clinical staging, overall survival (OS) and/or disease free survival (DFS) of patients with solid tumors, regardless of the sample size or follow-up period; 3. Studies with sufficient data available to estimate the hazard ratio (HR) and corresponding 95% confidence interval (CI) of the impact of IL-17 on clinical staging, OS and/or DFS; 4. Intratumoral or peripheral IL-17 expression level evaluated by immunohistochemical (IHC), enzyme linked immunosorbent assay (ELISA), flow cytometry (FCM) or reverse transcription-polymerase chain reaction (RT-PCR) method. The exclusion criteria included: 1. Patients diagnosed with hematological tumors or recurrent solid tumors; 2. Studies without sufficient information to estimate the correlation of IL-17 with clinical staging, OS or DFS; 3. Full-text published in other languages rather than English or Chinese. Studies were also excluded if they pertained to overlapping patients with other included studies while the one with the largest sample size was included for meta-analysis.
Two independent reviewers conducted the primary assessment by identifying the eligibility of the abstracts from database. Full-text review was done for further assessment when the eligibility was unclear from the abstracts. Any disagreement in assessment was discussed and resolved seriously. As there has not been a general agreed score system to weigh each study in an observational prognostic meta-analysis by a quality score, we therefore conducted sub-group analyses for instead as widely recommended.
Definitions and standardizations
We identified clinical staging according to the TMN staging system (Union for International Cancer Control, UICC), FIGO stage (International Federation of Gynecology and Obstetrics) or other worldwide used staging systems. Overall survival (OS) referred to the length of time from the date of initial definitive treatment to death or deadline of follow-up, and disease free survival (DFS) referred to the duration of survival without any documented local regional or distant relapse.
So far, most studies have detected the IL-17 expression level in tumor tissue and few in peripheral blood or ascites. As peripheral and intratumoral IL-17 presented consistent prognostic prediction value in current published studies, we included studies evaluating either peripheral or intratumoral expression of IL-17 and grouped them to perform meta-analysis respectively. When IL-17 was measured in both center and invasive margin of tumor tissue, we chose the latter because necrosis and hypoxia in the center part of tumor might influence the cytokines production and distribution in the microenvironment of tumor. We preferred HR value and corresponding 95% CI of multivariate Cox regression analysis rather than that of univariate analysis when both were reported in study. In the enrolling studies, IL-17 protein, IL-17 positive cells and IL-17 mRNA were measured by IHC, ELISA, RT-PCR or FCM with variety of antibodies. Some of the studies defined the cut-off value by the “minimum P value” approach while the others used the mean or median level of IL-17 to separate the IL-17 high or IL-17 low group, so that the definitions of IL-17 positive or IL-17 high could not be consolidated in the meta-analysis and the potential bias should be considered when conclusions were drawn.
Data extraction
Data extraction was carefully conducted in duplicate by two of us with a standard data collection form. From all of the enrolling studies, the following information was collected: first author, time of publication, country of origin, tumor location, number of patients (number of men), median age, clinical staging, median follow-up, IL-17 assessment, experimental methods, antibody applied, cut-off value of IL-17 high, prognostic analysis, HR estimation and result. Any difference in the data extraction was discussed and resolved seriously by two of us.
Statistical analysis
We divided the included studies into three groups for meta-analysis according to data regarding clinical staging, OS and DFS. A study was considered significant when the P value for comparing clinical staging status or survival distribution between IL-17 high and low group was inferior to 0.05. We identified the result of a study as “positive” when a high level of IL-17 predicted a late clinical staging or short survival, “negative” when a high level of IL-17 predicted an early clinical staging or prolonged survival, and “indeterminate” when there was no significant relationship between IL-17 and clinical staging or survival.
For the quantitative aggregation of results, HRs and corresponding 95% CIs in each study were used to estimate the impacts of IL-17 on clinical staging, OS or DFS of patients with solid tumors. We first extracted HRs and 95% CIs that were available from the original article directly. For those without reported HRs or 95 CIs, we used the published data including the number of patients at risk and the total number of events in each group from original articles to estimate HR and 95% CIs according to the methods described by Parmar’s team [57]. If the data were only available in the form of figures, we read Kaplan-Meier curves from the articles by Engauge Digitizer version 4.1 (free software down-loaded from http://sourceforge.net) and extracted relative data to calculate HRs and 95% CIs. An observed HR > 1 implied a worse outcome for the IL-17 high group relative to IL-17 low group and would be considered statistically significant if the 95% CI for HR did not overlap 1, with P < 0.05. Random effect meta-analysis using DerSimonian-Laird algorithm was carried out with the Stata version 11.0 (Stata Corporation, College Station, TX, USA) and potential published bias on the outcomes were illustrated and assessed by both Begg’s test and Egger’s test, in which P < 0.10 for Begg’s test or P < 0.05 for Egger’s test indicated there were statistically significant publication bias [58,59].
Results
Studies selection and characteristics
The initial computer-aided literature search from PubMed/MEDLINE and EMBASE retrieved 2076 records after duplicates removed. Then, 1878 records were excluded by screening abstracts and 198 records needed further assessment. The full-text of the remaining studies was obtained and read in detail. Ninety-one records were out of scope because of no exploitable survival statistics available for analysis, so were the 49 conference abstracts, 26 reviews and 2 animal experiments. Additionally, 2 candidate records published in Polish were excluded (Figure 1). Finally, a total of 28 studies (n = 2902 patients) were identified as eligible for inclusion in our meta-analysis.
Figure 1.
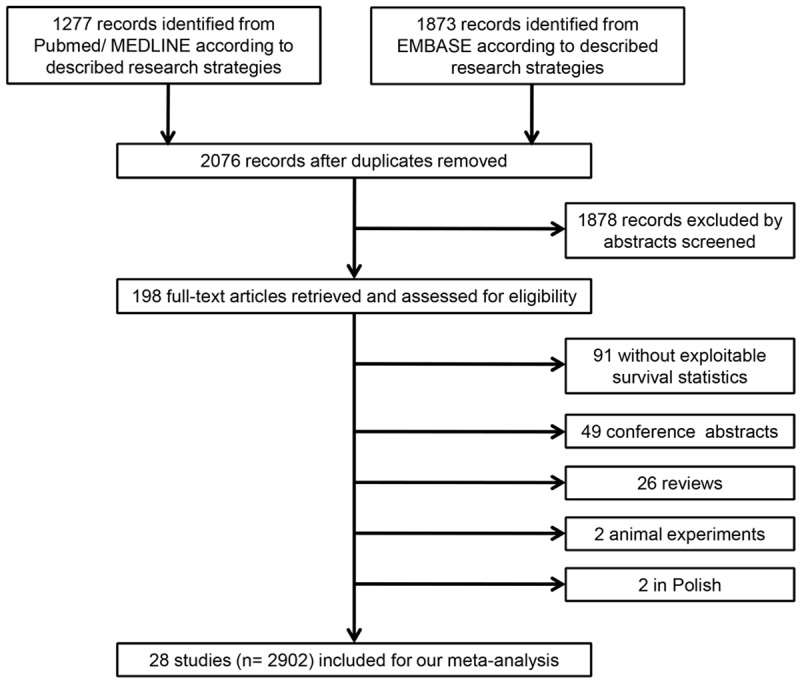
Flow chart of the literature search and selection of included studies.
The main features of the 28 included studies are listed in Table 1. The included studies were published between the year of 2009 and 2013 with one exception in 2001. Most studies enrolled were performed in Asia, while 3 in Europe and America. The median sample size for all studies was 102.5 patients (range from 17 to 300) and the median sample sizes for clinical staging, OS and DFS were 102, 104 and 105, respectively. Male subjects made up around 58.4% of all the studies (excepting 2 without data available), and the median age of all was 54.7 years old (excepting 5 studies not reported). For all studies included, 51.6% patients were in clinical staging I/II when diagnosed. Only 8 studies reported median follow-up period with an average of 2.9 years. However, 7 or more studies which did not report follow-up period estimated the prognostic value of IL-17 based on 5 year survival. Intratumoral IL-17 expression was assessed by intratumoral IL-17 positive stained cells with the method of IHC in 20 studies, and IL-17 positive stained cells in invasive margin of tumor was used in 1 study. The IL-17 protein in tumor tissue was measured in one study using IHC. Meanwhile, peripheral IL-17 was assessed by IL-17 protein in patient serum with the method of ELISA in 2 studies, and IL-17 protein in ascites in another one. In addition, T helper 17 (Th17) cells in peripheral blood were counted by FCM to assess the expression of IL-17 in one study. The antibodies applied for IL-17 measurement varied from each other, including mouse, goat or rabbit monoclonal or polyclonal anti-human IL-17 antibodies from different companies. The cut-off value for IL-17 high was different among the enrolling studies. The cut-off value of 16 studies was set at the median number or proportion of IL-17 positive stained cells or identified by the approach of “minimum P value”. In the 3 studies detecting peripheral IL-17 protein, the cut-off value was 0.9 and 1.0 pg/mL in patient serum and 220 pg/mL in ascites. Meanwhile, the cut-off value of 3 studies was 0 and the other 6 studies did not report. There were 17 studies available for prognostic analysis of IL-17 on clinical staging, 20 studies for OS and 13 studies for DFS. Of all, the results of 15 studies were positive whereas 7 negative and 8 indeterminate (2 studies reached negative results in OS analysis but indeterminate results in clinical staging analysis).
Table 1.
Characteristics of the eligible studies included in the meta-analysis
| First Author | Year | Country | Tumor Location | No. of patient (M/F) | Median Age (y) | Stage I/II (III/IV) | Median Follow-up (y) | Assessment of IL-17 | Method | Antibody Applied | Cut-off (IL-17 High) | Prognostic Analysis | Estimation of HR | Result |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gu FM [29] | 2012 | China | Bile duct | 123 (62/61) | 55 | 91 (32) | 1.1 | intratumoral IL-17 positive stained cells | IHC | Goat antihuman IL-17A | 111 cells counted per 1-mm core | OS/Stage | Reported in text/Data extrapolated | Positive |
| Cui XL [44] | 2013 | China | Brain | 41 (18/23) | 47 | 0 (41) | 1.1 | intratumoral IL-17 positive stained cells | IHC | Mouse anti-human IL-17 monoclonal antibody (Santa Cruz Biotechnology) | 15% positive cells | OS/DFS | Reported in text | Negative |
| Hu JH [49] | 2011 | China | Brain | 24 (18/6) | 41.3 | 12 (12) | NR | intratumoral mRNA of IL-17 | RT-PCR | NR | 0 | Stage | Data extrapolated | Indeterminate |
| Chen WC [30] | 2013 | Taiwan | Breast | 207 (0/207) | 51 | 137 (70) | 5.6 | intratumoral IL-17 positive stained cells | IHC | Goat polyclonal anti-human IL-17 antibody | 90 cells/5 HPFs | DFS/Stage | Reported in text | Positive |
| Liu JK [31] | 2011 | China | Colorectum | 52 (32/21) | NR | 0 (52) | NR | intratumoral IL-17 positive stained cells | IHC | Goat monoclonal anti-human IL-17 antibody | NR | OS | Reported in text | Positive |
| Tosolini M [32] | 2011 | France | Colorectum | 106 (?/?) | NR | 48 (58) | NR | IL-17 positive stained cells in invasive margin | IHC | Antibody against IL-17 (H-132; Santa Cruz Biotechnology) | NR (“minimum P value” approach) | DFS | Reported in text | Positive |
| Lv L [50] | 2011 | China | Esophagus | 181 (141/40) | 56 | 117 (64) | NR | intratumoral IL-17 positive stained cells | IHC | Goat antihuman IL-17 | 3.90 cells/HPF | OS/Stage | Reported in text/Data extrapolated | Indeterminate |
| Wang B [45] | 2013 | China | Esophagus | 215 (160/55) | 56 | 137 (78) | 2.5 | intratumoral IL-17 positive stained cells | IHC | Rabbit/goat polyclonal anti-human IL-17 antibody | 8.6 cells | OS/Stage | Reported in text/Data extrapolated | Negative |
| Zhang Y [33] | 2013 | China | Gallbladder | 104 (41/63) | 66.13 | 44 (60) | 3.3 | intratumoral IL-17 positive stained cells | IHC | Monoclonal antibody against IL-17 | NR | OS/DFS/Stage | Survival curve | Positive |
| Qiu LB [34] | 2011 | China | Hypophysis | 75 (27/48) | 51 | 35 (40) | NR | intratumoral IL-17 positive stained cells | IHC | Rabbit polyclonal anti human IL-17 antibody | 10% positive cells | Stage | Data extrapolated | Positive |
| Meng CD [51] | 2012 | China | Larynx | 32 (25/7) | 59 | 20 (12) | NR | intratumoral IL-17 positive stained cells | IHC | Rabbit polyclonal anti human IL-17 antibody | 5% positive cells | Stage | Data extrapolated | Indeterminate |
| Liao R [35] | 2013 | China | Liver | 300 (253/47) | 53 | 202 (98) | NR | intratumoral IL-17 positive stained cells | IHC | Antibody of IL-17 | 51 positive cells | OS/DFS | Survival curve | Positive |
| Lin SZ [46] | 2013 | China | Liver | 132 (122/10) | 51 | 82 (50) | NR | intratumoral IL-17 positive stained cells | IHC | Monoclonal antibody against IL-17 | NR (“minimum P value” approach) | OS/DFS | Reported in text | Negative |
| Wu JX [36] | 2012 | China | Liver | 105 (91/14) | 53 | 74 (31) | 1.7 | IL-17 protein in patient serum | ELISA | Quinsy’s human 9-plex assay kit | 0.9 pg/ml | OS/DFS | Reported in text/Survival curve | Positive |
| Zhang JP [37] | 2009 | China | Liver | 108 (95/13) | 46 | 82 (26) | NR | intratumoral IL-17 positive stained cells | IHC | Goat antihuman IL-17A | 70th percentile value | OS/DFS | Reported in text | Positive |
| Chang YH [52] | 2010 | Taiwan | Lung | 79 (52/27) | NR | 41 (38) | NR | intratumoral IL-17 positive stained cells | IHC | Rabbit polyclonal anti human IL-17 antibody | 10% cells | Stage | Data extrapolated | Indeterminate |
| Chen X [38] | 2010 | China | Lung | 52 (41/11) | 51.9 | 33 (19) | NR | intratumoral IL-17 positive stained cells | IHC | Rabbit polyclonal anti human IL-17 antibody | 5% positive cells | OS/DFS/stage | Reported in text/Data extrapolated | Positive |
| Zhang GQ [39] | 2012 | China | Lung | 102 (66/36) | 65 | 69 (33) | 2.5 | intratumoral IL-17 protein | IHC | Mouse anti-human IL-17A antibody | 0 | OS/Stage | Reported in text/Data extrapolated | Positive |
| Zhang YL [53] | 2010 | China | Nasopharynx | 106 (84/22) | 49 | 38 (68) | NR | intratumoral IL-17 positive stained cells | IHC | Goat monoclonal anti-human IL-17 (R&D Systems) | 5.60 cells/HPF | OS/DFS/Stage | Reported in text/Data extrapolated | Indeterminate |
| Droeser RA [47] | 2013 | Switzerland | Ovary | 94 (0/90) | NR | 11 (83) | NR | intratumoral IL-17 positive stained cells | IHC | Goat polyclonal anti-human IL-17 antibody | 1 positive cell/punch | DFS/Stage | Data extrapolated | Negative |
| Kato T [54] | 2001 | Japan | Ovary | 17 (0/17) | NR | 9 (8) | NR | intratumoral mRNA of IL-17 | RT-PCR | NR | 0 | Stage | Data extrapolated | Indeterminate |
| Kryczek I [55] | 2009 | America | Ovary | 85 (0/85) | 61 | 5 (80) | NR | IL-17 protein in ascites | ELISA | Anti-IL-17 (eBioscience) | 220 pg/mL | OS/Stage | Reported in text/Data extrapolated | Negative/Indeterminate |
| Lan CY [48] | 2013 | China | Ovary | 104 (0/104) | 53 | 0 (104) | NR | intratumoral IL-17 positive stained cells | IHC | Goat polyclonal anti-human IL-17 antibody | 35th percentile value | OS/DFS | Reported in text | Negative |
| He SB [40] | 2011 | China | Pancreas | 46 (31/15) | 61 | 22 (24) | NR | intratumoral IL-17 positive stained cells | IHC | Anti-IL-17-PE (PE-labeled mouse anti-human IL-17 antibody) | NR (median value) | OS | Survival curve | Positive |
| Chen JG [56] | 2011 | China | Stomach | 192 (129/63) | 58 | 79 (113) | 5.1 | intratumoral IL-17 positive stained cells | IHC | Goat anti-human IL-17 polyclonal antibody | 2.5 cells | OS/Stage | Reported in text/Data extrapolated | Negative/Indeterminate |
| Liu T [41] | 2012 | China | Stomach | 32 (21/11) | 54 | 9 (23) | NR | Th17 cells in peripheral blood | FCM | IL-17-PE | NR | OS | Survival curve | Positive |
| Yamada Y [42] | 2012 | Japan | Stomach | 85 (?/?) | 65.5 | 66 (29) | NR | IL-17 protein in patient serum | ELISA | Human IL-17 (IL-17A) ELISA Development Kit | 1 pg/mL | OS | Reported in text | Positive |
| Zhuang Y [43] | 2012 | China | Stomach | 103 (75/28) | 55 | 34 (69) | NR | intratumoral IL-17 positive stained cells | IHC | Anti-IL-17 (Santa Cruz Biotechnology) | 2.75% positive cells | OS/DFS/Stage | Reported in text/Survival curve/Data extrapolated | Positive |
Abbreviations: M, male; F, female; NR, not reported; Th17, T helper 17; IHC, immunohistochemistry; FCM, flow cytometry; ELISA, enzyme linked immunosorbent assay; RT-PCR, reverse transcription-polymerase chain reaction; PE, phycoerythrin; HPF, high power field; OS, overall survival; DFS, disease free survival; HR, hazard ratio.
More than half (N = 15) of the studies were focused on gastrointestinal tumors, including gastric cancer (N = 4), hepatocellular carcinoma (HCC) (N = 4), esophageal squamous cell carcinoma (N = 2), colorectal cancer (N = 2), gallbladder cancer (N = 1), intrahepatic cholangiocarcinoma (N = 1) and pancreatic cancer (N = 1). There were 4 studies showing the prognostic value of IL-17 in ovarian cancer while 3 studies in non-small cell lung cancer (NSCLC). The impact of IL-17 on survival of patients with breast cancer, glioblastoma, glioma, pituitary adenoma, laryngeal squamous carcinoma and nasopharyngeal carcinoma were also reported in the rest of involved studies respectively.
As shown in Table 2, the prognostic value of IL-17 on staging, OS and DFS was analyzed separately, and sub-group analysis was conducted as well according to tumor location.
Table 2.
The combined HR of sub-group meta-analysis in Clinical Staging, OS and DFS
| Sub-group Analysis | Clinical Staging | OS | DFS | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Tumor Location | No. of studies | HR (95% CI) | No. of studies | HR (95% CI) | No. of studies | HR (95% CI) |
| Overall solid tumor | 17 | 1.51 (0.98, 2.34) | 20 | 1.34 (0.87, 2.05) | 13 | 1.59 (1.24, 2.03)a,c |
| Gastrointestinal tumor | 6 | 1.87 (0.84, 4.19)c | 14 | 1.85 (1.24, 2.75)a | 7 | 2.41 (1.98, 2.92)a |
| Bile duct | 1 | 2.93 (1.25, 6.88)a | 1 | 1.59 (1.05, 2.41)a | - | - |
| Brain | 1 | 0.60 (0.08, 4.47) | 1 | 0.16 (0.04, 0.73)b | 1 | 0.23 (0.06, 0.89)b |
| Breast | 1 | 1.24 (0.59, 2.60) | - | - | 1 | 2.24 (1.06, 4.74)a |
| Colorectum | - | - | 1 | 4.02 (1.56, 10.37)a | 1 | 2.78 (1.38, 5.60)a |
| Esophagus | 2 | 0.63 (0.42, 0.94)b | 2 | 0.65 (0.50, 0.84)b | - | - |
| Gallbladder | 1 | 2.57 (1.15, 5.75)a | 1 | 2.34 (2.20, 2.49)a | 1 | 2.66 (2.47, 2.87)a |
| Hypophysis | 1 | 3.64 (1.39, 9.51)a | - | - | - | - |
| Larynx | 1 | 1.15 (0.27, 4.88) | - | - | - | - |
| Liver | - | - | 4 | 1.87 (1.23, 2.84)a | 4 | 1.65 (0.75, 3.60) |
| Lung | 3 | 2.33 (1.25, 4.32)a | 2 | 1.55 (1.02, 2.35)a | 1 | 2.35 (1.01, 5.34)a |
| Nasopharynx | 1 | 0.48 (0.22, 1.07) | 1 | 0.62 (0.30, 1.28) | 1 | 0.75 (0.39, 1.44) |
| Ovary | 3 | 0.71 (0.23, 2.22) | 2 | 0.26 (0.03, 2.31) | 2 | 0.33 (0.11, 1.00)b |
| Pancreas | - | - | 1 | 3.55 (3.30, 3.82)a | - | - |
| Stomach | 2 | 6.44 (0.23, 180.73) | 4 | 2.27 (0.44, 11.75) | 1 | 2.77 (2.31, 3.33)a |
Abbreviations: OS, overall survival; DFS, disease free survival; No., number; HR, hazard ratio; CI, confidence interval;
The combined HR was significantly positive.
The combined HR was significantly negative.
Publication bias was found in Begg’s test and/or Egger’s test.
IL-17 and clinical staging
The clinical staging status of tumor at the time of diagnosis plays an important role in survival prediction of patients with solid tumors and a late staging usually indicates more probability of invasion or metastasis and shorter survival compared to an early staging. We analyzed the impact of IL-17 on the clinical staging of solid tumors with the included studies which provided sufficient statistics to perform meta-analysis. Of the 17 studies, 6 studies reached positive results while the rest 11 studies indeterminate. As shown in Figure 2A, the combined HR of IL-17 on clinical staging was non-statistically significant (HR = 1.51, 95% CI: 0.98-2.34). Sub-group analysis was then performed according to the tumor location (Figure 3A). As a result, the combined HRs of IL-17 on clinical staging of gastrointestinal tumors (N = 6), ovarian cancer (N = 3) or gastric cancer (N = 2) were entirely not significant (HR = 1.87, 95% CI: 0.84-4.19; HR = 0.71, 95% CI: 0.23-2.22 and HR = 6.44, 95% CI: 0.23-180.73, respectively) (Figure 3D). However, a high expression of IL-17 was found significantly associated with a late staging (III/IV) of NSCLC (N = 3, HR = 2.33, 95% CI: 1.25-4.32) but an early staging (I/II) of esophageal squamous cell carcinoma (N = 2, HR = 0.63, 95% CI: 0.42-0.94). In addition, only one study suggested IL-17 was associated late clinical staging of intrahepatic cholangiocarcinoma (HR = 2.93, 95% CI: 1.25-6.88), gallbladder cancer (HR = 2.57, 95% CI: 1.15-5.75) and pituitary adenoma (HR = 3.64, 95% CI: 1.39-9.51).
Figure 2.
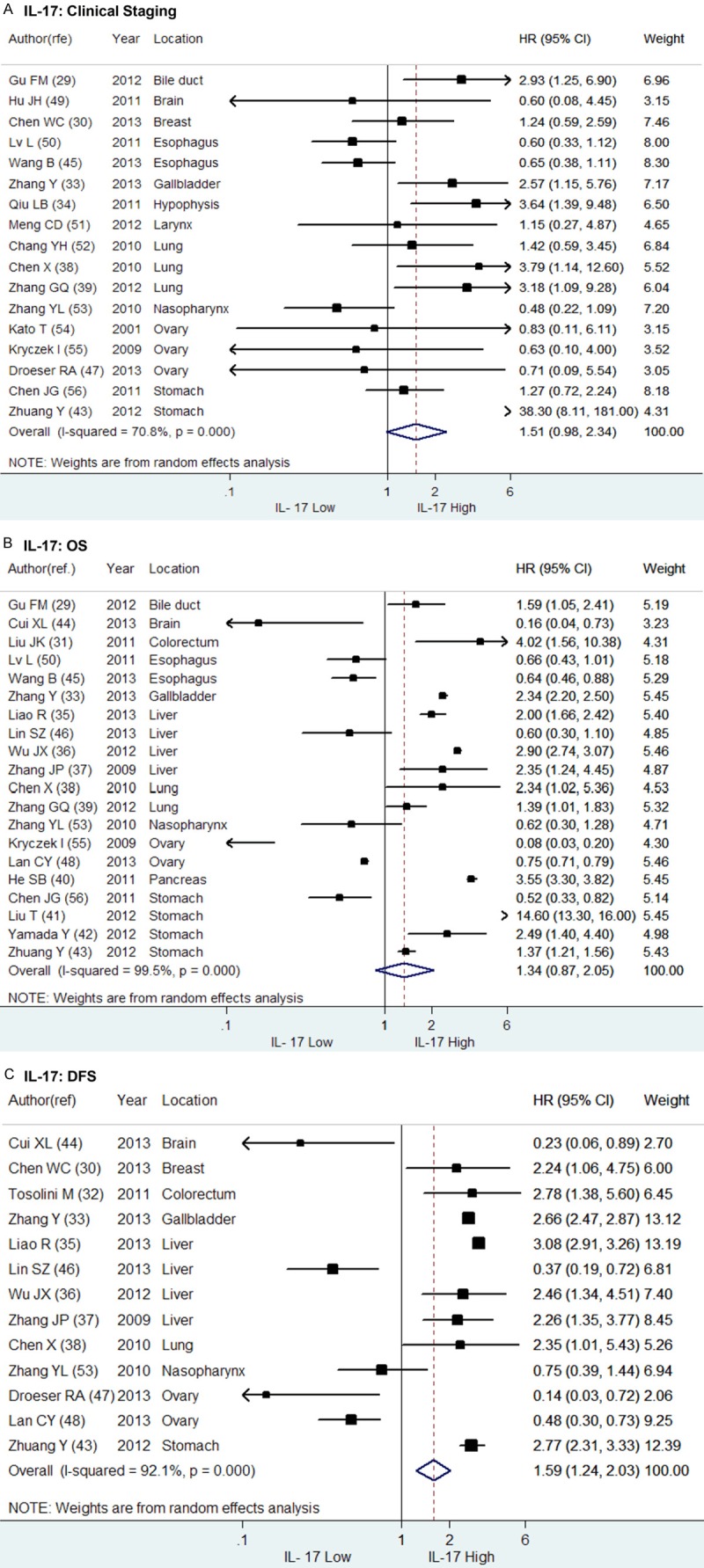
Forrest plots and meta-analysis of studies evaluating HR of IL-17 high compared to IL-17 low. The combined HRs of IL-17 high on (A) clinical staging, (B) overall survival (OS) and (C) disease free survival (DFS) were respectively aggregated above.
Figure 3.
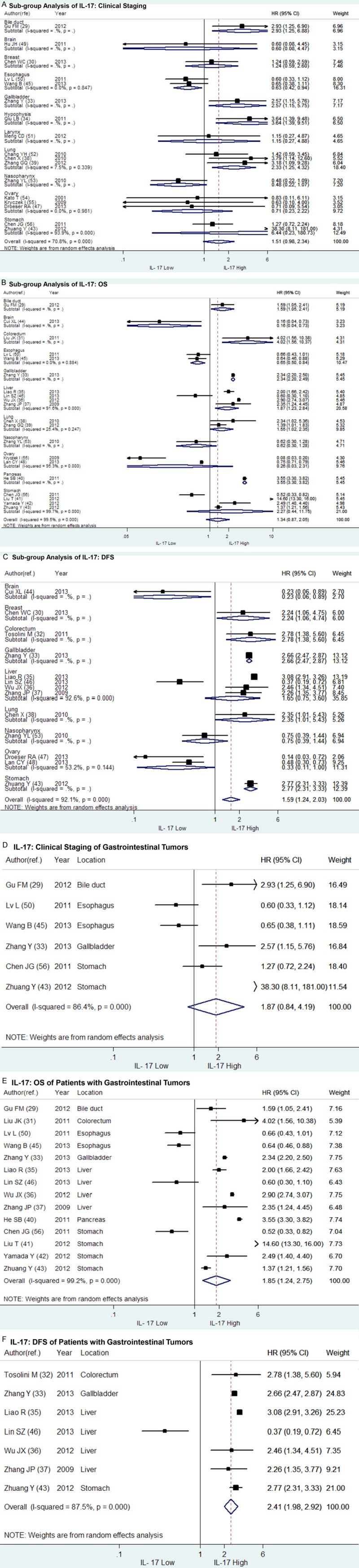
Forrest plots and sub-group meta-analysis of studies evaluating HR of IL-17 high compared to low. The combined HRs of IL-17 high on (A) clinical staging, (B) overall survival (OS) and (C) disease free survival (DFS) were aggregated in sub-groups according to tumor location. The combined HRs of IL-17 high on (D) clinical staging, (E) OS and (F) DFS of patients with gastrointestinal tumors were reported as well.
IL-17 and overall survival
A total of 20 studies were included in the meta-analysis of the prognostic value of IL-17 on OS, among which 12 studies provided positive result while 5 studies negative and 3 studies indeterminate. The combined HR of the 20 studies was not significant as shown in Figure 2B (HR = 1.35, 95% CI: 0.87-2.05). Sub-group meta-analysis was conducted according to tumor location (Figure 3B). As more than half of the enrolling 20 studies (N = 14) focused on gastrointestinal tumors, we firstly conducted a sub-group meta-analysis for the prediction value of IL-17 on OS of patients with gastrointestinal tumors. The combined HR of the 14 studies was statistically significant (HR = 1.85, 95% CI: 1.24-2.75) that suggested a high expression of IL-17 was associated with a poorer overall survival of patients with gastrointestinal tumors (Figure 3E). Similarly, in patients with hepatic cellular carcinoma (N = 4, HR = 1.87, 95% CI: 1.23-2.84) and NSCLC (N = 2, HR = 1.55, 95% CI: 1.02-2.35), IL-17 indicated shorter OS. However, IL-17 high suggested a prolonged OS in patients with esophageal squamous carcinoma (N = 2, HR = 0.65, 95% CI: 0.50-0.84). Besides, no relationship was found between IL-17 expression and OS in patients with gastric cancer (N = 4, HR = 2.27, 95% CI: 0.44-11.75) or ovarian cancer (N = 2, HR = 0.26, 95% CI: 0.03-2.31). As for patients with intrahepatic cholangiocarcinoma (HR = 1.59, 95% CI: 1.05-2.41), colorectal cancer (HR = 4.02, 95% CI: 1.56-10.37), gallbladder cancer (HR = 2.34, 95% CI: 2.20-2.49) and pancreatic cancer (HR = 3.55, 95% CI: 3.30-3.82), a high expression of IL-17 indicated poor outcome in only one independent study, respectively. Meanwhile, a better OS was found in glioblastoma patients with a high expression of IL-17 (N = 1, HR = 0.16, 95% CI: 0.04-0.73), but the relationship between IL-17 and OS of patients with nasopharyngeal carcinoma was indeterminate.
IL-17 and disease free survival
The data of DFS was obtained from 13 independent primary studies with different outcomes, including 8 positive, 4 negative and 1 indeterminate results. As shown in Figure 2C, the combined HR of the 13 studies was significantly positive (HR = 1.59, 95% CI: 1.24-2.03), suggesting a high expression of IL-17 was correlated with a poor DFS in patients with solid tumors. Furthermore, the sub-group analysis of the 7 studies focused on gastrointestinal tumors reached the same outcome (HR = 2.41, 95% CI: 1.98-2.92) (Figure 3F). However, the relation between IL-17 and DFS of patients with hepatic cellular carcinoma (N = 4, HR = 1.65, 95% CI: 0.75-3.60) was indeterminate. On the contrary, a borderline correlation was found in the meta-analysis of the 2 studies focused on ovarian cancer (HR = 0.33, 95% CI: 0.11-1.00), showing a high expression of IL-17 might indicate a better DFS in patients with ovarian cancer. Additionally, only one independent study respectively revealed that IL-17 implied shorter DFS in patients with breast cancer (HR = 2.24, 95% CI: 1.06-4.74) and NSCLC (HR = 2.35, 95% CI: 1.01-5.34), whereas a better DFS in patients with glioblastoma (HR = 0.23, 95% CI: 0.06-0.89). Meanwhile, no significant relationship was found between IL-17 and DFS in patients with nasopharyngeal carcinoma (HR = 0.75, 95% CI: 0.39-1.44) in another one study (Figure 3C).
Evaluation of published bias
As illustrated in Figure 4, both Begg’s test and Egger’s test were conducted to assess potential publication bias in the meta-analysis and sub-group analysis of IL-17 on clinical staging, OS and DFS respectively. Begg’s funnel plot did not show any significant asymmetry in the overall meta-analysis of IL-17 on clinical staging (P = 0.564 > 0.10), OS (P = 0.516 > 0.10) or DFS (P = 0.464 > 0.10). The Egger’s test found no significant bias in meta-analysis of IL-17 on clinical staging (P = 0.178 > 0.05) and OS (P = 0.738 > 0.05), but potential publication bias in meta-analysis of IL-17 on DFS (P = 0.006 < 0.05). For sub-group meta-analysis, potential publication bias were only found in the meta-analysis of IL-17 on clinical staging in patients with gastrointestinal tumors (N = 6, P = 0.015 < 0.10 for Begg’s test and P = 0.008 < 0.05 for Egger’s test). However, neither Begg’s test nor Egger’s test revealed any evidence of significant publication bias in the rest of sub-group meta-analysis of IL-17 on clinical staging, OS or DFS (data not shown).
Figure 4.
Begg’s test and Egger’s test assessed potential publication bias in studies evaluating IL-17 expression in patients with solid tumors. Begg’s test assessed potential publication bias in meta-analysis of IL-17 on (A) clinical staging, (B) OS and (C) DFS, and Egger’s test in meta-analysis of IL-17 on (D) clinical staging, (E) OS and (F) DFS. The result of Begg’s test showed no statistically significance (P = 0.564 > 0.10, P = 0.516 > 0.10 and P = 0.464 > 0.10, respectively). The Egger’s test showed no statistically significance in meta-analysis of IL-17 on clinical staging and OS (P = 0.178 > 0.05 and P = 0.730 > 0.05, respectively), but potential publication bias were found in meta-analysis of IL-17 on DFS (P = 0.006 < 0.05).
Discussion
Since IL-17 was first isolated and reported by Rouvier’s team in 1993, it has received much attention as a proinflammatory cytokine because of its potential role in immune defense against extracellular pathogens as well as in the autoimmune diseases. A considerable amount of primary studies have estimated the potential relationship between IL-17 and prognosis of patients with solid tumors, and the importance of IL-17 on survival was emphasized by both significant and non-significant outcomes. The present studies reported different or even controversial results. Then, it is necessary to analyze the variability of survival results and evaluate the prognostic value of IL-17 by the quantitative aggregation of survival statistics. As shown in Table 2, the overall meta-analysis of clinical staging and OS suggested that a high expression of IL-17 was correlated with a late clinical staging and a poor overall survival, but the combined HRs were not statistically significant. From the DFS of 13 primary studies, the overall meta-analysis provided the evidence that a high expression of IL-17 indicated a poor DFS in patients with solid tumors (HR = 1.59, 95% CI: 1.24-2.03), but the reliability was weakened by potential publication bias (P = 0.006 < 0.05 for Egger’s test). However, some inspiring outcomes were reached in the further analysis. In the sub-group meta-analysis of IL-17 in gastrointestinal tumors, IL-17 was found significantly correlated with poor OS and DFS, which suggested IL-17 did harm to patients with gastrointestinal tumors. Similar result was found in the OS meta-analysis of IL-17 in HCC patients. For NSCLC, IL-17 was found correlated with clinical staging, OS and DFS significantly, suggesting that IL-17 promoted tumor growth and progression in NSCLC. On the contrary, IL-17 was proved to be a beneficial factor and play a suppression role in the growth and progression of tumor in patients with esophageal squamous carcinoma. Meanwhile, IL-17 predicted better DFS in patient with ovarian cancer with a borderline HR of 0.33 (95% CI: 0.11-1.00). Although we found IL-17 was significantly correlated with the clinical staging, OS or DFS of several tumors in present meta-analysis, only one of the 28 enrolling studies assessed sensitivity and specificity of elevated serum IL-17 in predicting DFS and OS of HCC patients. Therefore, the validation of the prognostic prediction value of IL-17 in solid tumors should be conducted by large multicenter prospective studies before it can be applied to the diagnosis, treatment or prognostic prediction of cancers in the future.
Of all the results displayed above, some interesting differences or contradictions should be taken into discussion. In the analysis of IL-17 in gastrointestinal tumors, IL-17 was proved to predict worse OS and DFS, however an early staging and a better OS were found in esophageal squamous carcinoma patients with a high expression level of IL-17. With further investigation of the enrolling studies, we found adenocarcinoma predominated among the enrolling gastrointestinal tumors with the exception of esophageal squamous carcinoma, and the contradiction above might result from the variability of histopathological classification. However, the difference in histopathological classification may not be the only cause, because it cannot explain why IL-17 was a beneficial factor for DFS of patients with ovarian cancer as another kind of adenocarcinoma (HR = 0.33). Moreover, even in the same kind of tumor, opposite conclusions were drawn and published by different researchers. Take the four independent studies of HCC for example, Lin’s team suggested IL-17 was a beneficial factor in prediction of survival prognosis, which was completely opposite to the studies of Liao’s, Wu’s and Zhang’s team [35-37,46]. Although Wu’s team evaluated peripheral IL-17 expression in serum of HCC patients by ELISA, the methods in the other three studies were almost the same, evaluating the IL-17 expression by counting the intratumoral IL-17 positive cells stained with IHC. The selection of patient, size of sample, experimental error or other confounding factors may lead to the confused results. However, much more efforts are needed to explain the inconsistent results in spite of the potential heterogeneity between different tumors and studies.
As described above, 4 primary studies evaluated peripheral IL-17 in serum or ascites while the majority of enrolling studies measured the intratumoral IL-17 [36,41,42,55]. We included these 4 studies for two reasons. On one hand, IL-17 in patients with solid tumors is mainly secreted by infiltrated Th17 cells or IL-17 positive lymphocytes in tumor tissue and draining lymph nodes, and a group of studies has confirmed that intratumoral and peripheral IL-17 played a consistent role in predicting the prognosis of patients with solid tumors. We included valuable studies as many as possible in order to strengthen the reliability of the results in our meta-analysis. On the other hand, the potential predictive value of peripheral IL-17 is considered much more convenient and cost-effective in clinical practice because samples of peripheral blood, ascites or other body fluid are much easier to collect from patients than those of tumor tissues. Besides, the peripheral IL-17 can be detected before surgery and provide prognostic prediction for patients with unresectable tumors. In order to avoid the potential bias caused by the distribution of IL-17, we excluded these four studies and conducted meta-analysis again with the same method descried before. The results of overall meta-analysis for OS (HR = 1.22, 95% CI: 0.83-1.80) and DFS (HR = 1.53, 95% CI: 1.18-1.98) did not change, except for a borderline HR of clinical staging (HR = 1.57, 95% CI: 1.00-2.45) (Figure 5). However, the discrepancy should not be overestimated in consideration of the limited number of including studies. When aggregating the HRs of the 4 studies involving peripheral IL-17, we found no relation between peripheral IL-17 and OS (HR = 1.90, 95% CI: 0.59-6.16) (Figure 5D).
Figure 5.
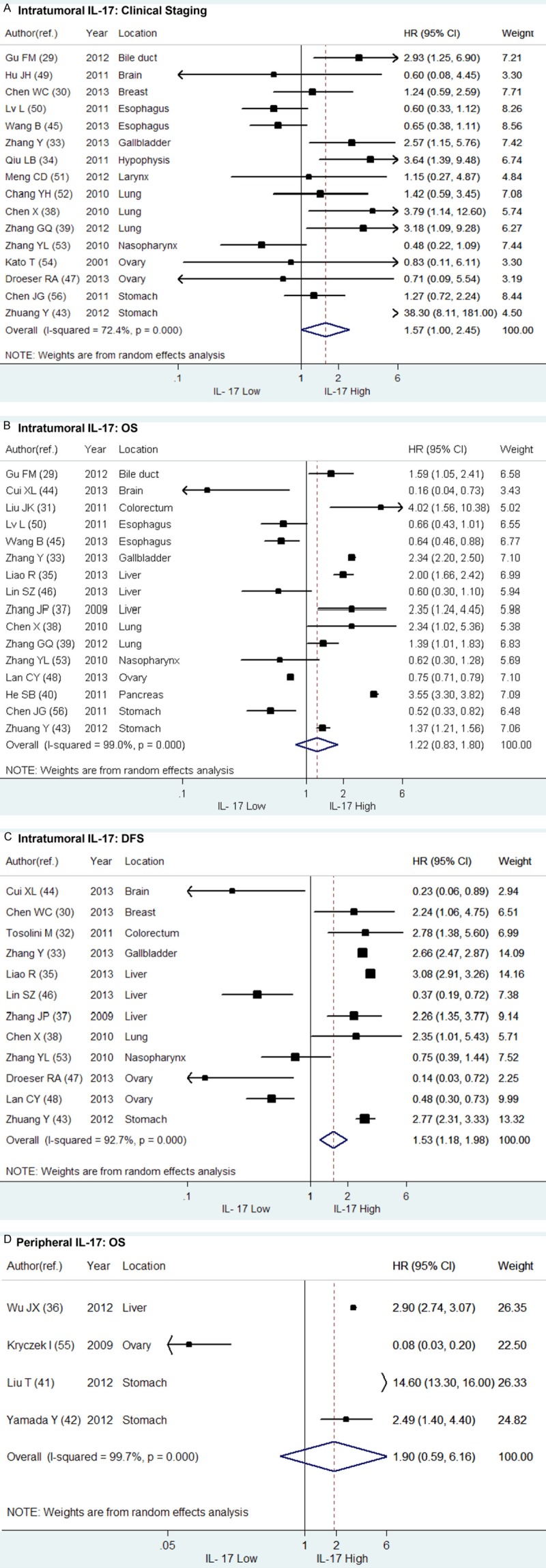
Forrest plots and meta-analysis of HR of high expression level of intratumoral and peripheral IL-17 compared to low in patients with solid tumors. The combined HRs of intratumoral IL-17 on (A) clinical staging, (B) overall survival (OS) and (C) disease free survival (DFS) were respectively aggregated above. The HR of peripheral IL-17 on (D) OS of patients with solid tumors was also evaluated.
The preliminary evidence from the meta-analysis suggested that IL-17 was correlated with tumor growth and progression and it was capable to indicate both better and worse survival outcomes in different kind of solid tumors. Although the interaction between IL-17 and tumorigenesis has not been elucidated clearly, several possible mechanisms were postulated. As reviewed by Xu’s and Hemdan’s team, IL-17 could induce the production of a series of cytokines such as vascular growth factor (VEGF), prostaglandin E1, prostaglandin E2, matrix metalloproteinase (MMP) and IL-6, which accelerated the angiogenesis in tumor tissues and promoted tumor growth and progression [7,60]. IL-17 also exerted the pro-tumor effect by promoting tumor progression mediated by myeloid-derived suppressor cells (MDSCs) and inhibiting the activities of CTLs [61]. Besides, an antiapoptotic function of IL-17 was also found in the tumorigenesis of breast cancer [25]. Recent studies demonstrated that IL-17 produced by transformed enterocytes could promote early colorectal tumorigenesis by activating ERK, p38 MAPK, and NF-κB signaling, and that combined treatment with chemotherapy and anti-IL-17 antibody could enhance the therapeutic responsiveness of established colon tumors in comparison with chemotherapy alone [62,63]. In contrast, IL-17 was found capable to inhibit regulatory T cells (Tregs) in the tumor microenvironment and promote the generation and activity of tumor antigen-targeted effector CTLs, presenting an antitumor effect [64]. Simultaneously, IL-17 was reported to synergize with interferon-γ (IFN-γ), co-expressed by Th17 cells, and induce more Th1-type chemokines CXCL9/10, which was correlated with the reduction in angiogenesis and progression of ovarian cancer [55]. In regard to the confused manifestation of IL-17 in tumor growth and progression, some researchers assumed that the definite function of IL-17 might depend on the immunogenicity of tumor, the immune status of the patient and the phase of disease [60]. Together with the results of our meta-analysis, the origination and histopathological classification of tumor should be taken into consideration as well in order to avoid the potential interference caused by tumor heterogeneity.
The results of meta-analysis are globally treated as golden standards [65]. However, results of this study should be interpreted with caution because some kind of potential bias always exists between the enrolling studies and cannot be eliminated completely. Several limitations of our meta-analysis should be considered. First of all, publication bias might exist although the difference in the results of Begg’s and Egger’s test may be attributed to the limited number of including studies. Due to resource limitation, we included literatures only published in English or Chinese from PubMed/MEDLINE and EMBASE, probably missing valuable data published in other language like Polish, Japanese, etc. Therefore, the prognostic significance of IL-17 could be overestimated because of a phenomenon called “file drawer problem”, which was described by Earleywine’s team that studies with positive results were more likely to be published in English while those with negative results were often published in native languages or even not submitted by journals [66]. Besides, the exclusion of studies with no accessible survival statistics could influence the accuracy of data aggregation as well. Secondly, the variability in tumor location, study design, assessment of IL-17, methods, antibodies, cut-off value and estimation of HR could contribute to the heterogeneity between studies. Therefore, we grouped the studies according to tumor location and conducted sub-group analysis. The potential interference caused by IL-17 location, intratumoral and peripheral, was also assessed, whereas the results were basically consistent no matter whether the 4 studies evaluating peripheral IL-17 were excluded or not. We did not define a prespecified cut-off value for IL-17 high because different measurements were used in the enrolling studies. As the median follow-up duration was not mentioned in most included studies, we did not limit the follow-up period to define the end point such as 5-year overall survival. Although there might be some difference between the HRs directly reported in the original articles and those calculated with methods suggested by Parmar’s team, the prediction value of IL-17 was consistent and it did not affect the results of quantitative aggregation of HRs. Thirdly, it might be inappropriate to extrapolate the results of this meta-analysis to populations worldwide because the majority of included studies were originated from Asia. Additionally, for each kind of tumor, both the number of included studies and the sample sizes were limited to some extent to perform a meta-analysis of high quality. More studies with larger sample sizes are needed to clarify the impact of IL-17 on solid tumors and evaluate the prognostic prediction value of IL-17 for a specified tumor.
Although some modest bias could not be excluded, the results of our meta-analysis revealed that IL-17 might correlate with poor OS and DFS in gastrointestinal tumors. Specifically, IL-17 was a detrimental factor for HCC and NSCLC patients, whereas a beneficial factor for patients with esophageal squamous cell carcinoma and ovarian cancer. In the future, more studies are needed to throw light upon the underlying mechanism of anti-tumor or pro-tumor effect of IL-17 and explore the potential clinical utility of IL-17 in the diagnosis, treatment and prognostic prediction of various human tumors.
Acknowledgements
We thank all authors for their publications included in our meta-analysis.
Disclosure of conflict of interest
None.
References
- 1.Rouvier E, Luciani M, Mattei M, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. The J Immunol. 1993;150:5445–56. [PubMed] [Google Scholar]
- 2.Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, Armitage RJ. Human IL-17: a novel cytokine derived from T cells. The J Immunol. 1995;155:5483–6. [PubMed] [Google Scholar]
- 3.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, Blanchard D, Gaillard C, Das Mahapatra B, Rouvier E, Golstein P, Banchereau J, Lebecque S. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aggarwal S, Gurney AL. IL-17: prototype member of an emerging cytokine family. J Leukoc Biol. 2002;71:1–8. [PubMed] [Google Scholar]
- 5.Moseley T, Haudenschild D, Rose L, Reddi A. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003;14:155–74. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 6.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–89. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 7.Xu S, Cao X. Interleukin-17 and its expanding biological functions. Cell and Mol Immunol. 2010;7:164–74. doi: 10.1038/cmi.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional Specialization of Interleukin-17 Family Members. Immunity. 2011;34:149–62. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Schnyder-Candrian S, Togbe D, Couillin I, Mercier I, Brombacher F, Quesniaux V, Fossiez F, Ryffel B, Schnyder B. Interleukin-17 is a negative regulator of established allergic asthma. The J Exp Med. 2006;203:2715–25. doi: 10.1084/jem.20061401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He D, Wu L, Kim HK, Li H, Elmets CA, Xu H. CD8+ IL-17-producing T cells are important in effector functions for the elicitation of contact hypersensitivity responses. J Immunol. 2006;177:6852–8. doi: 10.4049/jimmunol.177.10.6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–8. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 12.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–73. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 13.Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chabaud M, Durand JM, Buchs N, Fossiez F, Page G, Frappart L, Miossec P. Human interleukin-17: A T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 1999;42:963–70. doi: 10.1002/1529-0131(199905)42:5<963::AID-ANR15>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 15.Ye P, Garvey PB, Zhang P, Nelson S, Bagby G, Summer WR, Schwarzenberger P, Shellito JE, Kolls JK. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol. 2001;25:335–40. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- 16.Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. Resident Vδ1+ γδ T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J Immunol. 2007;178:4466–72. doi: 10.4049/jimmunol.178.7.4466. [DOI] [PubMed] [Google Scholar]
- 17.Luzza F, Parrello T, Monteleone G, Sebkova L, Romano M, Zarrilli R, Imeneo M, Pallone F. Up-regulation of IL-17 is associated with bioactive IL-8 expression in Helicobacter pylori-infected human gastric mucosa. J Immunol. 2000;165:5332–7. doi: 10.4049/jimmunol.165.9.5332. [DOI] [PubMed] [Google Scholar]
- 18.Numasaki M, Fukushi JI, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–7. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6–Stat3 signaling pathway. J Exp Med. 2009;206:1457–64. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benchetrit F, Ciree A, Vives V, Warnier G, Gey A, Sautes-Fridman C, Fossiez F, Haicheur N, Fridman WH, Tartour E. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood. 2002;99:2114–21. doi: 10.1182/blood.v99.6.2114. [DOI] [PubMed] [Google Scholar]
- 21.Hsieh HG, Loong CC, Lui WY, Chen A, Lin CY. IL-17 expression as a possible predictive parameter for subclinical renal allograft rejection. Transpl Int. 2001;14:287–98. doi: 10.1007/s001470100344. [DOI] [PubMed] [Google Scholar]
- 22.Fabrega E, Lopez-Hoyos M, San Segundo D, Casafont F, Pons-Romero F. Changes in the serum levels of interleukin-17/interleukin-23 during acute rejection in liver transplantation. Liver Transpl. 2009;15:629–33. doi: 10.1002/lt.21724. [DOI] [PubMed] [Google Scholar]
- 23.Antonysamy M, Fanslow W, Fu F, Li W, Qian S, Troutt A, Thomson AW. Transplant Proc. Elsevier; 1999. Evidence for a role of IL-17 in alloimmunity: a novel IL-17 antagonist promotes heart graft survival; p. 93. [DOI] [PubMed] [Google Scholar]
- 24.Numasaki M, Lotze MT, Sasaki H. Interleukin-17 augments tumor necrosis factor-(alpha)-induced elaboration of proangiogenic factors from fibroblasts. Immunol Lett. 2004;93:39–43. doi: 10.1016/j.imlet.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 25.Nam JS, Terabe M, Kang MJ, Chae H, Voong N, Yang YA, Laurence A, Michalowska A, Mamura M, Lonning S, Berzofsky JA, Wakefield LM. Transforming growth factor (beta) subverts the immune system into directly promoting tumor growth through interleukin-17. Cancer Res. 2008;68:3915–23. doi: 10.1158/0008-5472.CAN-08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K, Gattinoni L, Wrzesinski C, Hinrichs CS, Kerstann KW, Feigenbaum L, Chan CC, Restifo NP. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–73. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787–98. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kryczek I, Wei S, Szeliga W, Vatan L, Zou W. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood. 2009;114:357–9. doi: 10.1182/blood-2008-09-177360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gu FM, Gao Q, Shi GM, Zhang X, Wang J, Jiang JH, Wang XY, Shi YH, Ding ZB, Fan J, Zhou J. Intratumoral IL-17+ cells and neutrophils show strong prognostic significance in intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2012;19:2506–14. doi: 10.1245/s10434-012-2268-8. [DOI] [PubMed] [Google Scholar]
- 30.Chen WC, Lai YH, Chen HY, Guo HR, Su IJ, Chen HH. Interleukin-17-producing cell infiltration in the breast cancer tumour microenvironment is a poor prognostic factor. Histopathology. 2013;63:225–33. doi: 10.1111/his.12156. [DOI] [PubMed] [Google Scholar]
- 31.Liu J, Duan Y, Cheng X, Chen X, Xie W, Long H, Lin Z, Zhu B. IL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinoma. Biochem Biophys Res Commun. 2011;407:348–54. doi: 10.1016/j.bbrc.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 32.Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman WH, Pagès F, Galon J. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, Th2, Treg, Th17) in patients with colorectal cancer. Cancer Res. 2011;71:1263–71. doi: 10.1158/0008-5472.CAN-10-2907. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Huang Y, Qin M. Tumor-infiltrating FoxP3+ and IL-17 producing T cells affect the progression and prognosis of gallbladder carcinoma after surgery. Scand J Immunol. 2013;78:516–22. doi: 10.1111/sji.12109. [DOI] [PubMed] [Google Scholar]
- 34.Qiu L, He D, Fan X, Li Z, Liao C, Zhu Y, Wang H. The expression of interleukin (IL)-17 and IL-17 receptor and MMP-9 in human pituitary adenomas. Pituitary. 2011;14:266–75. doi: 10.1007/s11102-011-0292-5. [DOI] [PubMed] [Google Scholar]
- 35.Liao R, Sun J, Wu H, Yi Y, Wang JX, He HW, Cai XY, Zhou J, Cheng YF, Fan J, Qiu SJ. High expression of IL-17 and IL-17RE associate with poor prognosis of hepatocellular carcinoma. J Exp Clin Cancer Res. 2013;32:3. doi: 10.1186/1756-9966-32-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu J, Du J, Liu L, Li Q, Rong W, Wang L. Elevated Pretherapy Serum IL17 in Primary Hepatocellular Carcinoma Patients Correlate to Increased Risk of Early Recurrence after Curative Hepatectomy. PLoS One. 2012;77:e50035. doi: 10.1371/journal.pone.0050035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang JP, Yan J, Xu J, Pang XH, Chen MS, Li L, Wu C, Li SP, Zheng L. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol. 2009;50:980–9. doi: 10.1016/j.jhep.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 38.Chen X, Wan J, Liu J, Xie W, Diao X, Xu J, Zhu B, Chen Z. Increased IL-17-producing cells correlate with poor survival and lymphangiogenesis in NSCLC patients. Lung Cancer. 2010;69:348–54. doi: 10.1016/j.lungcan.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 39.Zhang GQ, Han F, Fang XZ, Ma XM. CD4+, IL17 and Foxp3 expression in different pTNM stages of operable non-small cell lung cancer and effects on disease prognosis. Asian Pac J Cancer Prev. 2012;13:3955–60. doi: 10.7314/apjcp.2012.13.8.3955. [DOI] [PubMed] [Google Scholar]
- 40.He S, Fei M, Wu Y, Zheng D, Wan D, Wang L. Distribution and clinical significance of Th17 cells in the tumor microenvironment and peripheral blood of pancreatic cancer patients. Int J Mol Sci. 2011;12:7424–37. doi: 10.3390/ijms12117424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu T, Peng L, Yu P, Zhao Y, Shi Y, Mao X, Chen W, Cheng P, Wang T, Chen N, Zhang J, Liu X, Li N, Guo G, Tong W, Zhuang Y, Zou Q. Increased circulating Th22 and Th17 cells are associated with tumor progression and patient survival in human gastric cancer. J Clin Immunol. 2012;32:1332–9. doi: 10.1007/s10875-012-9718-8. [DOI] [PubMed] [Google Scholar]
- 42.Yamada Y, Saito H, Ikeguchi M. Prevalence and clinical relevance of Th17 cells in patients with gastric cancer. J Surg Res. 2012;178:685–91. doi: 10.1016/j.jss.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 43.Zhuang Y, Peng LS, Zhao YL, Shi Y, Mao XH, Chen W, Pang KC, Liu XF, Liu T, Zhang JY, Zeng H, Liu KY, Guo G, Tong WD, Shi Y, Tang B, Li N, Yu S, Luo P, Zhang WJ, Lu DS, Yu PW, Zou QM. CD8+ T cells that produce interleukin-17 regulate myeloid-derived suppressor cells and are associated with survival time of patients with gastric cancer. Gastroenterology. 2012;143:951–62.e8. doi: 10.1053/j.gastro.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 44.Cui X, Xu Z, Zhao Z, Sui D, Ren X, Huang Q, Qin J, Hao L, Wang Z, Shen L, Lin S. Analysis of CD137l and IL-17 expression in tumor tissue as prognostic indicators for gliblastoma. Int J Bio Sci. 2013;9:134–41. doi: 10.7150/ijbs.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang B, Li L, Liao Y, Li J, Yu X, Zhang Y, Xu J, Rao H, Chen S, Zhang L, Zheng L. Mast cells expressing interleukin 17 in the muscularis propria predict a favorable prognosis in esophageal squamous cell carcinoma. Cancer Immunol Immunother. 2013;62:1575–85. doi: 10.1007/s00262-013-1460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin SZ, Chen KJ, Xu ZY, Chen H, Zhou L, Xie HY, Zheng SS. Prediction of recurrence and survival in hepatocellular carcinoma based on two cox models mainly determined by FoxP3+ regulatory T cells. Cancer Prev Res (Phila) 2013;6:594–602. doi: 10.1158/1940-6207.CAPR-12-0379. [DOI] [PubMed] [Google Scholar]
- 47.Droeser RA, Guth U, Eppenberger-Castori S, Stadlmann S, Hirt C, Terracciano L, Singer G. High IL-17-positive tumor immune cell infiltration is indicative for chemosensitivity of ovarian carcinoma. J Cancer Res Clin Oncol. 2013;139:1295–302. doi: 10.1007/s00432-013-1441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lan C, Huang X, Lin S, Huang H, Cai Q, Lu J, Liu J. High density of IL-17-producing cells is associated with improved prognosis for advanced epithelial ovarian cancer. Cell Tissue Res. 2013;352:351–9. doi: 10.1007/s00441-013-1567-0. [DOI] [PubMed] [Google Scholar]
- 49.Hu J, Mao Y, Li M, Lu Y. The profile of Th17 subset in glioma. Int Immunopharmacol. 2011;11:1173–9. doi: 10.1016/j.intimp.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 50.Lv L, Pan K, Li XD, She KL, Zhao JJ, Wang W, Chen JG, Chen YB, Yun JP, Xia JC. The accumulation and prognosis value of tumor infiltrating IL-17 producing cells in esophageal squamous cell carcinoma. PLoS One. 2011;6:e18219. doi: 10.1371/journal.pone.0018219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meng CD, Zhu DD, Jiang XD, Li L, Sha JC, Dong Z, Kong H. Overexpression of interleukin-l7 in tumor-associated macrophages is correlated with the differentiation and angiogenesis of laryngeal squamous cell carcinoma. Chin Med J (Engl) 2012;125:1603–7. [PubMed] [Google Scholar]
- 52.Chang YH, Yu CW, Lai LC, Tsao CH, Ho KT, Yang SC, Lee H, Cheng YW, Wu TC, Shiau MY. Up-regulation of interleukin-17 expression by human papillomavirus type 16 E6 in nonsmall cell lung cancer. Cancer. 2010;116:4800–9. doi: 10.1002/cncr.25224. [DOI] [PubMed] [Google Scholar]
- 53.Zhang YL, Li J, Mo HY, Qiu F, Zheng LM, Qian CN, Zeng YX. Different subsets of tumor infiltrating lymphocytes correlate with NPC progression in different ways. Mol Cancer. 2010;9:4. doi: 10.1186/1476-4598-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kato T, Furumoto H, Ogura T, Onishi Y, Irahara M, Yamano S, Kamada M, Aono T. Expression of IL-17 mRNA in ovarian cancer. Biochem Biophys Res Commun. 2001;282:735–8. doi: 10.1006/bbrc.2001.4618. [DOI] [PubMed] [Google Scholar]
- 55.Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, Chang A, Coukos G, Liu R, Zou W. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–9. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen JG, Xia JC, Liang XT, Pan K, Wang W, Lv L, Zhao JJ, Wang QJ, Li YQ, Chen SP, He J, Huang LX, Ke ML, Chen YB, Ma HQ, Zeng ZW, Zhou ZW, Chang AE, Li Q. Intratumoral expression of IL-17 and its prognostic role in gastric adenocarcinoma patients. Int J Bio Sci. 2011;7:53–60. doi: 10.7150/ijbs.7.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–34. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 58.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 59.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hemdan NY. Anti-cancer versus cancer-promoting effects of the interleukin-17-producing T helper cells. Immunol Lett. 2013;149:123–33. doi: 10.1016/j.imlet.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 61.He D, Li H, Yusuf N, Elmets CA, Li J, Mountz JD, Xu H. IL-17 promotes tumor development through the induction of tumor promoting microenvironments at tumor sites and myeloid-derived suppressor cells. J Immunol. 2010;184:2281–8. doi: 10.4049/jimmunol.0902574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang K, Kim MK, DiCaro G, Wong J, Shalapour S, Wan J, Zhang W, Zhong Z, Sanchez-Lopez E, Wu LW, Taniguchi K, Feng Y, Fearon E, Grivennikov SI, Karin M. Interleukin-17 receptor a signaling in transformed enterocytes promotes early colorectal tumorigenesis. Immunity. 2014;41:1052–63. doi: 10.1016/j.immuni.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Round JL. Quenching the fire fueling cancer in the gut. Science Translational Medicine. 2015;7:269. [Google Scholar]
- 64.Yamamoto M, Kamigaki T, Yamashita K, Hori Y, Hasegawa H, Kuroda D, Moriyama H, Nagata M, Ku Y, Kuroda Y. Enhancement of anti-tumor immunity by high levels of Th1 and Th17 with a combination of dendritic cell fusion hybrids and regulatory T cell depletion in pancreatic cancer. Oncol Rep. 2009;22:337–43. [PubMed] [Google Scholar]
- 65.Stewart LA, Parmar MK. Meta-analysis of the literature or of individual patient data: is there a difference? Lancet. 1993;341:418–22. doi: 10.1016/0140-6736(93)93004-k. [DOI] [PubMed] [Google Scholar]
- 66.Earleywine M, Pollock V. The file drawer problem in the meta-analysis of subjective responses to alcohol. replies. Am J Psychiatry. 1993;150:1435–6. doi: 10.1176/ajp.150.9.1435c. [DOI] [PubMed] [Google Scholar]