Abstract
Objective: C-reactive protein (CRP) has been reported, with controversy, to be associated with poor survival of patients with non-small cell lung cancer (NSCLC). This meta-analysis aimed to evaluate the prognostic role of CRP in NSCLC. Methods: We searched PubMed, EMBASE and CNKI databases for published studies that evaluated the prognostic role of CRP in NSCLC up to March 1, 2014. The data were analyzed using STATA software (Version 12.0; Stata Corporation). Hazard ratios (HRs) with a 95% CI and 5-year survival rates were calculated to evaluate the relationships between CRP levels and the prognosis of NSCLC patients. Results: Eight studies were included, totaling 1668 NSCLC patients. The results revealed that elevated CRP values might predict poor 5-year overall survival rates (RR=2.15, 95% CI: 1.78-2.59) and poor 5-year disease-specific survival rates (RR=2.12, 95% CI: 1.56-2.88). The pooled HR between stage I/II and stage III/IV patients was 0.98 (95% CI: 0.26-3.63, P=0.976), which indicated that the difference between the survival rates of the patients with elevated CRP and those with undetectable CRP was not significant. In our survival analysis, the results of Egger’s testing did not demonstrate evidence of publication bias (P=0.099). Conclusion: Elevated CRP level is relevant to poorer survival of NSCLC patients and might be used as a prognostic biomarker for NSCLC.
Keywords: C-reactive protein, non-small cell lung cancer, prognosis
Introduction
Primary lung cancer is the leading cause of cancer-related death worldwide [1]. Most lung cancer cases (85%) are categorized as non-small cell lung cancer (NSCLC), with the remaining cases constituting small cell lung cancer (SCLC) [2]. The cellular effectors of inflammation are integral parts of the tumor local environment [3]. Among the many laboratory markers of systemic inflammation is serum C-reactive protein concentration (CRP) [4].
CRP is an acute-phase reactant [5]. Elevated preoperative serum CRP has been identified to be a significant prognostic factor in patients with colorectal, esophageal and hepatic carcinoma [5]. Many studies have shown that NSCLC patients with elevated preoperative serum CRP levels experienced worse survival than patients with undetectable CRP levels [5-9]. However, some investigators reported dissimilar results, demonstrating that elevated CRP was unrelated to shorter survival [10]. CRP value is much easier to get to predict the prognosis of patients with NSCLC than other subjects such as cancer type and tumor size and so on. Therefore, this meta-analysis was conducted to study whether elevated preoperative CRP level represented an independent factor for worse survival.
Materials and methods
We searched PubMed, EMBASE and CNKI databases for published studies that evaluated the prognostic role of CRP in NSCLC up to May 1, 2014. The following search terms using MeSH Major Topic and Text Word were used: “C-reactive protein” and “lung cancer”. We also supplemented this search by reviewing the reference lists of all of the retrieved publications and by identifying additional relevant articles.
The following inclusion criteria were employed: (1) the relationship between preoperative serum CRP value and the prognosis of the patients had been analyzed; (2) 5-year survival rates or HRs could be obtained; and (3) the minimum sample size was 30. Following the search, 8 articles were entered into our analysis (Figure 1).
Figure 1.
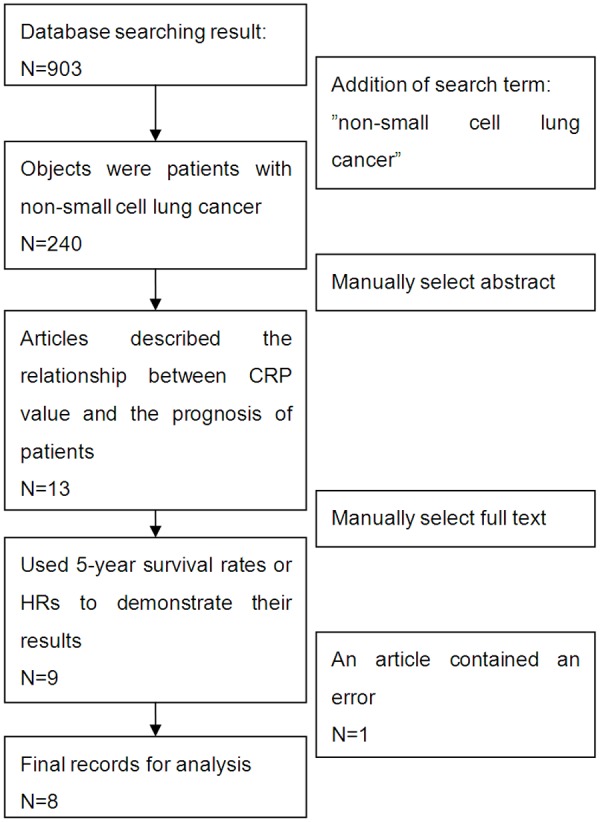
Flow chart of study selection process.
Statistical analysis
The data were analyzed using STATA software (Version 12.0; Stata Corporation). HRs with a 95% CI and 5-year survival rates were calculated to evaluate the relationships between CRP levels and the prognosis of NSCLC patients. A random-effects model was used if heterogeneity existed. Otherwise, a fixed-effects model was employed. Moreover, subgroup analyses were used to investigate potential sources of heterogeneity. Begg’s funnel plot was used to evaluate publication bias in our selected studies. A value of P<0.05 was considered statistically significant.
Results
Study characteristics
The main study characteristics of the 8 articles [5-12] are summarized in Table 1.
Table 1.
Characteristics of the studies included in this meta-analysis
| Study | Country | Elevated CRP/normal CRP | Cut-off value (mg/L) | Study design | Median follow-up, mo (range) | Stage | Prognosis factor *(Y/N) | |
|---|---|---|---|---|---|---|---|---|
| 5 | Koch, 2009 | Sweden | 206/83 | 10 | Retrospective | 42.9 (14.2-74.3) | IIIB-IV | Y |
| 6 | HARA, 2007 | Japan | 38/165 | 50 | Retrospective | - | I-IIIA | Y |
| 7 | Shen, 2011 | Chinese | 36/69 | 50 | Retrospective | 67.3 (0.7-122.2) | I | Y |
| 8 | Masago, 2010 | Japan | 26/53 | 100 | Retrospective | 22.8 (-) | IIIB-IV | Y |
| 9 | Tomita, 2012 | Japan | 62/239 | 50 | Retrospective | - | I, II, III | Y |
| 10 | Fan, 2013 | Chinese | 75/51 | 7.1 | Prospective | - | I, II, III | Y |
| 11 | Hara, 2010 | Japan | 130/146 | 50 | Retrospective | - | I-III A | Y |
| 12 | Alifano, 2011 | France | 64/225 | 20 | Prospective | 89.2 (82.6-92.9) | I, II, III, IV | Y/N |
The prognosis prognostic factor is described as yes (Y) and no (N).
Meta-analysis results
Due to indistinct heterogeneity between the selected studies (P=0.921, I2=0.0%), a fixed-effects model was used to explore the relationship between CRP levels and the survival of NSCLC patients. The results revealed that elevated CRP values might predict poor 5-year overall survival rates (RR=2.15, 95% CI: 1.78-2.59) (Figure 2). Figure 3 shows that elevated CRP might be related to poor 5-year disease-specific survival rates (RR=2.12, 95% CI: 1.56-2.88) (Figure 3).
Figure 2.
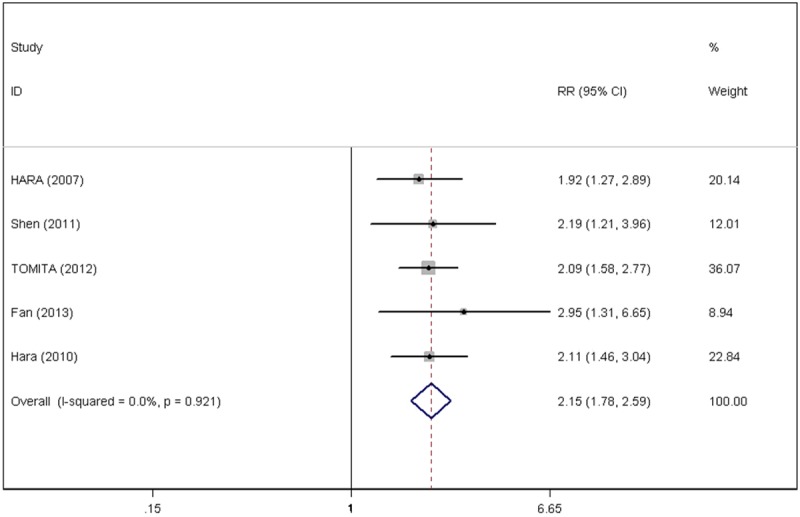
Forest plots of the C-reactive protein value and 5-year overall survival rate.
Figure 3.
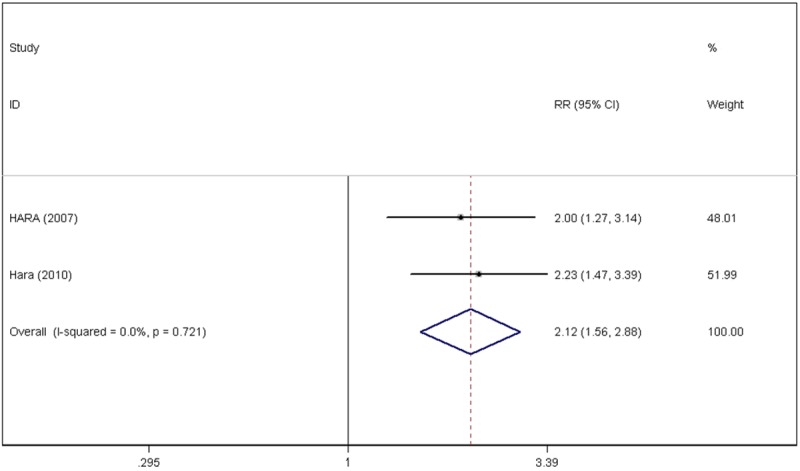
Forest plots of the C-reactive protein value and 5-year disease-specific survival rate.
In one primary study, the investigators demonstrated that the stage I and stage II patients with elevated CRP had worse survival than patients with undetectable CRP. However, the survival difference was not significant in stages III and IV patients [10]. The pooled HR between stage I/II and stage III/IV patients was calculated in this article. Because the heterogeneity testing results yielded P=0.006 and I2=86.8%, a random-effects model was used, determining the pooled HR as 0.98 (95% CI: 0.26-3.63, P=0.976), which demonstrated that the difference in the survival rates between patients with elevated CRP and undetectable CRP was not significant (Figure 4).
Figure 4.
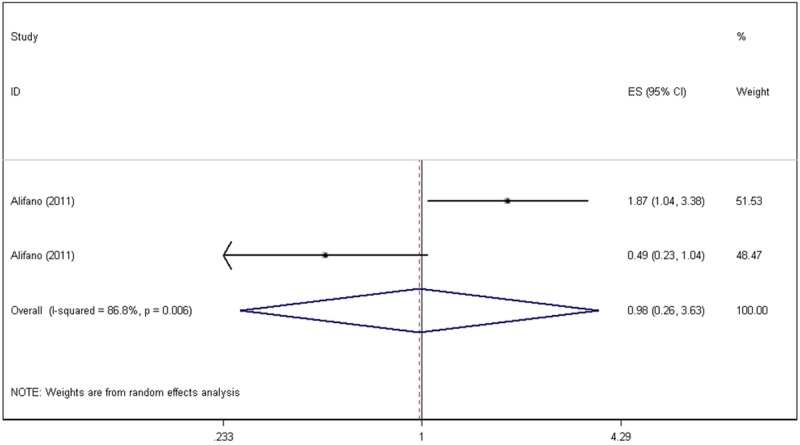
Forest plots of a particular primary publication’s hazard ratios for high C-reactive protein values.
Publication bias
Begg’s funnel plot was used to evaluate publication bias in our selected studies. As shown in Figure 5, as regards the 5-year overall survival, 5-year disease-specific survival meta-analyses, the P values were 0.099 and 1.000, respectively. The results of Egger’s test conducted on our survival analysis did not show evidence of publication bias (Figure 5).
Figure 5.
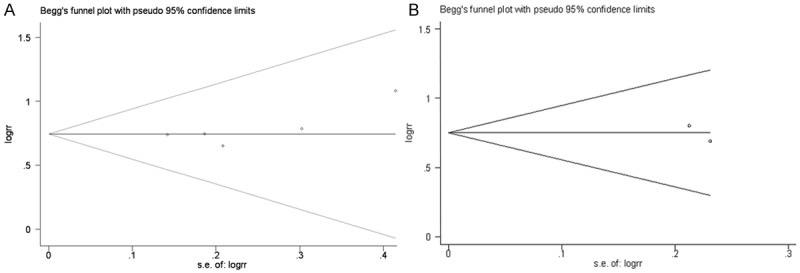
Begg’s funnel plots for publication bias testing: (A) 5-year overall survival, (B) 5-year disease-specific survival.
Discussion
An increasing number of studies have shown that an elevated serum CRP value was associated with survival of patients with non-small cell lung cancer. However, the nature of this association currently remains controversial. Some investigators have reported a significant association between CRP elevation and NSCLC survival, whereas others have reached the opposite conclusion. Thus, we conducted a meta-analysis to determine whether elevated CRP levels were related to worse survival.
Our study included 8 studies that contained 1668 patients with non-small cell lung cancer. We observed that elevated CRP values might predict worse survival in NSCLC patients, whereas normal CRP values might portend a better prognosis. However, in Alifano’s study [10], the pooled HR did not support a significant difference in the survival rates between patients with elevated CRP and undetectable CRP. The study data revealed that patients who were stages I/II with elevated CRP had worse survival than the patients with undetectable CRP. However, the difference was not significant in stages III and IV patients. Because the pooled HR is 0.98, we considered that the controversial conclusions might have resulted from the insufficient sample size (the number of stages III and IV patients with elevated CRP level was only 19). A further study is expected to support that report’s findings.
CRP, discovered in 1930, is a representative acute-phase reactant whose levels rapidly increase in response to most types of inflammation [13] and is accepted as one of the most widely used systemic inflammation markers in vivo [14]. Furthermore, CRP was reported to be an informative biomarker that reflects disease progression and the efficacy of therapeutic intervention [15]. Serum CRP levels begin to increase within 4-6 h after the onset of inflammation, with a peak concentration at 36-50 h. After inflammation resolution, serum levels decrease with a half-life of less than 12 h [8]. The mechanism by which cancer occurs along with increased CRP levels is widely known. CRP production by the liver is induced by cytokines such as interleukin-1 (IL-1), TNF, and mostly IL-6. Some studies in NSCLC cell lines and in lung surgical specimens have shown that at least some NSCLC tumors are able to produce IL-6 and TNF-α themselves [5]. Elevated CRP values were also detected in NSCLC patients with larger tumor sizes, the latter being both an important staging factor and prognostic factor [7]. Elevated CRP has also been associated with increased weight loss, reduced performance status, increased fatigue and decreased survival [16]. According to these findings, elevated CRP values may predict poor survival, consistent with the results of this study.
There were some limitations to this meta-analysis. Owing to the strict selection criteria and the limited number of published studies concerning CRP level and NSCLC prognosis, only 8 published articles were eligible for our meta-analysis. A key finding of this meta-analysis was that the cut-off value of CRP in the original documents was not absolute, ranging from 7.1 to 100 mg/L because different methods and kits for measuring CRP were used in the respective hospitals. It was beyond the scope of this study to stratify the cases by absolute CRP value. As a result, we classified all of the cases according to the original publication, which in general might cause heterogeneity and bias. Notwithstanding, we attempted to control the systematic error in our study; however, our study also contained some confounding factors, the distribution of which between the experimental group and the control group might have affected the results, such as age [12,17], gender [18-20], and smoking status [20,21]. Yet, because obtaining their original data appeared improbable, we believed that a further study was necessary to substantiate their conclusions. Finally, publication bias is often a concern in a meta-analysis because null results tend to be unpublished [16].
In conclusion, this meta-analysis clarified that CRP is an independent prognostic factor for patients with NSCLC.
Acknowledgements
The authors gratefully acknowledge the faculty of the Department of Epidemiology of the School of Public Health of Tianjin Medical University for their instruction and copyediting. We are also indebted to the physicians at the National Clinical Research Center for Cancer and the Key Laboratory of Cancer Prevention and Therapy of Tianjin Medical University Cancer Institute and Hospital for their contributions.
Disclosure of conflict of interest
None.
References
- 1.Alberg AJ, Ford JG, Samet JM American College of Chest Physicians. Epidemiology of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132:29S–55S. doi: 10.1378/chest.07-1347. [DOI] [PubMed] [Google Scholar]
- 2.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367–80. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 4.Jafri SH, Shi R, Mills G. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): a retrospective review. BMC Cancer. 2013;13:158. doi: 10.1186/1471-2407-13-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hara M, Matsuzaki Y, Shimuzu T, Tomita M, Ayabe T, Enomoto Y, Onitsuka T. Preoperative serum C-reactive protein level in non-small cell lung cancer. Anticancer Res. 2007;27:3001–4. [PubMed] [Google Scholar]
- 6.Koch A, Fohlin H, Sorenson S. Prognostic significance of C-reactive protein and smoking in patients with advanced non-small cell lung cancer treated with first-line palliative chemotherapy. J Thorac Oncol. 2009;4:326–32. doi: 10.1097/JTO.0b013e31819578c8. [DOI] [PubMed] [Google Scholar]
- 7.Shen L, Li ZM, Lu S. Clinical significance of C-reactive protein in patients with stage I non-small cell lung cancer. Chin J Oncol. 2011;33:442–46. [PubMed] [Google Scholar]
- 8.Masago K, Fujita S, Togashi Y, Kim YH, Hatachi Y, Fukuhara A, Nagai H, Irisa K, Sakamori Y, Okuda C, Mio T, Mishima M. Clinical significance of pretreatment C-reactive protein in patients with advanced nonsquamous, non-small cell lung cancer who received gefitinib. Oncology. 2010;79:355–62. doi: 10.1159/000323486. [DOI] [PubMed] [Google Scholar]
- 9.Hara M, Yonei A, Ayabe T, Tomita M, Nakamura K, Onitsuka T. Postoperative serum C-reactive protein levels in non-small cell lung cancer patients. Ann Thorac Cardiovasc Surg. 2010;16:85–90. [PubMed] [Google Scholar]
- 10.Alifano M, Falcoz PE, Seegers V, Roche N, Schussler O, Younes M, Antonacci F, Forgez P, Dechartres A, Massard G, Damotte D, Régnard JF. Preresection serum C-reactive protein measurement and survival among patients with resectable non-small cell lung cancer. J Thorac Cardiovasc Surg. 2011;142:1161–7. doi: 10.1016/j.jtcvs.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 11.Tomita M, Shimizu T, Ayabe T, Nakamura K, Onitsuka T. Elevated preoperative inflammatory markers based on neutrophil-to-lymphocyte ratio and C-reactive protein predict poor survival in resected non-small cell lung cancer. Anticancer Res. 2012;32:3535–8. [PubMed] [Google Scholar]
- 12.Zhi-ming F, Chao-hong H, Hong-gui H. Correlation between C-reactive protein levels and long-term prognosis of patients with non-small cell lung cancer. Hainan Med J. 2013;24:536–38. [Google Scholar]
- 13.Shiels MS, Pfeiffer RM, Hildesheim A, Engels EA, Kemp TJ, Park JH, Katki HA, Koshiol J, Shelton G, Caporaso NE, Pinto LA, Chaturvedi AK. Circulating inflammation markers and prospective risk for lung cancer. J Natl Cancer Inst. 2013;105:1871–80. doi: 10.1093/jnci/djt309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tillett WS, Francis T. Serological Reactions In Pneumonia with a Non-Protein Somatic Fraction Of Pneumococcus. J Exp Med. 1930;52:561–71. doi: 10.1084/jem.52.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hotta K, Sho M, Fujimoto K, Shimada K, Yamato I, Anai S, Konishi N, Hirao Y, Nonomura K, Nakajima Y. Prognostic significance of CD45RO+ memory T cells in renal cell carcinoma. Br J Cancer. 2011;105:1191–6. doi: 10.1038/bjc.2011.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott HR, McMillan DC, Forrest LM, Brown DJ, McArdle CS, Milroy R. The systemic inflammatory response, weight loss, performance status and survival in patients with inoperable non-small cell lung cancer. Br J Cancer. 2002;87:264–7. doi: 10.1038/sj.bjc.6600466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sigel K, Bonomi M, Packer S, Wisnivesky J. Effect of age on survival of clinical stage I non-small-cell lung cancer. Ann Surg Oncol. 2009;16:1912–7. doi: 10.1245/s10434-009-0475-8. [DOI] [PubMed] [Google Scholar]
- 18.Gasperino J. Age Is a Prognostic Factor Affecting Survival in Lung Cancer Patients. Med Hypotheses. 2011;76:328–31. [Google Scholar]
- 19.Salmeron D, Chirlaque MD, Isabel Izarzugaza M, Sanchez MJ, Marcos-Gragera R, Ardanaz E, Galceran J, Mateos A, Navarro C. Lung cancer prognosis in Spain: the role of histology, age and sex. Respir Med. 2012;106:1301–8. doi: 10.1016/j.rmed.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Tas F, Cliftci R, Kilic L. Age Is a Prognostic Factor Affecting Survival in Lung Cancer Patients. Oncology Lett. 2013;6:1507–13. doi: 10.3892/ol.2013.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim C, Chapman RS, Hu W, He X, Hosgood HD, Liu LZ, Lai H, Chen W, Berndt SI, Hosgood HD, Lee KM, Zheng T, Blair A, Chapman RS. Smoky coal, tobacco smoking, and lung cancer risk in Xuanwei, China. Lung Cancer. 2014;84:31–5. doi: 10.1016/j.lungcan.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]


