Abstract
The value of FDG-positron emission tomography/computed tomography (PET/CT) for detecting prostate cancer is unknown. We aimed to investigate the clinical value of incidental prostate FDG uptake on PET/CT scans. We reviewed 6128 male patients who underwent FDG-PET/CT scans and selected cases that reported hypermetabolic lesion in the prostate. The patients who have prior history of prostate carcinoma or prostate surgery were excluded from the study. We have analyzed the correlation between PET/CT findings and serum prostate-specific antigen (PSA) levels, imaging (USG), urological examinations and biopsy. Incidental 18F-FDG uptake of the prostate gland was observed in 79 patients (1.3%). While sixteen of them were excluded due to inadequate clinical data, the remaining 63 patients were included for further analysis. The patients were divided into two groups; 8 patients (12.7%) in the malignant group and 55 patients (87.3%) in the benign group. The SUVmax values were not significantly different between the two groups. In 6 (75%) patients with prostate cancer, FDG uptake was observed focally in the peripheral zone of the prostate glands. There was no significant correlation between the SUVmax and the PSA levels. Incidental 18F-FDG uptake in the prostate gland is a rare condition, but a substantial portion of it is associated with the cancer. Benign and malignant lesions of the prostate gland in FDG-PET/CT imaging could not be reliably distinguished. The peripheral focally FDG uptake of prostate glands should be further examined with the clinical and labaratory evaluations.
Keywords: Prostate cancer, 18F-fluorodeoxyglucose, positron emission tomography/computed tomography
Introduction
Prostate cancer is the most common cancer of men in the United States and worldwide [1]. Positron emission tomography/computed tomography (PET/CT) with 18F-fluorodeoxy-glucose (FDG) is widely used in the imaging work-up of various malignancies for diagnosing, staging, restaging and surveillance [2]. As noted in the few previous studies, prostate tumors are characterized by slow glycolysis and low FDG-avidity on PET images and it is not possible to distinguish benign and malignant lesions of the prostate glands by the amount of FDG uptake. Also, urinary excretion of FDG is an important additional problem. For these reasons, FDG-PET/CT is generally not preferred in prostate cancers [3-5].
In daily practice, focal hypermetabolic lesions in sites that are not associated with clinical diagnoses are often observed on whole-body FDG-PET/CT images. Although many of these incidental lesions were associated with a physiologic or benign process, some of them may be associated with a secondary cancer, unexpected metastasis or other pathological lesions with further evaluation. Physiological or benign incidental focal uptake is often found in the thyroid, bowel, uterus, ovary, and other organs [6]. Due to the patient management and particularly early cancers that require radical treatment, the detection of second primary cancer is an important prognostic factor [7]. In previous PET studies, second primary cancers were detected in 1.2-4.8% of patients with known cancer and they were found in varied locations such as thyroid, lung, colon, oesophagus, breast, parotid gland and other organs [8-10].
Incidental uptake of FDG is occasionally observed in the prostate gland, but benign and malignant lesions could not be reliably distinguished based on the SUV alone. The aim of our study was to examine the frequency and clinical significance of incidental hypermetabolic lesions in prostate gland on FDG-PET/CT scans. Also, the availability of this imaging technique will be investigated for the differential diagnosis of malignant and benign lesions in prostate gland.
Material and methods
Patients
The database of 6128 male patients with known or suspected malignancy, who underwent FDG-PET/CT whole-body scan during the period January 2007 to December 2014 in our department, were retrospectively reviewed. Among these, 143 patients who have a previous history of prostate carcinoma or prostate surgery were excluded from analysis. Therefore, 5985 patients were included in this study. Seventy-nine (1.3%) of patients with FDG-PET/CT whole-body scan reports mentioning incidental focal hypermetabolic lesions of the prostate gland were selected, and further assessment was recommended for these lesions. While sixteen of them were excluded due to inadequate clinical data, the remaining 63 patients (age range 41-83, mean age 60.1±8.0 years) demonstrating incidental focal FDG uptake of prostate with further diagnostic work-up were selected and included in study. The indications of these patients for FDG-PET/CT are listed in Table 1.
Table 1.
PET/CT indications for the 63 patients with incidental hypermetabolism in prostate gland
| Indication for PET/CT | Number of patients |
|---|---|
| Lung cancer | 15 |
| Head and neck cancer | 11 |
| Colorectal cancer | 9 |
| Lymphoma | 8 |
| Gastric cancer | 6 |
| Other (Carcinoma of unknown primary, Melanoma, Pancreas, Oesophagus) | 14 |
Patient preparation and PET/CT imaging protocol
All patients fasted, except for glucose-free oral hydration, for at least 6 h before the iv injection of 370-555 MBq (10-15 mCi) of FDG. At the time of the tracer injection, blood glucose levels were checked and confirmed to be less than 150 mg/dl in all patients.
All patients were examined using a PET/CT system (Siemens Biograph 2 dual slice PET/CT) combining a dedicated, full-ring PET scanner with lutetium oxyorthosilicate (LSO) crystals.
As related to the indications, PET imaging was performed 60 minutes after injection, extending from the vertex to the pelvis or feet, with 5-8 bed positions of 3 min each. CT images were used for attenuation correction and fusion; no iv contrast medium was used.
PET, CT, and fused whole-body images displayed in axial, coronal, and sagittal planes were available for review. A semiquantitative analysis of FDG activity was measured as the maximal standardized value uptake of FDG (SUVmax) using the software programe.
Image analysis
The PET/CT images were carefully searched for the hypermetabolic lesions of the prostate gland by two nuclear medicine physicians. The image review was performed without information about other assessments of the prostate gland. According to the FDG uptake pattern of the prostate gland, lesions were classified as diffuse or focal hypermetabolic lesions. The focal hypermetabolic lesion was defined as showing well-circumscribed areas of prostate glands which were more intense than adjacent prostatic parenchyma uptake of 18F-FDG. Also, focal hypermetabolic lesions were classified as central or peripheral based on the distance of central prostatic urethra.
The maximum standardised uptake value (SUVmax) in prostate was measured from transaxial views.
Data and statistical analysis
The final clinical diagnoses of the hypermetabolic lesions were determined based on the serum prostate-specific antigen (PSA) levels, imaging studies for prostate (USG), urological examinations, and biopsy. There was a period of 1 month between these further evaluations and PET/CT imaging. The biopsy was performed for 12 patients who had elevated PSA levels (> 4.0 ng/ml) or an abnormal nodule with palpation or USG results. The patients were categorized into benign or malignant groups. In this classification for the patients without biopsy, patients with the PSA levels within the normal range and the ones with no abnormality on imaging or physical examination were considered to be in the benign group. Statistical analyses were performed using the SPSS version 13. According to the obtained results, the frequency of abnormal prostate hypermetabolism and prostate cancer were evaluated simply. Clinical characteristics, age, serum PSA levels and SUVmax were compared using independent t-test for the two groups. Fisher’s exact test was used to assess whether FDG uptake pattern and location differed between the groups. The correlations among SUVmax, serum PSA, and Gleason score were evaluated using Pearson correlation coefficient. A P-value of < 0.05 was considered statistically significant.
Results
Among 5985 patients who underwent FDG-PET/CT scans, 79 cases (1.3%) demonstrated incidental abnormal hypermetabolism of the prostate gland. Sixteen of them were excluded due to inadequate clinical data. Therefeore, 63 patients (age range 41-83, mean age 60.1±8.0 years) with further diagnostic work-up were selected and included in the study. In 9 of the 63 patients, PSA levels were measured within 1 month before the PET imaging. The remaining 54 patients underwent further evaluation with PSA level within 1 month after the PET imaging. Based on the PSA level results, other assessments such as USG and urological examination were performed. In accordance with these assessments, the biopsy was performed for 12 patients who had elevated PSA levels (> 4.0 ng/ml) or an abnormal nodule with palpation or USG results. While prostate cancer was confirmed in 8 patients, BPH was confirmed in the remaining four patients by biopsy.
The fifty-one patients were not pathologically confirmed due to normal serum PSA levels, and they were considered to have no malignancy in the prostate gland.
In accordance with the PSA levels and biopsy results, these 63 patients who were included in the study were divided into 2 groups as malignant and benign. Consequently, there were 8 patients (12.7%) in the malignant group and 55 patients (87.3%) in the benign group.
Malignant group
Among the 63 patients, 8 (12.7%) patients (age range 58-71, mean age 62.6±4.1 years) were diagnosed prostate cancer by biopsy. All of them were reported to have prostate-origin adenocarcinoma and there were no cases of metastasis from other malignancies to prostate gland. Gleason score of the 7 (87.5%) patients was greater than 6, and one patient had Gleason score 6. The mean PSA level for the malignant group was 47.6±24.4 ng/ml (range 10.3-89.6 ng/ml). The mean SUVmax value of the patient with prostate cancer was 4.7±2.3 (range 3.1-10.2). While FDG uptake in the prostate gland showed focal feature in 7 patients (6 of them were located peripherally, one of them was located both centrally and peripherally), only one patient had diffuse uptake. Figure 1 shows a hypermetabolic lesion in the peripheral portion of the left prostate lobe in 76 years old male with a history colon cancer.
Figure 1.
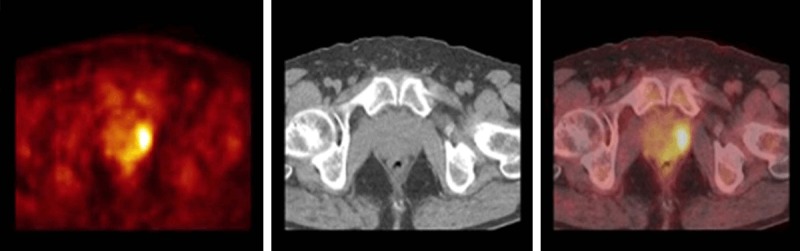
FDG-PET/CT images of a 76-year-old male with a history colon cancer. Axial PET, CT and PET/CT fusion images at the prostate gland level are shown. There is a focal hypermetabolic activity (SUVmax = 6.1) in the peripheral portion of the left prostate lobe. The PSA level was increased (48 ng/mL) and this lesion was confirmed to be prostate cancer with biopsy.
Benign group
Among the 63 patients, 55 (87.3%) patients (age range 41-83, mean age 59.8±8.4 years) have constituted the benign group. While 4 of these patients were diagnosed by biopsy, the other 51 patients were considered benign based on their PSA levels within the normal range and having no abnormality on imaging or physical examination. The PSA levels for the 4 patients who were diagnosed benign by biopsy were 4.7, 6.0, 7.1 and 9.4 ng/ml, respectively. In 3 of these patients the FDG uptake in the prostate gland showed focal feature (2 of them were located centrally, one of them was located peripherally) and one patient had diffuse uptake.
The mean PSA level for all patients in the benign group was 2.3±1.6 ng/ml (range 0.3-9.4 ng/ml). The mean SUVmax for the benign group was 4.0±1.0 (range 2.9-8.2).
While FDG uptake in the prostate gland had focal feature in 46 patients (63% of them were centrally located) and was diffused in 9 patients in the benign groups.
The mean SUVmax for the benign group with diffuse FDG uptake was 3.7±1.1 (range 2.9-6.1). Figure 2 shows diffuse FDG uptake in the prostate gland in 59 years old male with a history lung cancer.
Figure 2.
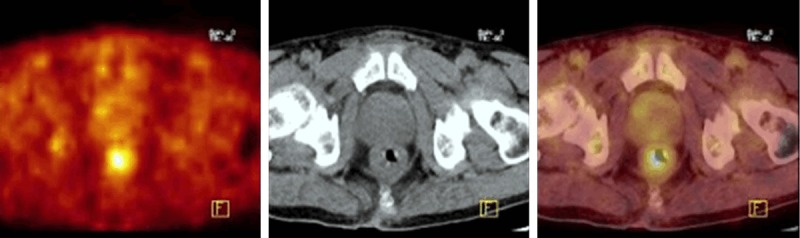
FDG-PET/CT images of a 59-year-old male with a history lung cancer. Axial PET, CT and PET/CT fusion images at the prostate gland level are shown. There is a diffuse FDG uptake (SUVmax = 3.4) in the prostate gland. The PSA level was increased (13 ng/mL) and this lesion was confirmed to be benign prostat hyperplasia with biopsy.
The some outcomes in the malignant and benign groups are summarized Table 2.
Table 2.
Comparison of the some outcomes between the malignant and benign group
| Malignant group (n = 8) | Benign group (n = 55) | P value | All (n = 89) | |
|---|---|---|---|---|
| Age (mean-SD), years | 62.6±4.1 | 59.8±8.4 | *P > 0.05 | 60.1±8.0 |
| SUVmax (mean-SD) | 4.7±2.3 | 4.0±1.0 | *P > 0.05 | 4.1±1.2 |
| PSA level (mean-SD) ng/mL | 47.6±24.4 | 2.3±1.6 | *P < 0.05 | 8.1±17.3 |
| Gleason score (6/7/≥ 8) | 1/4/3 | - | - | 1/4/3 |
| FDG uptake pattern (focal/diffuse) | 7/1 | 46/9 | **P > 0.05 | 53/10 |
| FDG uptake location (peripheral/other (central-both)) | 6/0-1 | 12/28-6 | **P < 0.05 | 18/28-7 |
FDG, fluorodeoxyglucose; PSA, prostate-specific antigen; SUVmax, maximum standardised uptake value; SD, standart deviation;
P, according to independent t-test;
P, according to the fisher’s exact test.
The statistical comparison of the benign and malignant groups
The mean age of the patients in the malignant group was found slightly higher than the benign group, but this difference was not statistically significant (The T value is 0.927 and the P-Value is 0.178).
We compared the PSA levels and SUVmax between two groups. The patients with prostate cancer had significantly higher PSA levels than the benign group (P < 0.01). The SUVmax values were not significantly different between the two groups (The T value = 1.5 and the P-Value = 0.069).
PSA level was not statistically correlated with SUVmax in the malignant groups (R2 = 0.045, r = 0.214, P = 0.610) (Figure 3), but statistically significant weak correlation was found in the benign group (R2 = 0.236, r = 0.48, P = 0.0002) (Figure 4).
Figure 3.

The correletions of SUVmax and PSA levels in the malignant group according to the Pearson Correlation.
Figure 4.
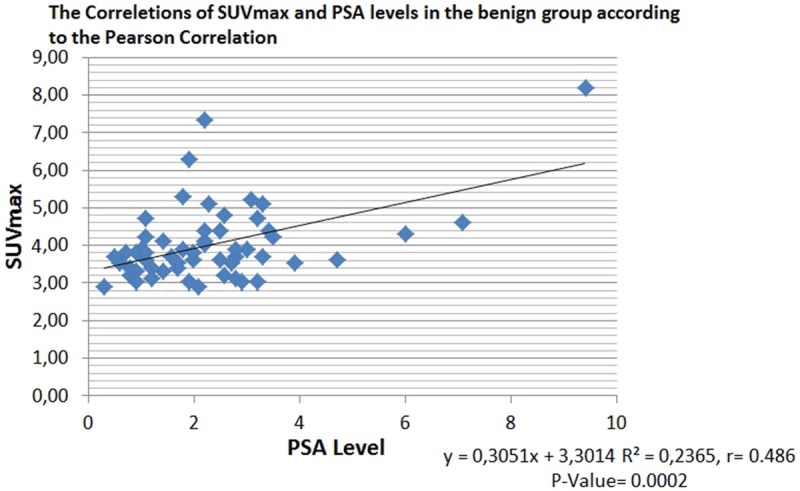
The correletions of SUVmax and PSA levels in the benign group according to the Pearson Correlation.
Gleason score was not statistically correlated with SUVmax in the prostate cancers (R2 = 0.280, r = 0.53, P = 0.176) (Figure 5).
Figure 5.
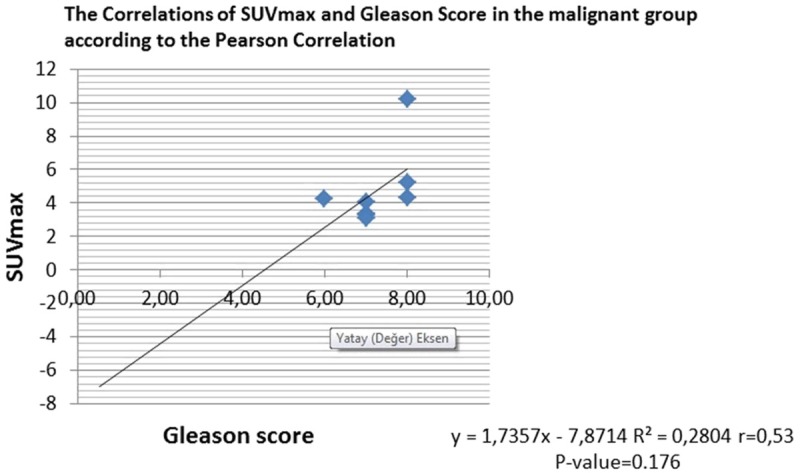
The correlations of SUVmax and Gleason Score in the malignant group according to the Pearson Correlation.
In patients with prostate cancer there was no statistical difference between patients with focal and diffuse uptake (P-Value = 1, Table 2); however, prostat cancer was significantly higher in the hypermetabolic lesions showing peripheral focal feature (P-Value = 0.004422, Table 2).
Discussion
FDG-PET/CT scans have been used for the staging and restaging of various primary and metastatic cancers, but it has not been preferred in prostate cancer because of the previously mentioned reasons. Also, due to the variability of FDG uptake in the prostate gland, there is no cut off values of SUV for distinguishing malignant and benign lesions. In some studies [11,12], SUVmax values for normal prostate gland have been reported as 2.7±1.2 (range 1.4-6.2) and 1.6±0.4 (range 1.1-3.7). As can be seen, there are different results for the SUVmax values of prostate gland in the studies. In addition, some common benign prostate diseases, such as benign prostatic hypertrophy (BPH) and prostatitis, can show focal or diffuse FDG uptake which can be confused with malignancy [13].
As noted in previous studies, incidental hypermetabolic lesions in the prostate gland are rarely seen on FDG-PET/CT imaging, but a significant portion of them are associated with malignancy [14-16]. In present study, the incidence of hypermetabolic lesions in the prostate gland was 1.3%, and 12.7% of them were diagnosed prostate cancer with further assessment. Although the incidence of hypermetabolic lesion in the prostate gland was similar to the ones reported in other studies that have been mentioned in the previous paragraph, the incidence of prostate cancer was found to be different in our study. These diffrences were considered to be due to the use of different confirmation methods and the absence of histopathological evaluation for all cases.
As noted in previous studies, the incidence of cancer in the prostate gland and the degree of FDG uptake can vary related to age [12,17]. The mean age of the patients in the malignant group was found slightly higher in our study, but this difference was not statistically significant.
Because of the variability of FDG uptake in the normal prostate gland and the detecting of FDG uptake in the benign postate diseases such as benign prostatic hyperplasia and prostatitis, SUVmax is insufficient to distinguish between benign and malignant processes in prostate gland. Reinicke et al. [18] reported that carcinogenesis in prostate tissue is not associated with GLUT-1 expression, and glucose might not play an important role in prostate cancer cell metabolism. Accordingly, the FDG uptake in the prostate gland is not very informative.
We compared the PSA levels and SUVmax between malignant and benign groups and our results revealed that while there was significantly higher PSA levels in the patients with prostate cancer, there was no significant difference between groups’ for the SUVmax values. These results are consistent with the findings of previous studies.
The measurement of serum PSA level is a useful and easy accessible labaratory test for the assesment of malign and benign disease in the prostat gland. The existence of a correlation between PSA levels and SUVmax values was evaluated in several studies but it is still a controversial issue. According to our results, there was no significant correlation in the malignant group but a weak correlation was found in the benign group for the SUVmax and PSA levels. Similar to our findings, some studies [15,19] reported that there was no correlation between the SUVmax and PSA level.
Gleason score is one of the most important prognostic factors for both localized and advanced prostate cancer [20]. In our study, 87.5% of the patients with prostate cancer diagnosed by biopsy had Gleason score greater than 6, and therefore they were intermediate or high risk of recurrence after definitive therapy according to the National Comprehensive Cancer Network Guideline Panel [21]. The presence of a significant correlation between the PSA levels and Gleason scores has been reported in the studies [15,22], especially in cases that has Gleason score > 6. We have not evaluated the relationship between the PSA levels and Gleason scores because it is not the purpose of our study. On the other hand, we found that there was no statistically significant correlation between SUVmax and Gleason’s scores for the prostat cancers, and these results are similar to the findings of other studies [15,23].
Prostate cancer is characterized as a multifocal disease, and most of them are peripherally located in the prostate gland [24]. In our sudy, FDG uptake in the prostate gland showed focal feature in malignant and benign groups, 87.5% and 83.6% respectively. The localization of focal hypermetabolic lesions in the prostate gland were 85.7% periferally in the malignant group, and 63% centrally in the benign group. Compared with the benign group, the hypermetabolic lesions in the prostate gland were significantly more peripherally located in the malignant group, and these findings were consistent with the results of other studies [14,25].
Our present study has several limitations which can be listed as follows: i) the study uses retrospective analysis and this is the main limitation, ii) the number of patients is small, especially in the malignant group, iii) histological confirmation is not performed in all patients iv) there is possibility of the false negative results in the further evaluation such as PSA, USG and examination, because the biopsy was performed according to the these evalutions, v) and, also there is a possibility of false negative biopsy results.
Because of these limitations, the incidence of prostate cancer could not be obtained accurately in the current study. Despite this, we considered that this study will contribute to the evaluation of hypermetabolic lesions in the prostate gland.
Conclusions
This study shows that incidental hypermetabolic lesions in the prostate gland on FDG-PET/CT scan is a rare condition (1.3%), but a substantial portion (12.7%) of them is associated with prostate cancer.
Although, FDG-PET/CT is not a reliable imaging method to distinguish benign and malignant lesions of the prostate gland, we recommend that further evaluation would be appropriate to peripheral focal hypermetabolic lesions on FDG-PET/CT for malignancy.
We think that there is a need for further prospective studies with a larger number of histopathologically confirmed patients to better clarify the issue.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Ma J, Zhou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Bar-Shalom R, Valdivia AY, Blaufox D. PET imaging in oncology. Semin Nucl Med. 2000;30:150–185. doi: 10.1053/snuc.2000.7439. [DOI] [PubMed] [Google Scholar]
- 3.Ravizzini G, Turkbey B, Kurdziel K, Choyke PL. New horizons in prostate cancer imaging. Eur J Radiol. 2009;70:212–26. doi: 10.1016/j.ejrad.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powels T, Murray I, Brock C, Oliver T, Avril N. Molecular positron emission tomography and PET/CT imaging in urological malignancies. Eur Urol. 2007;51:1520–1521. doi: 10.1016/j.eururo.2007.01.061. [DOI] [PubMed] [Google Scholar]
- 5.Scher B, Seitz M, Albinger W, Tiling R, Scherr M, Becker HC, Souvatzogluou M, Gildehaus FJ, Wester HJ, Dresel S. Value of 11C-choline PET and PET/CT in patients with suspected prostate cancer. Eur J Nucl Med Mol Imaging. 2007;34:45–53. doi: 10.1007/s00259-006-0190-7. [DOI] [PubMed] [Google Scholar]
- 6.Choi JY, Lee KS, Kim HJ, Shim YM, Kwon OJ, Park K, Baek CH, Chung JH, Lee KH, Kim BT. Focal thyroid lesions incidentally identified by integrated 18FFDG PET/CT: clinical significance and improved characterization. J Nucl Med. 2006;47:609–15. [PubMed] [Google Scholar]
- 7.Leön X, Ferlito A, Myer CM 3rd, Saffiotti U, Shaha AR, Bradley PJ, Brandwein MS, Anniko M, Elluru RG, Rinaldo A. Second primary tumors in head and neck cancer patients. Acta Otolaryngol. 2002;122:765–778. [PubMed] [Google Scholar]
- 8.Ishimori T, Patel PV, Wahl RL. Detection of unexpected additional primary malignancies with PET/CT. J Nucl Med. 2005;46:752–7. [PubMed] [Google Scholar]
- 9.Even-Sapir E, Lerman H, Gutman M, Lievshitz G, Zuriel L, Polliack A, Inbar M, Metser U. The presentation of malignant tumours and pre-malignant lesions incidentally found on PET-CT. Eur J Nucl Med Mol Imaging. 2006;33:541–52. doi: 10.1007/s00259-005-0056-4. [DOI] [PubMed] [Google Scholar]
- 10.Choi JY, Lee KS, Kwon OJ, Shim YM, Baek CH, Park K, Lee KH, Kim BT. Improved detection of second primary cancer using integrated [18F] fluorodeoxyglucose positron emission tomography and computed tomography for initial tumor staging. J. Clin. Oncol. 2005;23:7654–9. doi: 10.1200/JCO.2005.01.4340. [DOI] [PubMed] [Google Scholar]
- 11.Zincirkeser S, Sahin E, Halac M, Sager S. Standardized uptake values of normal organs on 18F-fluorodeoxyglucose positron emission tomography and computed tomography imaging. J Int Med Res. 2007;35:231–6. doi: 10.1177/147323000703500207. [DOI] [PubMed] [Google Scholar]
- 12.Jadvar H, Ye W, Groshen S, Conti PS. [F-8] -fluorodeoxyglucose PET-CT of the normal prostate gland. Ann Nucl Med. 2008;22:787–793. doi: 10.1007/s12149-008-0177-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathews D, Oz OK. Positron emission tomography in prostate and renal cell carcinoma. Curr Opin Urol. 2002;12:381–5. doi: 10.1097/00042307-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Han EJ, H OJ, Choi WH, Yoo IR, Chung SK. Significance of incidental focal uptake in prostate on 18-fluoro-2-deoxyglucose positron emission tomography CT images. Br J Radiol. 2010;83:915–20. doi: 10.1259/bjr/19887771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang I, Chong A, Jung SI, Hwang EC, Kim SO, Kang TW, Kwon DD, Park K, Ryu SB. Is further evaluation needed for incidental focal uptake in the prostate in 18-fluoro-2-deoxyglucose positron emission tomography-computed tomography images? Ann Nucl Med Feb. 2013;27:140–5. doi: 10.1007/s12149-012-0663-7. [DOI] [PubMed] [Google Scholar]
- 16.Kang PM, Seo WI, Lee SS. Incidental abnormal FDG uptake in the prostate on 18 fluoro-2-deoxyglucose positron emission tomography-computed tomography scans. Asian Pac J Cancer Prev. 2014;15:8699–703. doi: 10.7314/apjcp.2014.15.20.8699. [DOI] [PubMed] [Google Scholar]
- 17.Hankey BF, Feuer FJ, Clegg LX, Hayes RB, Legler JM, Prorok PC, Ries LA, Merrill RM, Kaplan RS. Cancer surveillance series: Interpreting trends in prostate cancer- part I: Evidence of the effects of screening in recent prostate cancer incidence, mortality and survival rates. J Natl Cancer Inst. 1999;91:1017. doi: 10.1093/jnci/91.12.1017. [DOI] [PubMed] [Google Scholar]
- 18.Reinicke K, Sotomayor P, Cisterna P, Delgado C, Nualart F, Godoy A. Cellular distribution of Glut-1 and Glut-5 in benign and malignant human prostate tissue. J Cell Biochem. 2012;113:553–62. doi: 10.1002/jcb.23379. [DOI] [PubMed] [Google Scholar]
- 19.Nathan M, Rohren E. FDG uptake in the prostate gland on PET/CT: a worrisome sign for prostate cancer regardless of PSA level. J Nucl Med. 2006;47(Suppl 1):180P. [Google Scholar]
- 20.Gleason DF, Mellinger GT. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol. 1974;111:58–64. doi: 10.1016/s0022-5347(17)59889-4. [DOI] [PubMed] [Google Scholar]
- 21.Mohler J, Bahnson RR, Boston B, Busby JE, D’Amico A, Eastham JA, Enke CA, George D, Horwitz EM, Huben RP, Kantoff P, Kawachi M, Kuettel M, Lange PH, Macvicar G, Plimack ER, Pow-Sang JM, Roach M 3rd, Rohren E, Roth BJ, Shrieve DC, Smith MR, Srinivas S, Twardowski P, Walsh PC. NCCN clinical practice guidelines in oncology: prostate cancer. J Natl Compr Canc Netw. 2010;8:162–200. doi: 10.6004/jnccn.2010.0012. [DOI] [PubMed] [Google Scholar]
- 22.Li QK, Chen L, Ao MH, Chiu JH, Zhang Z, Zhang H, Chan DW. Serum Fucosylated Prostate-specific Antigen (PSA) Improves the Differentiation of Aggressive from Non-aggressive Prostate Cancers. Theranostics. 2015;5:267–76. doi: 10.7150/thno.10349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang PM, Seo WI, Lee SS, Bae SK, Kwak HS, Min K, Kim W, Kang DI. Incidental Abnormal FDG Uptake in the Prostate on 18-fluoro-2-Deoxyglucose Positron Emission Tomography-Computed Tomography Scans. Asian Pac J Cancer Prev. 2014;15:8699–8703. doi: 10.7314/apjcp.2014.15.20.8699. [DOI] [PubMed] [Google Scholar]
- 24.McNeal JE. The zonal anatomy of the prostate. Prostate. 1981;2:35–49. doi: 10.1002/pros.2990020105. [DOI] [PubMed] [Google Scholar]
- 25.Cho SK, Choi JY, Yoo J, Cheon M, Lee JY, Hyun SH, Lee EJ, Lee KH, Kim BT. Incidental Focal (18)F-FDG Uptake in the Prostate: Clinical Significance and Differential Diagnostic Criteria. Nucl Med Mol Imaging. 2011;45:192–6. doi: 10.1007/s13139-011-0092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


