Abstract
Interleukin (IL-21) is a member of the type I cytokine family with sequence homology to IL-2, IL-4, and IL-15. IL-21 has been reported to improve symptoms of allergic rhinitis in mice. In this study we examined whether IL-21 signaling involved in allergic airway inflammation and remodeling in vivo by using ovalbumin (OVA)-induced chronic asthma model. We showed IL-21 level was increased in the serum of asthma models but not bronchoalveolar lavage fluid (BALF). Intranasal administration with recombinant mouse IL-21 or anti-IL-21 receptor (IL-21R) antibody did not affect OVA-induced chronic airway inflammation and airway remodeling in vivo and also not affect the expression of IL-13 and TGF-β in BALF. Moreover, expression of IL-13 and TGF-β was not affect by intranasal administration with recombinant mouse IL-21 or anti-IL-21R antibody. These results indicated that IL-21 signaling might not play an important role in airway inflammation and remodeling.
Keywords: IL-21, airway remodeling, airway inflammation
Introduction
Asthma is a chronic disease characterized by airway inflammation, airway hyperresponsiveness (AHR) and airway remodeling. However, the mechanisms that underlie the development of airway inflammation and airway remodeling are poorly understood. Allergic airway inflammation mainly manifests as airway infiltration of leukocytes such as eosinophils, lymphocytes, and macrophages [1]. Airway remodeling refers to pathologic structural changes such as subepithelial fibrosis, increase of smooth muscle mass in the airway, and increased vascularization [2]. Airway remodeling has been considered to result in more rapid decline of lung function in some asthmatic patients [3]. However, there is no effective therapeutic agent preventing airway remodeling, which urges us to look for new therapeutic targets for asthma.
Interleukin (IL)-21 is a member of the type I cytokine family with sequence homology to IL-2, IL-4, and IL-15 [4]. The IL-21 receptor complex is composed of two subunits. The common γ-chain subunit is shared with IL-2, IL-4, IL-7, IL-9, IL-13, IL-15 and the private IL-21 receptor (IL-21R) [4,5]. IL-21 is predominantly produced by activated CD4+ T cells and natural killer (NK) T cells [6]. IL-21R is expressed by a variety of lymphohematopoietic cells [4]. IL-21 exerts a variety of regulatory effects. Several studies have indicated that IL-21 regulates the differentiation, growth, and activation of Th1 [7-10], Th2 [11], CTL [12] and NK cells [10].
Some previous studies reported that IL-21 suppressed IgE production by B cells. The mice deficient for the IL-21 [13] or the IL-21R [14] gene exhibited enhanced IgE production in response to Antigen (Ag) immunization. Similarly, Hiromura Y et al showed that IL-21 administration into the nostril improved symptoms of allergic rhinitis in mice through preventing of IgE production by B cells and eotaxin production by fibroblasts [15].
In our previous study, we have demonstrated that administration of IL-21 alone may not alleviate acute allergic lung inflammation [16]. Nevertheless, the effect of IL-21/IL-21R signaling on chronic airway inflammation and airway remodeling has not been explored. Consequently, the goal of the current study is to investigate the effect of IL-21/IL-21R signaling on chronic airway inflammation and airway remodeling. In this study, we used a chronic allergen-induced asthma model and administered them with recombinant mouse IL-21 (rmIL-21) and anti-IL-21R antibody (anti-IL-21R Ab) to examine the in vivo effects of IL-21/IL-21R signaling.
Methods
Animals and experimental protocol
Female BALB/c mice were sensitized by intraperitoneal injections of ovalbumin (OVA) (Sigma, St. Louis, MO) on day 0 (0.02 mg/mouse) and day 14 (0.2 mg/mouse). On day 21, mice were exposed to intranasal OVA challenges (1 mg in 0.2 ml saline) or saline used as control 3 times a week for 4 weeks [17]. Intranasal administration of IL-21 (20 ng/mouse, PeproTech, Rocky Hill, NJ), anti-IL-21R Ab (1 µg/mouse, Santa Cruz) or control IgG was performed under light anesthesia 30 minutes before each OVA challenge. All experiment procedures were performed according to the requirements of the Animal Care and Ethics Committee of Tongji Hospital.
Bronchoalveolar lavage and lung histology
Bronchoalveolar lavage fluid (BALF) was collected by cannulating the upper part of the trachea, followed by lavage with 1 ml of PBS. Different cell counts were performed on Wright-Giemsa-stained cytospins. The supernatant was stored at -80°C. To evaluate the extent of inflammation, lung sections were stained with hematoxylin and eosin. Gomori trichrome stain was used to evaluate collagen deposition.
Determination of AHR
Airway responsiveness to inhaled methacholine was measured with FlexiVent (SCIREQ Scientific Respiratory Equipment, Inc. Montreal, PQ, Canada) [18]. Mice were anesthetized by intraperitoneal injection of pentobarbital, then tracheostomized and connected to the flexiVent. Baseline airway resistance was measured in each mouse after nebulization of PBS (vehicle for methacholine) for 10 seconds using an Aeroneb ultrasonic nebulizer (SCIREQ). After baseline measurements, mice were exposed to increasing concentrations of nebulized methacholine.
Measurement of BALF and serum cytokines
The levels of IL-13 and TGF-β in BALF and serum were determined by ELISA (Ebioscience) according to the manufacturer’s instructions.
Statistical analysis
Data were expressed as means ± SEM. Variance analysis was used for statistical comparisons between groups, followed by the Tukey test using GraphPad Prism 5 Software; p values of less than 0.05 were considered to indicate statistical significance.
Results
IL-21 level is increased in plasma from OVA-challenged mice
To determine whether chronic allergic airway inflammation led to change of IL-21 level, mice were sensitized and challenged with OVA and IL-21 level was determined. As shown in Figure 1, compared with those from Saline-challenged mice, levels of IL-21 in serum from OVA-challenged mice were increased significantly while the level of IL-21 from BALFs was not different.
Figure 1.
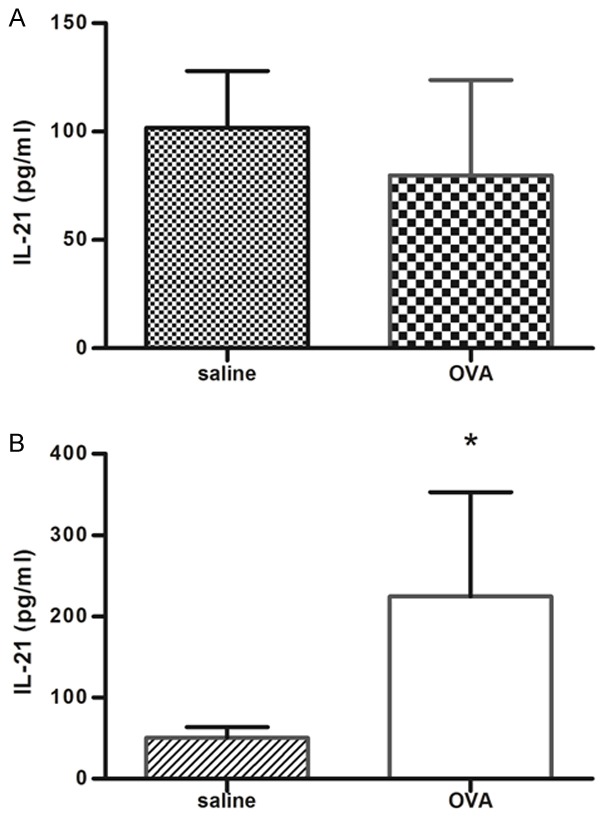
IL-21 is increased in plasma but not BALF from OVA-challenged mice. IL-21 concentrations in BALF (A) and plasma (B) were assessed 24 hours after the last challenge, as described in Methods. Results were presented as means ± SEM. N = 4-6 mice per group; *P < 0.05 compared with saline-challenged group.
Administration of IL-21 or anti-IL-21R Ab does not reduce lung inflammation and alleviate airway remodeling
We next determine the effect of IL-21 or anti-IL-21R Ab on lung inflammation and airway remodeling. Compared with control mice challenged with saline, OVA-challenged mice exhibited a significant increase in total leukocyte numbers detected in BALF 24 h after last provocation; differential cell counts revealed that this was due primarily to infiltration of eosinophils, and a small amount of macrophages and lymphocytes (Figure 2). IL-21 administration had no effect on total cell counts and eosinophils counts, as well as macrophages, lymphocytes and neutrophils counts. As compared with IgG, treatment with anti-IL-21R Ab did not affect the counts of total cells, eosinophils, macrophages, lymphocytes and neutrophils.
Figure 2.
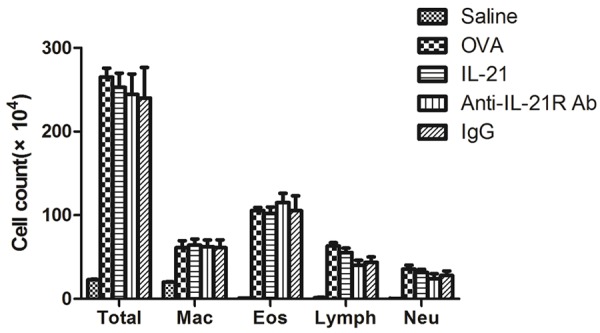
IL-21 or Anti-IL-21R Ab treatment does not affect leukocyte accumulation in BALF. The number of inflammatory cells in BALF was determined 24 hours after the last challenge, as described in Methods. Saline; normal control mice treated with saline only; OVA; OVA-sensitized/challenged mice; IL-21; IL-21 treatment and OVA-sensitized/challenged mice; IgG; IgG treatment and OVA-sensitized/challenged mice; Anti-IL-21R Ab; Anti-IL-21R Ab treatment and OVA-sensitized/challenged mice. All data represent means ± SEM. N = 4-6 mice per group.
To evaluate the collagen deposition, lung sections from mice were stained with Gomori trichrome (Figure 3). As shown in Figure 3, the accumulation of collagen in lung tissues was significantly increased in the OVA-challenged mice compared with saline-challenged mice. Mice treated with IL-21 showed no significant change of collagen deposition compared with saline-challenged mice. In addition, the extent of collagen deposition was not affected by anti-IL-21R Ab treatment, compared with those after treatment with control IgG.
Figure 3.
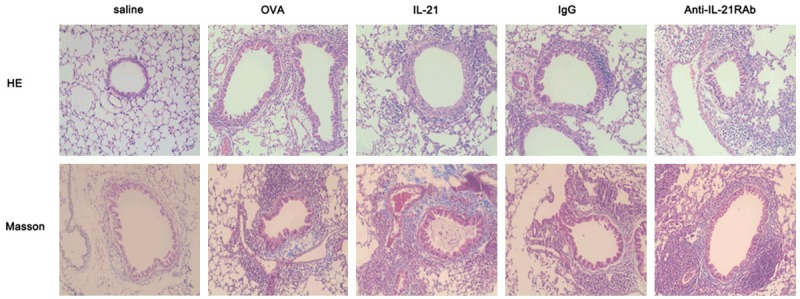
IL-21 or Anti-IL-21R Ab treatment does not affect OVA-induced airway remodeling. Sections were stained with haematoxylin and eosin (top panel) or Gomori trichrome (bottom panel). Representative results of six different experiments are shown (original magnification: × 200).
Administration of IL-21 or anti-IL-21R Ab does not reduce the production of IL-13 and TGF-β
IL-13 and TGF-β concentrations in BALF were measured by ELISA 24 hours after last provocation. The production of IL-13 and TGF-β were unchanged by IL-21 or anti-IL-21R Ab treatment (Figure 4).
Figure 4.
IL-21 or Anti-IL-21R Ab treatment does not affect OVA-induced airway hyperresponsiveness (AHR). Mice were first exposed to nebulized saline, followed by increasing doses (3 to 25) mg/ml) of nebulized methacholine for 3 minutes each. Breathing indices were read for 3 minutes after each nebulization, and enhanced pause values were determined. All data represent means ± SEM. N = 4-6 mice per group.
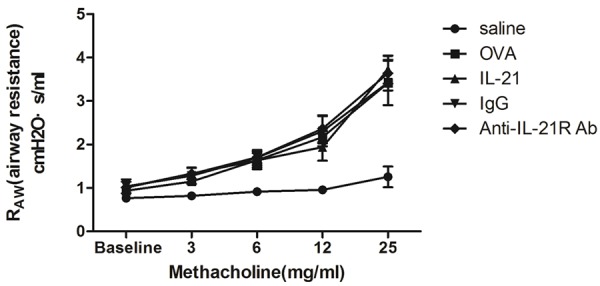
Treatment with IL-21 or anti-IL-21R Ab does not reduced AHR in chronic allergic asthma
To determine the effects of IL-21 or anti-IL-21R Ab on lung function, airway resistance was measured 24 h after the last provocation. In OVA-challenged mice, airway resistance was increased in a dose-dependent manner after exposure to methacholine (Figure 5). IL-21 treatment did not inhibit the increased airway reactivity to methacholine. Anti-IL-21R Ab treatment did not alter the methacholine-induced AHR, compared with control IgG treatment.
Figure 5.
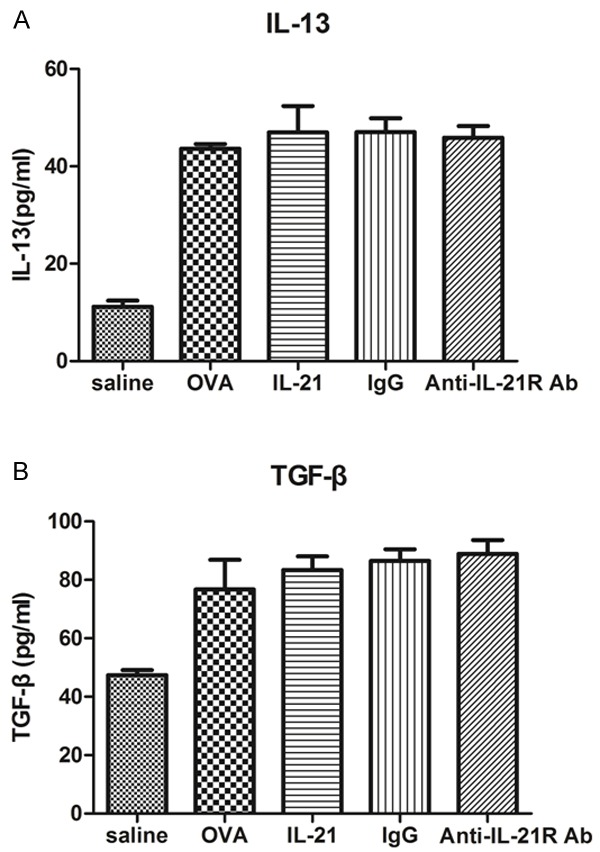
IL-21 or Anti-IL-21R Ab treatment does not affect cytokine production in BALF. IL-13 and TGF-β concentrations in BALF were assessed 24 hours after last challenge, as described in Methods. Results were presented as means ± SEM. N = 4-6 mice per group.
Discussion
In the present study, we investigated the impact of IL-21 signaling on the regulation of chronic allergic airway inflammation and remodeling induced by OVA exposure. We showed that either IL-21 or anti-IL21R Ab: (1) did not affect OVA-induced airway inflammation and airway remodeling; (2) did not effect on OVA-induced AHR; (3) did not modulate the expression of inflammation and remodeling related cytokines. Taken together, these data suggested that IL-21 might not play a critical role during the development of allergic airway remodeling.
Our previous study showed that the serum IL-21 production was increased in asthma patients, herein; we found that IL-21 was up-regulated in the serum of OVA-challenged mice but not BALF. Therefore, we hypothesized that IL-21 may involve in the pathogenesis of asthma. To test our hypothesis, we treated mice with IL-21 or anti-IL-21R Ab before every OVA provocation. Hiromura and coworkers documented that IL-21 suppressed eosinophils infiltration in nasal-associated lymphoid tissue through preventing eotaxins production by nasal fibroblasts [15]. To validate the effect of IL-21 as a positive control in our study, we repeated their study by using an allergic rhinitis model treated with IL-21 before challenge, and we found decreased eosinophilic infiltration of the nasal mucosa. In previous study we found exogenous administration of IL-21 may not alleviate allergic lung inflammation [16]. In this study, we demonstrated that intranasal administration of IL-21 had no effect on allergen-induced chronic airway inflammation and remodeling. Similarly, expression of IL-13 and TGF-β in the BALF were not modified by IL-21 or anti-IL-21R Ab. One should note that, asthma and allergic rhinitis may have much in common, however, there might be some other factors that would contribute to this discrepancy and more data were needed to interpret this discrepancy.
In order to further test our hypothesis, we examined whether IL-21 or anti-IL-21R Ab could affect OVA-induced AHR. However, as seen in the chronic asthma model, neither IL-21 nor anti-IL-21R Ab exerted effect on airway resistance. Based on the evidence we concluded that IL-21 signaling did not involve in the OVA-induced AHR.
Taken together, our findings suggest that the chronic airway inflammation and remodeling response triggered by OVA exposure occurred independently of IL-21 activity. Our study is the first study to report that IL-21 has little effect on development of chronic airway inflammation and airway remodeling. However, more research is needed to prove our results further.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81170021 and No. 30900647).
Disclosure of conflict of interest
None.
References
- 1.Busse WW, Lemanske RJ. Asthma. N Engl J Med. 2001;344:350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 2.Vignola AM, Mirabella F, Costanzo G, Di Giorgi R, Gjomarkaj M, Bellia V, Bonsignore G. Airway remodeling in asthma. Chest. 2003;123:417S–422S. doi: 10.1378/chest.123.3_suppl.417s. [DOI] [PubMed] [Google Scholar]
- 3.Lange P, Parner J, Vestbo J, Schnohr P, Jensen G. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med. 1998;339:1194–1200. doi: 10.1056/NEJM199810223391703. [DOI] [PubMed] [Google Scholar]
- 4.Parrish-Novak J, Foster DC, Holly RD, Clegg CH. Interleukin-21 and the IL-21 receptor: novel effectors of NK and T cell responses. J Leukoc Biol. 2002;72:856–863. [PubMed] [Google Scholar]
- 5.Parrish-Novak J, Foster DC, Holly RD, Clegg CH. Interleukin-21 and the IL-21 receptor: novel effectors of NK and T cell responses. J Leukoc Biol. 2002;72:856–863. [PubMed] [Google Scholar]
- 6.Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, Johnston J, Madden K, Xu W, West J, Schrader S, Burkhead S, Heipel M, Brandt C, Kuijper JL, Kramer J, Conklin D, Presnell SR, Berry J, Shiota F, Bort S, Hambly K, Mudri S, Clegg C, Moore M, Grant FJ, Lofton-Day C, Gilbert T, Rayond F, Ching A, Yao L, Smith D, Webster P, Whitmore T, Maurer M, Kaushansky K, Holly RD, Foster D. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 7.Suto A, Wurster AL, Reiner SL, Grusby MJ. IL-21 inhibits IFN-gamma production in developing Th1 cells through the repression of Eomesodermin expression. J Immunol. 2006;177:3721–3727. doi: 10.4049/jimmunol.177.6.3721. [DOI] [PubMed] [Google Scholar]
- 8.Strengell M, Matikainen S, Siren J, Lehtonen A, Foster D, Julkunen I, Sareneva T. IL-21 in synergy with IL-15 or IL-18 enhances IFN-gamma production in human NK and T cells. J Immunol. 2003;170:5464–5469. doi: 10.4049/jimmunol.170.11.5464. [DOI] [PubMed] [Google Scholar]
- 9.Wurster AL, Rodgers VL, Satoskar AR, Whitters MJ, Young DA, Collins M, Grusby MJ. Interleukin 21 is a T helper (Th) cell 2 cytokine that specifically inhibits the differentiation of naive Th cells into interferon gamma-producing Th1 cells. J Exp Med. 2002;196:969–977. doi: 10.1084/jem.20020620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strengell M, Sareneva T, Foster D, Julkunen I, Matikainen S. IL-21 up-regulates the expression of genes associated with innate immunity and Th1 response. J Immunol. 2002;169:3600–3605. doi: 10.4049/jimmunol.169.7.3600. [DOI] [PubMed] [Google Scholar]
- 11.Pesce J, Kaviratne M, Ramalingam TR, Thompson RW, Urban JJ, Cheever AW, Young DA, Collins M, Grusby MJ, Wynn TA. The IL-21 receptor augments Th2 effector function and alternative macrophage activation. J Clin Invest. 2006;116:2044–2055. doi: 10.1172/JCI27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smyth MJ, Hayakawa Y, Cretney E, Zerafa N, Sivakumar P, Yagita H, Takeda K. IL-21 enhances tumor-specific CTL induction by anti-DR5 antibody therapy. J Immunol. 2006;176:6347–6355. doi: 10.4049/jimmunol.176.10.6347. [DOI] [PubMed] [Google Scholar]
- 13.Ozaki K, Spolski R, Feng CG, Qi CF, Cheng J, Sher A, Morse HR, Liu C, Schwartzberg PL, Leonard WJ. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 14.Shang XZ, Ma KY, Radewonuk J, Li J, Song XY, Griswold DE, Emmell E, Li L. IgE isotype switch and IgE production are enhanced in IL-21-deficient but not IFN-gamma-deficient mice in a Th2-biased response. Cell Immunol. 2006;241:66–74. doi: 10.1016/j.cellimm.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Hiromura Y, Kishida T, Nakano H, Hama T, Imanishi J, Hisa Y, Mazda O. IL-21 administration into the nostril alleviates murine allergic rhinitis. J Immunol. 2007;179:7157–7165. doi: 10.4049/jimmunol.179.10.7157. [DOI] [PubMed] [Google Scholar]
- 16.Cheng S, Chen H, Wang A, Bunjhoo H, Cao Y, Xie J, Xu Y, Xiong W. Exclusion of IL-21 in the pathogenesis of OVA-induced asthma in mice. Int J Clin Exp Med. 2014;7:3202–3208. [PMC free article] [PubMed] [Google Scholar]
- 17.Yamabayashi C, Koya T, Kagamu H, Kawakami H, Kimura Y, Furukawa T, Sakagami T, Hasegawa T, Sakai Y, Matsumoto K, Nakayama M, Gelfand EW, Suzuki E, Narita I. A Novel Prostacyclin Agonist Protects to Airway Hyperresponsiveness and Remodeling in Mice. Am J Respir Cell Mol Biol. 2012;47:170–7. doi: 10.1165/rcmb.2011-0350OC. [DOI] [PubMed] [Google Scholar]
- 18.Kramer EL, Mushaben EM, Pastura PA, Acciani TH, Deutsch GH, Khurana HG, Korfhagen TR, Hardie WD, Whitsett JA, Le Cras TD. Early growth response-1 suppresses epidermal growth factor receptor-mediated airway hyperresponsiveness and lung remodeling in mice. Am J Respir Cell Mol Biol. 2009;41:415–425. doi: 10.1165/rcmb.2008-0470OC. [DOI] [PMC free article] [PubMed] [Google Scholar]


