Abstract
Matrine has been proved to inhibit proliferation and induce apoptosis of human lung cancer cells. However, less studies involved in evaluating the effects and mechanism of matrine in cell migration and invasion of lung cancer. This study was aim to investigate the involvement of miR-133a in matrine’s anti-invasion and anti-metastasis in lung cancer. MTT assay was used to assess the inhibition of proliferation effects of matrine in NCI-H1299 cells. Migration and invasion abilities of NCI-H1299 cells were investigated by Transwell assays. Expression of miR-133a was detected by real-time PCR. Anti-miR technique was applied to inhibit miR-133a in matrine treated HCI-H1299 cells. Real-time PCR and Western blotting were performed to evaluate the activation of EGFR/Akt/MMP-9 pathway. As results, matrine treatment significantly inhibited proliferation, migration and invasion of NCI-H1299 cells in a concentration-dependent manner, accompanied by significantly elevation of miR-133a expression. However, matrine failed to inhibit the metastatic ability when cells transfected with anti-miR-133a. Matrine treatment also suppressed activation of EGFR/Akt/MMP-9 pathway. The inhibitory effects of matrine on activation of EGFR pathway were also reversed by anti-miR-133a transfection in NCI-H1299 cells. In conclusion, matrine inhibited the invasion and metastasis of lung cancer cell by elevating expression of miR-133a which further suppressed activation of EGFR/Akt/MMP-9 pathway.
Keywords: Lung cancer, invasion; metastasis, microrna, matrine
Introduction
Lung cancer is among the most common cancers worldwide with a morbidity of approximate 50 per 100 thousand. Lung cancer is also the leading cause of cancer-related deaths, killing around 1.5 million people every year [1]. To date, advanced achievement has been made in treatment of lung. However, many patients with lung cancer showed resistance to exiting treatments, such as chemotherapy and radiotherapy [2]. Fortunately, there are many extracts from Traditional Chinese Medicines showing better therapies to human lung cancer, especially for non-small cell lung cancer (NSCLC) [3].
As referred as C15H24N20, matrine has a wide spectrum of biological activities including antivirus [4], anti-inflammation [5], antifibrosis [6], et al. Clinical application of matrine, as the major active component of Sophora flavescens, has a long history in traditional Chinese herbal medicine with ideal curative effects in treatments of hepatic fibrosis, atherosclerosis, arrhythmias, infectious diseases and so on [7,8]. Recent in vivo and in vitro studies verified matrine’s anti-cancer activities in kinds of cancers such as gastric cancer, prostate cancer, lung cancer, glioma and cervical cancer [9]. In lung cancer, matrine has been proved to inhibit proliferation and induce apoptosis of human lung cancer cells [2,3,10]. However, less studies involved in evaluating the effects and mechanism of matrine in cell migration and invasion of lung cancer.
Over 90% patients with lung cancer died because of invasion and metastasis rather than the primary malignant lesions [11]. Because many biological processes take place during invasion and metastasis, the mechanisms are still not completely clear. MicroRNAs (miRNAs) have been considered playing critical roles in regulating many cellular biological procedures including cell migration and invasion by targeting the 3’-UTR of down-stream specific genes for either degradation of mRNA or inhibition of translation [12]. Studies revealed the enrichment or insufficiency of different kinds of miRNAs in malignant lung tumors due to their roles in positively or negatively regulating oncogenes or cancer suppressors to affect invasion and metastasis [13]. In previous studies, miR-133a was identified down-expression in malignant lung cancer and inhibited cell migration and invasion in NSCLC [14,15]. In addition, it was believed that miR-133a inhibited invasion and metastasis of malignant cells by targeting directly the epidermal growth factor receptor (EGFR) [16].
Since matrine could change miRNA expression profiles in human lung cancer cells [2], we hypothesize that matrine may suppress migration and invasion of lung cancer cell through miR-133a/EGFR pathway. Thus, in this study, the effects of matrine and miR-133a on invasion and metastasis capabilities of non-small lung cancer cells NCI-H1299 were investigated. Furthermore, the regulation effect of matrine on expression of miR-133a, which then inhibited migration and invasion by attenuating the EGFR signaling pathway in NCI-H1299 was also examined to demonstrate that matrine suppresses invasion and metastasis of NCI-H1299 cells by increasing miR-133a expression.
Materials and methods
Cell culture and matrine treatment
Human non-small lung cancer NCI-H1299 cell line (ATCC) was donated by Cancer Research Center of Xi’an Jiaotong University. Cells were maintained in RPMI 1640 culture medium (Gibco, New York, USA) containing 10% fetal bovine serum (FBS, Hyclone, Logan, USA), 100 μg/ml streptomycin (Sigma, St. Louis, USA), 100 U/ml penicillin (Sigma, St. Louis, USA) and 2 mmol/L gluatmine (Sigma, St. Louis, USA) in cell culture flasks (Corning Inc., Corning, NY). Cells were incubated in humidified environment with 5% dioxide atmosphere at 37°C. Same amount of cultured NCI-H1299 cells were treated by matrine (Sigma, St. Louis, USA) at serial concentrations of 0, 10, 20, 50, 100, 200 µg/ml for 24 hours.
Cell transfection
5×105 NCI-H1299 cells were planted and maintained on a 6-well plate (Corning Inc., Corning, NY) to achieve 80%-90% confluent, then proceed to transfection. Cells were transfected with siRNA (100 pmol/well) using Lipofectamine 2000 Reagent (Life Technologies, Grand Island, USA) according to manufacturer’s instruction. miR-133a inhibitor (anti-miR-133a) and miR-Inhibitors-Negative Control (Control) were purchased from AngRang Inc. (Xi’an, China). After 48 hours of transfection, cells were starved for migration and invasion assays. All siRNAs were purchased from Santa Cruz Biotechnology (Santa Cruz, USA).
Cell viability assay
Colorimetric 3-(4,5-dimethylthiazol-2-yl) 2,5-diphenyltetrazolium bromide (MTT) assay was applied to evaluate the cell viability of NCI-H1299 cells. MTT (Sigma, St. Louis, USA) solution at concentration of 5 mg/ml was used to incubate washed cells which were planted on a 96-well plate for 4 hours at 37°C. After washing, 150 μL dimethylsulfoxide (DMSO, Sigma, St. Louis, USA) was added to the wells. 540 nm absorbance (A540) value of each well was then detected by a plate reader (Bio-Rad). The proliferation inhibitory rate was calculated as [1-A540 (experimental well)/A540 (control well)] ×100%.
Cell migration and invasion assays
The migration and invasion abilities of NCI-H1299 cells were assessed by using
Transwell plates with 8 μm pore size membranes (Corning Inc., Corning, NY) according to protocols described previously [17]. Briefly, NCI-H1299 cells were cultured in RPMI 1640 medium for 24 hours and the medium were collected as conditioning medium. For invasion assay, Matrigel (0.1 mg/mL) was coated on the top surface of the transwell chambers. Treated cells were seeded to the chambers and incubated in humidified environment with 5% CO2 at 37°C for 48 hours. The cells passed through polyethylene membrane (migrated cells) or passed through Matrigel (invaded cells) were fixed by methanol. Then the crystal violet staining applied to indicate the migration or invasion under a light microscopy (Motic, Xiamen, China).
Quantitative real-time PCR
Total RNA was extracted by using Trizol reagent (Invitrogen, Carlsbad, USA) in accordance with manufacturer’s instructions. SuperScript III Reverse Transcriptase (Invitrogen, Carlsbad, USA) was used to perform the reverse transcription and synthesizes cDNA. All-in-oneTM qPCR kit (GeneCopoeia, Rockville, USA) was used to perform quantitative real-time PCR according to protocols provided by the manufacturer. The primers for miR-133a, EGFR, MMP-9 and GAPDH were designed and provided by TaKaRa. U6 and GAPDH were used as the internal reference.
Western blotting analysis
NCI-H1299 cells were lyzed by RIPA lysis buffer (Beyotime, Shanghai, China) on ice and total protein was extracted by protein extraction kit (Beyotime, Shanghai, China) according to the manufacturer’s instructions. A BCA kit (Thermo, Pittsburg, USA) was used to detect the concentration of extracted protein. 50 μg proteins were electrophoresed vertically through sodium dodecylsulfate-polyacrylamide gels and the separated proteins were transferred electronically to poly vinylidene difluoride (PVDF) membranes. Then the non-specific interactions were blocked by 5% defatted milk-TBST solution incubation at 37°C for 1 hour. After washing, specific antibodies against EGFR (Abcam, Cambridge, USA), AKT (Cell Signaling Tech, Danvers, USA), phosphorylated AKT (Cell Signaling Tech, Danvers, USA), MMP-9 (Abcam, Cambridge, USA) and GAPDH (Abcam, Cambridge, USA) were applied to incubate the membranes at 4°C for 12 hours to detect corresponding proteins. The membranes were then washed and incubated with horseradish peroxidase-conjugated second antibodies (Bioss) for 1 hour at 37°C. Immunoblots were then detected by ECL reagents (Invitrogen, Carlsbad, USA) and finally exposed to X-ray films. Software Quantity One (Bio-Rad) was used to analyze relative protein expressions while GAPDH was introduced as the internal reference.
Statistical considerations
The result values were expressed as mean ± standard deviation and further analyzed by SPSS (ver.16.0, SPSS). The Student’s t-test was utilized to assess the statistical significance of the difference between two treatments. A P value of less than 0.05 was considered significant.
Results
Matrine inhibited NCI-H1299 cells proliferation, migration and invasion
Figure 1A demonstrated the effects of matrine at serial concentrations (0, 10, 20, 50, 100, 200 µg/ml) on proliferation of NCI-H1299 cells by MTT assay. Concentrations of matrine above 50 µg/ml began to show significant cytotoxicity, inhibiting the proliferation of NCI-H1299 cells. So concentrations below 50 µg/ml were appropriate for the subsequent experiments concerning invasion and metastasis. As shown in Figure 1B and 1C, matrine significantly inhibited migration (Figure 1B) and invasion (Figure 1C) of NCI-H1299 cells at the concentration of 20 µg/ml.
Figure 1.
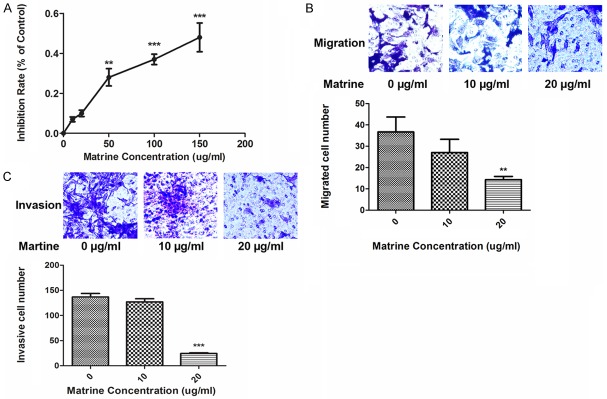
Effects of matrine on proliferation, migration, and invasion of lung cancer NCI-H1299 cells. (A) As demonstrated, MTT assay was applied to assess the effects of matrine on proliferation of NCI-H1299 cells. The line chart shows the inhibition rates of NCI-1299 cells incubated by serial concentrations of matrine (0, 10, 20, 50, 100, 150 µg/ml respectively). Values are represented in a mean ± SD manner of three independent experiments (**P<0.01, ***P<0.001). (B and C) The effect of matrine on cell migration (B) and invasion (C) evaluated by transwell assays in NCI-H1299 cells. The results are from three independent experiments (**P<0.01, ***P<0.001).
MiR-133a involved in matrine’s anti-migration and -invasion activities in NCI-H1299 cells
Since miR-133a has been proved to inhibit cell migration and invasion in NSCLC [14,18], we further tested whether miR-133a also involved in matrine’s anti-migration and -invasion activities in NCI-H1299 cells. A miR-133a inhibitor (anti-miR-133a) was used to block miR-133a expression in NCI-H1299 cells (Figure 2A). Transwell assay was used to evaluate cells migration and invasion. As results, down-regulation of miR-133a reversed matrine’s anti-migration (Figure 2B) and -invasion (Figure 2C) effects in NCI-H1299 cells.
Figure 2.
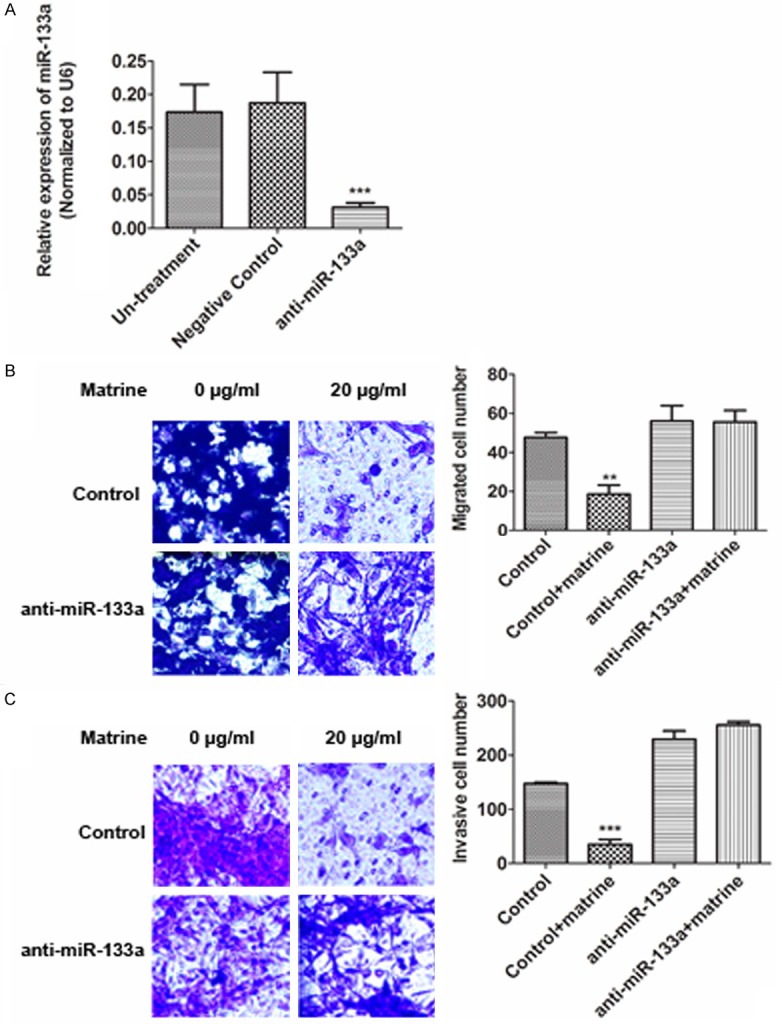
The effect of down-regulation of miR-133a on cell migration and invasion inhibited by matrine. A. Reduced miR-133a expression by anti-miR-133a siRNA in NCI-H1299 cells detected by real-time PCR. B and C. Transwell assays were used to assess the effect of down-regulation of miR-133a on cell migration and invasion inhibited by matrine (20 µg/ml). Values are represented in a mean ± SD manner of three independent experiments (**P<0.01, ***P<0.001).
Matrine elevated expression of miR-133a in NCI-H1299 cells
The present study analyzed the effects of matrine on the expression levels of miR-133a. As shown in Figure 3, after treatment of matrine, the expression level of miR-133a increased significantly in NCI-H1299 cells in a concentration- (Figure 3A) and time- (Figure 3B) dependent manner according to the results of quantitative real-time PCR.
Figure 3.
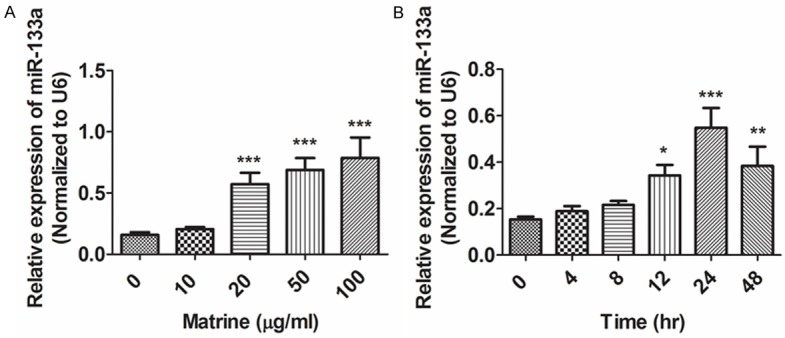
Effects of matrine on expression levels of miR-133a in lung cancer NCI-H1299 cells. NCI-H1299 cells were treated with different concentrations of matrine for 24 hr (A) or with matrine (20 μg/ml) for different times (B). Real-time PCR was used to evaluate expression of miR-133a Representative results are shown from three independent experiments. (*P<0.05, **P<0.01, ***P<0.001).
Matrine attenuated EGFR signaling pathway but reversed by anti-miR-133a
EGFR is one of the down-stream target of miR-133a [14], therefore, we hypothesized that matrine inhibited NCI-H1299 cells migration and invasion through suppressing EGFR signaling pathway. As shown in Figure 4, matrine decreased the expression of EGFR and MMP-9 at both transcriptional (Figure 4A) and translational levels (Figure 4B), as well as the ratio of p-AKT/t-AKT in NCI-H1299 cells in a concentration-dependent manner (Figure 4B). However, the suppressive effects of matrine on EGFR signaling pathway was then reversed by anti-miR-133a transfection (Figure 4B), demonstrated the effects of miR-133a on matrine inhibiting activation of EGFR signaling pathway.
Figure 4.
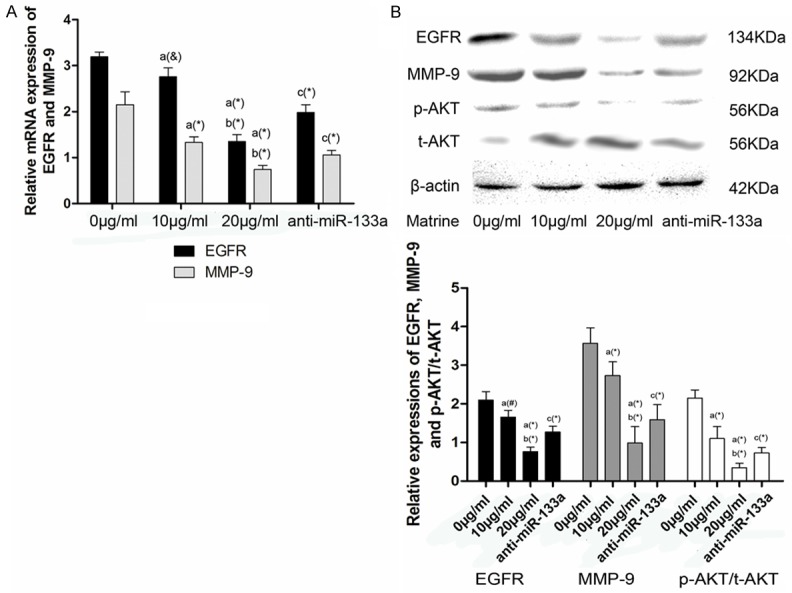
Effects of matrine and anti-miR-133a on activation of EGFR signaling pathway in NCI-1299 cells. A. Columns in this part showed relative mRNA expression levels of EGFR and MMP-9 in NCI-1299 cells treated by matine at concentrations of 0, 10 and 20 μg/ml, and 20 μg/ml after anti-miR-133a transfection respectively. Values are represented in a mean ± SD manner. a differences are significantly from 0.0 mg/ml; b differences are significantly from 0.2 mg/ml; c differences are significantly from 0.4 mg/ml. (&P<0.05, #P<0.01, *P<0.005). B. demonstrated the protein immunoblots of EGFR, MMP-9, p-AKT, t-AKT and β-actin in NCI-1299 cells treated by matine at concentrations of 0, 10 and 20 μg/ml, and 20 μg/ml after anti-miR-133a transfection, respectively. Columns represented relative protein expression levels of EGFR, MMP-9 and p-AKT/t-AKT in NCI-1299 cells. (#P<0.01, *P<0.005).
Discussion
In this study, we found evidences supporting the mechanisms of matrine’s anti-invasion and anti-metastasis effects were correlated with miR-133a in NCI-H1299 cells. Accompanied by invasion and migration inhibitions in NCI-H1299 cells, the expression of miR-133a was elevated by matrine treatment. Furthermore, the EGFR signaling pathway was targeted and inhibited by increased miR-133a. Notably, the application of anti-miR-133a which was the specific inhibitor of miR-133a, impaired the anti-invasion and anti-metastasis effects of matrine. These results supported our hypothesis that matrine inhibited invasion and metastasis of non-small lung cancer cells by stimulating expression of miR-133a which down-regulated EGFR pathway.
In recent years, monomers extracted from Chinese herbs became research objects in many studies because they exerted various pharmacological activities in many pathological conditions. It was suggested that some of the monomers such as curcumin [19], baicalein [20] and resveratrol [21] inhibited tumor malignancy of many human cancers. Matrine, an alkaloid compound which is extracted from plants Sopora (Leguminosae), is among these anti-cancer drugs [3]. Previous studies suggested matrine had significant inhibition effect on cancer cell proliferation and could induce apoptosis in many different human malignant cell lines [22,23]. Matrine also inhibited the invasion and metastasis of cancer cells via multiple pathways such as cell adhesion and extracellular matrix degradation [24]. However, the exact molecular mechanisms of matrine’s anti-metastasis and anti-invasion effects are still unclear.
MiRNAs were recognized as a novel family of biological regulators in recent years, which could regulate specific gene expression mainly by completely or incompletely targeting and binding to corresponding mRNAs [25]. Various biological processes under physiological and pathological conditions including cell differentiation, apoptosis, fibrosis, metabolism, are highly related to the machinery of miRNAs [26]. A recent study conducted by Liu et al. suggested that matrine changed miRNA expression profiles [2]. Their results suggested that significant changes of 206 out of 730 miRNAs were involved in apoptosis induced by matrine in A549 cells [2]. Thus, if the expression miRNAs involved in invasion and metastasis could be regulated by matrine treatment became the aim of the present study.
MiR-133a whose two copies are located respectively on chromosome 18 and chromosome 20 belongs to the miR-133 family [27]. The miR-133 has long recognized as the muscular specific regulators participating in differentiation and myogenesis in skeletal and cardiac muscles [28]. Nevertheless, results from several recent studies indicated that miR-133 played an important role in human cancer [16]. As a cancer suppressor, the expression level of miR-133a was found low in malignant tumors including liver cancer, prostate cancer, tongue squamous cell carcinoma and bladder cancer [29]. It was reported that miR-133 showed its inhibitory capacity against cancer cell proliferation by targeting and binding directly to an oncogene pyruvate kinase type M2 [30]. Liu et al.’s study found that miR-133a reduced migration and invasion of prostate cancer cells by direct targeting and inhibiting expression EGFR and its signaling pathway [16]. In this study, we found that the treatment of matrine elevated expression level of miR-133a in NCI-H1299 cells in a concentration- dependent manner, suggesting that regulation of miR-133 expression may be correlated with matrine’s anti-cancer activity.
Belongs to the ErbB receptor family, EGFR was described expressing in many kinds of normal cells and cancer cell, playing a part in regulating cell mobility, adhesion, growth, invasion and adhesion [31]. EGFR and its down-stream molecules phosphoinositide-3 kinase (PI3K) and AKT form a signaling pathway [32]. MMP-9 which belongs to MMPs family, are among the effectors of AKT. Over expression of MMP-9 were found in many different kinds of invasive and metastatic cancers because of its role in degradation of the extra cellular matrix (ECM) [33]. Thus, activation of EGFR/AKT/MMP-9 pathway could initiate the invasion and metastasis in malignant tumors. In the present study, our result demonstrated that accompanied by elevation of miR-133a in NCI-H1299 cells, matrine treatment also down-regulated the expression of EGFR pathway to inhibit the invasion and migration. More importantly, matrine failed to down-regulate the expression of EGFR pathway in NCI-H1299 cells treated by anti-miR-133a, which also impaired the inhibitory effects of matrine against migration and invasion.
In summary, the present study is the first study reviling the involvement of miR-133a in matrine’s anti-invasion and anti-metastasis effects in lung cancer. Based on the results in this study, we concluded that matrine inhibited migration and invasion of NCI-H1299 cells via elevating expression of miR-133a to suppress activation of EGFR pathway. However, how matrine treatment regulated miR-133a expression level in lung cancer cells is still unknown. Our further work would concentrate on that mechanism.
Disclosure of conflict of interest
None.
References
- 1.Kim AW, Faber LP, Warren WH, Shah ND, Basu S, Liptay MJ. Bilobectomy for non-small cell lung cancer: a search for clinical factors that may affect perioperative morbidity and long-term survival. J Thorac Cardiovasc Surg. 2010;139:606–611. doi: 10.1016/j.jtcvs.2009.05.044. [DOI] [PubMed] [Google Scholar]
- 2.Liu YQ, Li Y, Qin J, Wang Q, She YL, Luo YL, He JX, Li JY, Xie XD. Matrine reduces proliferation of human lung cancer cells by inducing apoptosis and changing miRNA expression profiles. Asian Pac J Cancer Prev. 2014;15:2169–2177. doi: 10.7314/apjcp.2014.15.5.2169. [DOI] [PubMed] [Google Scholar]
- 3.Tan C, Qian X, Jia R, Wu M, Liang Z. Matrine induction of reactive oxygen species activates p38 leading to caspase-dependent cell apoptosis in non-small cell lung cancer cells. Oncol Rep. 2013;30:2529–2535. doi: 10.3892/or.2013.2727. [DOI] [PubMed] [Google Scholar]
- 4.Ma ZJ, Li Q, Wang JB, Zhao YL, Zhong YW, Bai YF, Wang RL, Li JY, Yang HY, Zeng LN, Pu SB, Liu FF, Xiao DK, Xia XH, Xiao XH. Combining Oxymatrine or Matrine with Lamivudine Increased Its Antireplication Effect against the Hepatitis B Virus In Vitro. Evid Based Complement Alternat Med. 2013;2013:186573. doi: 10.1155/2013/186573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suo Z, Liu Y, Ferreri M, Zhang T, Liu Z, Mu X, Han B. Impact of matrine on inflammation related factors in rat intestinal microvascular endothelial cells. J Ethnopharmacol. 2009;125:404–409. doi: 10.1016/j.jep.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 6.Zhang JP, Zhang M, Zhou JP, Liu FT, Zhou B, Xie WF, Guo C. Antifibrotic effects of matrine on in vitro and in vivo models of liver fibrosis in rats. Acta Pharmacol Sin. 2001;22:183–186. [PubMed] [Google Scholar]
- 7.Gao HY, Li GY, Lou MM, Li XY, Wei XY, Wang JH. Hepatoprotective effect of Matrine salvianolic acid B salt on Carbon Tetrachloride-Induced Hepatic Fibrosis. J Inflamm (Lond) 2012;9:16. doi: 10.1186/1476-9255-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xin HB, Liu SF. [Effects of matrine on myocardial contraction and arrhythmia in isolated heart atria] . Zhongguo Yao Li Xue Bao. 1987;8:501–505. [PubMed] [Google Scholar]
- 9.Liu Y, Xu Y, Ji W, Li X, Sun B, Gao Q, Su C. Anti-tumor activities of matrine and oxymatrine: literature review. Tumour Biol. 2014;35:5111–9. doi: 10.1007/s13277-014-1680-z. [DOI] [PubMed] [Google Scholar]
- 10.Niu H, Zhang Y, Wu B, Zhang Y, Jiang H, He P. Matrine induces the apoptosis of lung cancer cells through downregulation of inhibitor of apoptosis proteins and the Akt signaling pathway. Oncol Rep. 2014;32:1087–1093. doi: 10.3892/or.2014.3273. [DOI] [PubMed] [Google Scholar]
- 11.Sathiakumar N, Delzell E, Morrisey MA, Falkson C, Yong M, Chia V, Blackburn J, Arora T, Kilgore ML. Mortality following bone metastasis and skeletal-related events among patients 65 years and above with lung cancer: a population-based analysis of U. S. Medicare beneficiaries, 1999-2006. Lung India. 2013;30:20–26. doi: 10.4103/0970-2113.106127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baer C, Claus R, Plass C. Genome-wide epigenetic regulation of miRNAs in cancer. Cancer Res. 2013;73:473–477. doi: 10.1158/0008-5472.CAN-12-3731. [DOI] [PubMed] [Google Scholar]
- 13.Shi M, Liu D, Duan H, Shen B, Guo N. Metastasis-related miRNAs, active players in breast cancer invasion, and metastasis. Cancer Metastasis Rev. 2010;29:785–799. doi: 10.1007/s10555-010-9265-9. [DOI] [PubMed] [Google Scholar]
- 14.Wang LK, Hsiao TH, Hong TM, Chen HY, Kao SH, Wang WL, Yu SL, Lin CW, Yang PC. MicroRNA-133a suppresses multiple oncogenic membrane receptors and cell invasion in non-small cell lung carcinoma. PLoS One. 2014;9:e96765. doi: 10.1371/journal.pone.0096765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mataki H, Enokida H, Chiyomaru T, Mizuno K, Matsushita R, Goto Y, Nishikawa R, Higashimoto I, Samukawa T, Nakagawa M, Inoue H, Seki N. Downregulation of the microRNA-1/133a cluster enhances cancer cell migration and invasion in lung-squamous cell carcinoma via regulation of Coronin1C. J Hum Genet. 2014;60:53–61. doi: 10.1038/jhg.2014.111. [DOI] [PubMed] [Google Scholar]
- 16.Tao J, Wu D, Xu B, Qian W, Li P, Lu Q, Yin C, Zhang W. microRNA-133 inhibits cell proliferation, migration and invasion in prostate cancer cells by targeting the epidermal growth factor receptor. Oncol Rep. 2012;27:1967–1975. doi: 10.3892/or.2012.1711. [DOI] [PubMed] [Google Scholar]
- 17.Chen K, Zhang S, Ji Y, Li J, An P, Ren H, Liang R, Yang J, Li Z. Baicalein inhibits the invasion and metastatic capabilities of hepatocellular carcinoma cells via down-regulation of the ERK pathway. PLoS One. 2013;8:e72927. doi: 10.1371/journal.pone.0072927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mataki H, Enokida H, Chiyomaru T, Mizuno K, Matsushita R, Goto Y, Nishikawa R, Higashimoto I, Samukawa T, Nakagawa M, Inoue H, Seki N. Downregulation of the microRNA-1/133a cluster enhances cancer cell migration and invasion in lung-squamous cell carcinoma via regulation of Coronin1C. J Hum Genet. 2015;60:53–61. doi: 10.1038/jhg.2014.111. [DOI] [PubMed] [Google Scholar]
- 19.Yoysungnoen P, Wirachwong P, Changtam C, Suksamrarn A, Patumraj S. Anti-cancer and anti-angiogenic effects of curcumin and tetrahydrocurcumin on implanted hepatocellular carcinoma in nude mice. World J Gastroenterol. 2008;14:2003–2009. doi: 10.3748/wjg.14.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen CH, Huang TS, Wong CH, Hong CL, Tsai YH, Liang CC, Lu FJ, Chang WH. Synergistic anti-cancer effect of baicalein and silymarin on human hepatoma HepG2 Cells. Food Chem Toxicol. 2009;47:638–644. doi: 10.1016/j.fct.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 21.Sheth S, Jajoo S, Kaur T, Mukherjea D, Sheehan K, Rybak LP, Ramkumar V. Resveratrol reduces prostate cancer growth and metastasis by inhibiting the Akt/MicroRNA-21 pathway. PLoS One. 2012;7:e51655. doi: 10.1371/journal.pone.0051655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song S, Zhu S, Zhang Z, Mo Z, Ke Q, Luo Z. A study on the inhibitory effect of matrine on gastric cancer SGC-7901 cells. Afr J Tradit Complement Altern Med. 2013;10:435–438. doi: 10.4314/ajtcam.v10i6.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shao H, Yang B, Hu R, Wang Y. Matrine effectively inhibits the proliferation of breast cancer cells through a mechanism related to the NF-kappaB signaling pathway. Oncol Lett. 2013;6:517–520. doi: 10.3892/ol.2013.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu XY, Fang H, Yang ZG, Wang XY, Ruan LM, Fang DR, Ding YG, Wang YN, Zhang Y, Jiang XL, Chen HC. Matrine inhibits invasiveness and metastasis of human malignant melanoma cell line A375 in vitro. Int J Dermatol. 2008;47:448–456. doi: 10.1111/j.1365-4632.2008.03627.x. [DOI] [PubMed] [Google Scholar]
- 25.Fujiwara T, Yada T. miRNA-target prediction based on transcriptional regulation. BMC Genomics. 2013;14(Suppl 2):S3. doi: 10.1186/1471-2164-14-S2-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alshalalfa M. miRNA regulation in the context of functional protein networks: principles and applications. Wiley Interdiscip Rev Syst Biol Med. 2014;6:189–199. doi: 10.1002/wsbm.1251. [DOI] [PubMed] [Google Scholar]
- 27.Care A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, Bang ML, Segnalini P, Gu Y, Dalton ND, Elia L, Latronico MV, Hoydal M, Autore C, Russo MA, Dorn GW 2nd, Ellingsen O, Ruiz-Lozano P, Peterson KL, Croce CM, Peschle C, Condorelli G. MicroRNA-133 controls cardiac hypertrophy. Nat Med. 2007;13:613–618. doi: 10.1038/nm1582. [DOI] [PubMed] [Google Scholar]
- 28.Dong DL, Chen C, Huo R, Wang N, Li Z, Tu YJ, Hu JT, Chu X, Huang W, Yang BF. Reciprocal repression between microRNA-133 and calcineurin regulates cardiac hypertrophy: a novel mechanism for progressive cardiac hypertrophy. Hypertension. 2010;55:946–952. doi: 10.1161/HYPERTENSIONAHA.109.139519. [DOI] [PubMed] [Google Scholar]
- 29.Ambs S, Prueitt RL, Yi M, Hudson RS, Howe TM, Petrocca F, Wallace TA, Liu CG, Volinia S, Calin GA, Yfantis HG, Stephens RM, Croce CM. Genomic profiling of microRNA and messenger RNA reveals deregulated microRNA expression in prostate cancer. Cancer Res. 2008;68:6162–6170. doi: 10.1158/0008-5472.CAN-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong TS, Liu XB, Chung-Wai Ho A, Po-Wing Yuen A, Wai-Man Ng R, Ignace Wei W. Identification of pyruvate kinase type M2 as potential oncoprotein in squamous cell carcinoma of tongue through microRNA profiling. Int J Cancer. 2008;123:251–257. doi: 10.1002/ijc.23583. [DOI] [PubMed] [Google Scholar]
- 31.Jiang L, Lan T, Chen Y, Sang J, Li Y, Wu M, Tao Y, Wang Y, Qian H, Gu L. PKG II inhibits EGF/EGFR-induced migration of gastric cancer cells. PLoS One. 2013;8:e61674. doi: 10.1371/journal.pone.0061674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lotsch D, Steiner E, Holzmann K, Spiegl-Kreinecker S, Pirker C, Hlavaty J, Petznek H, Hegedus B, Garay T, Mohr T, Sommergruber W, Grusch M, Berger W. Major vault protein supports glioblastoma survival and migration by upregulating the EGFR/PI3K signalling axis. Oncotarget. 2013;4:1904–1918. doi: 10.18632/oncotarget.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng H, Takahashi H, Murai Y, Cui Z, Nomoto K, Niwa H, Tsuneyama K, Takano Y. Expressions of MMP-2, MMP-9 and VEGF are closely linked to growth, invasion, metastasis and angiogenesis of gastric carcinoma. Anticancer Res. 2006;26:3579–3583. [PubMed] [Google Scholar]


