Abstract
Rapamycin is helpful in the treatment of certain cancers by inhibiting mTOR (mammalian target of rapamycin) pathway. Here, rapamycin mediated apoptosis were investigated in human retinoblastoma Y79 cells. The MTT assay showed that the IC50 value of rapamycin against Y79 cells was 0.136 ± 0.032 μmol/L. Flow cytometry analysis indicated that the percentage of apoptotic cells was increased from 2.16 ± 0.41% to 12.24 ± 3.10%, 20.16 ± 4.22%, and 31.32 ± 5.78% after 0.1, 0.2, and 0.4 μmol/L rapamycin or without rapamycin treatment for 48 hours. Flow cytometry analysis showed that rapamycin induced mitochondrial membrane potential (∆Ψm) collapse in Y79 cells in a concentration-dependent manner. Western blot assay showed that rapamycin led to release of cytochrome c from mitochondrial membranes to cytosol. Further Western blot assays showed that rapamycin induced activation of caspase-9 and caspase-8 and the cleavage of caspase-3. Rapamycin induced cleavages of caspase-3 and apoptosis was inhibited by both Z-LETD-FMK and Z-IETD-FMK treatment. Together, all these results illustrated that rapamycin induced apoptosis in human retinoblastoma Y79 cells involvement of both intrinsic and extrinsic pathways.
Keywords: Retinoblastoma, rapamycin, intrinsic apoptosis signaling pathway, extrinsic apoptosis signaling pathway
Introduction
Retinoblastoma (Rb) is the most common intraocular malignancy in children [1]. Chemotherapy has become an integral part of the current management of retinoblastoma [2]. However, present chemotherapy treatments result in noteworthy complications including second malignancies (e.g., acute myeloid leukemia) [3]. Therefore, there is an urgent need to identify new therapeutic strategies to improve the clinical outcome of patients with retinoblastoma [4].
The mammalian target of rapamycin (mTOR) has emerged as a critical effector in cell growth, proliferation, survival, angiogenesis, and autophagy [5]. Rapamycin is a macrolide produced by the bacteria Streptomyces hygroscopicus [6]. Which was originally developed as an antifungal agent [7]. However, this use was abandoned when it was identified as have potent immunosuppressive and antiproliferative properties [8,9]. Now rapamycin is helpful in the treatment of certain cancers by inhibiting mTOR (mammalian target of rapamycin) pathway [10,11]. Over the years, apoptosis has turned out to be the major mechanism to eliminate cancer cells. Which involves both intrinsic or mitochondrial pathways and extrinsic or death receptor pathways [12]. In this study, we investigated rapamycin mediated toxicity and apoptosis in human retinoblastoma Y79 cells.
Materials and methods
Chemicals and reagents
RPMI 1640 media were purchased from Gibco BRL. Fetal bovine serum (FBS) was purchased from Life Technologies Corporation. Annexin V-FITC/propidium iodide (PI) Apoptosis Detection Kit was a product from Beyotime Corporation. Rapamycin, penicillin, streptomycin, MTT, DiOC6, Z-LETD-FMK, Z-IETD-FMK and other chemicals were purchased from Sigma Chemical Co. All antibodies were obtained from Cell Signaling Technology Inc.
Cell lines and cell culture
The human retinoblastoma cell line Y79 was obtained from American Type Culture Collection (ATCC). Y79 cells were incubated in RPMI 1640 media supplemented with 10% FBS, 1% penicillin, and streptomycin. Cells were cultured at 37°C in saturating humidity of 5% CO2 and 95% air.
Cell proliferation assays
The effect of rapamycin on proliferation of Y79 cells was determined by MTT assay. Briefly, cells were seeded in 96-well plates at 3000 cells per well and grown overnight. Then, various concentrations of rapamycin were added to the wells for 72 hours. Cell number was determined later 10 μL MTT (1 mg/ml) was added to each well and incubated at 37°C for an additional four hours. After media were removed, DMSO was added to dissolve purple crystals of formazan. Optical Density (OD) value was read at 490 nm by Thermo Scientific Fluoroskan Ascent FL (Thermo Fisher Scientific Inc.). Finally, the half inhibitory concentration (IC50) was calculated as the relative viability against untreated control cells [13]. All experiments were replicated at least three times.
Cell apoptosis analyses
Y79 cells were harvested at 48 hours after incubation with rapamycin as indicated concentration. Collected cells were stained with Annexin V-FITC and PI in the dark, according to the manufacturer’s protocol. Then, cells were classified by flow cytometry (Beckman Coulter, Inc). Finally, number of apoptotic cells was quantified by CellQuest software. All experiments were replicated at least three times.
Mitochondrial membrane potential (ΔΨm) assays
To observe the changes in mitochondrial membrane potential (ΔΨm), Y79 cells were stained by mitochondrial tracking fluorescent dye DiOC6 and measured by flow cytometry. Briefly, Y79 cells were treated with rapamycin as indicated concentration for 48 hours. Then, cells were harvested and incubated with 40 nmol/L DiOC6 at 37°C in the dark for 20 min. Finally, mean fluorescence intensity (MFI) was obtained by flow cytometry analysis (Beckman Coulter, Inc). All experiments were replicated at least three times.
Western blot analyses
Y79 cells were harvested at 48 hours after incubation with rapamycin as indicated concentration. Cells were lysed and equal amounts of protein lysate was separated on 8-12% SDS-PAGE gel and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore. USA). Membranes were blocked with 5% nonfat milk powder (w/v) for 2 h, and then incubated with primary antibodies at 4°C overnight. Thereafter, HRP-conjugated secondary antibody was incubated for one hour. Finally, the immunoblotted proteins were visualized by Western Blot Detection System (Millipore, USA). GAPDH was used as an endogenous control. All experiments were replicated at least three times.
Western blot analysis of cytosolic cytochrome c
Y79 cells were harvested at 48 hours after incubation with rapamycin as indicated concentration. The pellets were suspended with 5-fold volume cytosol extraction buffers containing DTT and protease inhibitors (Abcam plc. USA). After incubated on ice for 10 minutes, the pellets were homogenized in an ice-cold Dounce tissue grinder. Then, the cells were centrifuged at 700 g for 10 minutes at 4°C. The supernatant was collected into a fresh 1.5 ml tube, and centrifuged at 10,000 g for 30 minutes at 4°C. The final supernatant was used as cytosolic fraction. Finally, cytosolic cytochrome c was identified by Western blot analysis as described before.
Statistical analysis
Data were statistically analyzed by SPSS Statistics 16.0 software. Independent t-test was used between two groups, and the comparisons of multiple groups were performed with a one-way analysis of variance (ANOVA) followed by LSD-t (for equal variances assumed) or Dunnett’s (for equal variances not assumed) test. The significance determined at P < 0.05.
Results
Effects of rapamycin on Y79 cells viability
The sensitivity of human retinoblastoma Y79 cells to rapamycin was determined by performing cell proliferation assay. The MTT assay showed that rapamycin inhibited Y79 cell proliferation in a concentration-dependent manner. The IC50 value was 0.136 ± 0.032 μmol/L. The results indicated that rapamycin was effective in inhibiting the growth of human retinoblastoma Y79 cells.
Rapamycin induced apoptosis in Y79 cells
To determine the apoptotic effect of rapamycin on human retinoblastoma Y79 cells, we treated cells with 0.1, 0.2, and 0.4 μmol/L rapamycin or without rapamycin for 48 hours. Apoptosis was assessed by Annexin V/PI double staining, and apoptotic cell populations were quantified by flow cytometry as showed in Figure 1A. The results showed that the percentage of apoptotic cells was increased from 2.16 ± 0.41% to 12.24 ± 3.10%, 20.16 ± 4.22%, and 31.32 ± 5.78% (Figure 1B). The results showed that rapamycin induced apoptosis in human retinoblastoma Y79 cells in a dose-dependent manner.
Figure 1.
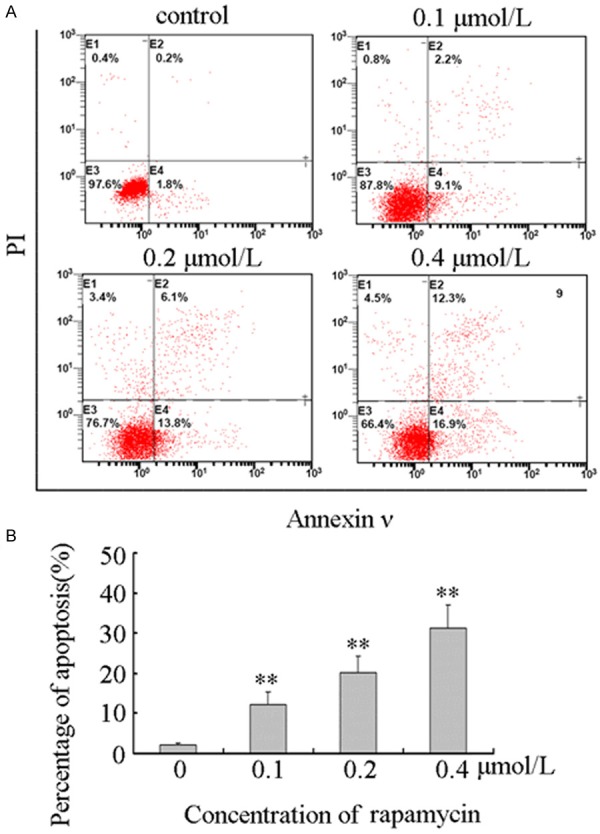
Rapamycin induced apoptosis in Y79 cells. A. Rapamycin induced apoptosis in Y79 cells. Y79 cells were plated in six-well culture plates and treated for 48 hours with vehicle control or 0.1, 0.2, and 0.4 μmol/L rapamycin, respectively. Cells were harvested and stained with Annexin V-FITC and PI as described in “Materials and methods”. Stained apoptotic cells were classified by flow cytometry. All these experiments were replicated at least thrice, and a representative example of apoptotic histograms was shown. B. The number of apoptotic cells was quantified by CellQuest software. The percentage of apoptotic cells was increased from 2.16 ± 0.41% to 12.24 ± 3.10%, 20.16 ± 4.22%, and 31.32 ± 5.78%. Columns, means of triplicate determinations; bars, SDs; **, P < 0.01 as compared with respective controls.
Effects of rapamycin on mitochondrial membrane potential (ΔΨm) in human retinoblastoma Y79 cells
DiOC6 is a lipophilic cationic dye that specifically accumulates into mitochondrial matrix depending on ∆Ψm [14]. In apoptotic cells, the Δψm collapses. So the mean fluorescence intensity (MFI) of DiOC6 was reduced by flow cytometry detection [15]. To observe the effects of rapamycin on ΔΨm, Y79 cells were treated with 0.1, 0.2, and 0.4 μmol/L rapamycin or without rapamycin for 48 hours. As showed in Figure 2, the MFI of DiOC6 was decreased from 8.24 ± 2.13 to 6.51 ± 1.42, 4.36 ± 1.05, and 3.18 ± 0.73. The results indicated that rapamycin can lead to dissipation of ΔΨm in human retinoblastoma Y79 cells.
Figure 2.

Rapamycin induced mitochondrial membrane potential (∆Ψm) collapse in Y79 cells. Rapamycin induced ∆Ψm collapse in Y79 cells. Y79 cells were plated in six-well culture plates and treated for 48 hours with vehicle control or 0.1, 0.2, and 0.4 μmol/L rapamycin, respectively. Cells were harvested and stained with DiOC6 as described in “Materials and methods”. The mean fluorescence intensity (MFI) of DiOC6 was obtained by flow cytometry analysis. The MFI was decreased from 8.24 ± 2.13 to 6.51 ± 1.42, 4.36 ± 1.05, and 3.18 ± 0.73. Data were shown as means ± SD of triplicate determinations. Columns, means of triplicate determinations; bars, SDs; *, P < 0.05, **, P < 0.01 as compared with respective controls.
Rapamycin induced apoptosis through intrinsic pathways in human retinoblastoma Y79 cells
ΔΨm is critical for proper cellular functions. Disruption of ΔΨm might alter the membrane dynamics of mitochondria leading to release of cytochrome c, formation of apoptosome complex, and activation of caspase-9. Which initiated a cascade of caspase activation leading to apoptosis [16]. To observe the release of cytochrome c and activation of caspase-9, Y79 cells were treated with 0.1, 0.2, and 0.4 μmol/L rapamycin or without rapamycin for 48 hours. Cytosolic cytochrome c was identified by Western blot analysis. As showed in Figure 3A, cytosolic cytochrome c was increased after rapamycin treatment. Released cytochrome C triggered caspase-9 and caspase-3 activation (Figure 3B).
Figure 3.
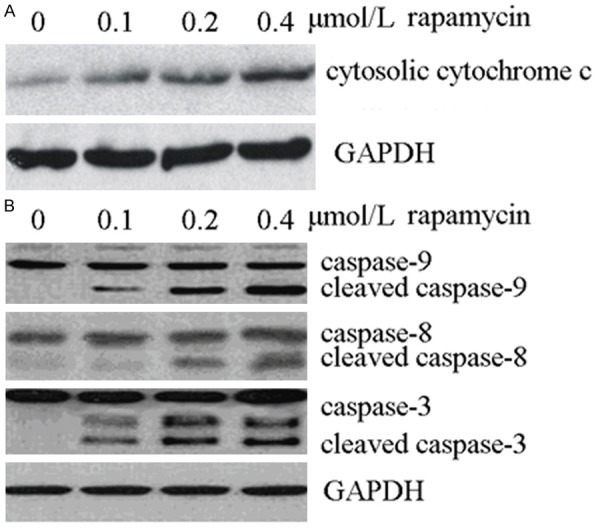
Rapamycin induced apoptosis through intrinsic and extrinsic signaling pathways in Y79 cells. A. Rapamycin induced release of cytochrome c from mitochondrial membrane to cytosol. Y79 cells were plated in six-well culture plates and treated for 48 hours with vehicle control or 0.1, 0.2, and 0.4 μmol/L rapamycin, respectively. Cells were harvested and cytosolic protein was extracted as described in “Materials and methods”. Cytosolic cytochrome c was identified by Western blot analysis. GAPDH protein levels were used as a cytosolic control. All these experiments were replicated at least thrice, and a representative experiment was shown in each panel. B. Rapamycin induced activation of caspase-9 and caspase-8 and the cleavage of caspase-3. Y79 cells were plated in six-well culture plates and treated for 48 hours with vehicle control or 0.1, 0.2, and 0.4 μmol/L rapamycin, respectively. Cells were harvested and total protein was extracted as described in “Materials and methods”. The whole-cell lysate was assayed by Western blot and corresponding antibodies. GAPDH protein levels were used as a loading control. All these experiments were replicated at least thrice, and a representative experiment was presented in each panel.
Rapamycin induced apoptosis via the extrinsic signaling pathways in human retinoblastoma Y79 cells
In order to investigate whether rapamycin would induce apoptosis through extrinsic pathways, we revealed the activation of caspase-8. As showed in Figure 3B, cleaved caspase-8 was detected after rapamycin treatment. These results suggested that extrinsic pathways involved in rapamycin mediated apoptosis in human retinoblastoma Y79 cells.
Rapamycin induced apoptosis through both intrinsic and extrinsic signaling pathways in human retinoblastoma Y79 cells
The cross-talk was found between intrinsic and extrinsic apoptosis signaling pathways [17]. In order to investigate which apoptotic signaling pathways rapamycin involved, Z-LETD-FMK (a selective caspase-9 inhibitor) and Z-IETD-FMK (a selective caspase-8 inhibitor) were used to block the intrinsic or extrinsic apoptosis signaling pathway. As showed in Figure 4A, Z-LETD-FMK only inhibited rapamycin-mediated activation of caspase-9, and Z-IETD-FMK only inhibited rapamycin-mediated activation of caspase-8. When both Z-LETD-FMK and Z-IETD-FMK were used, rapamycin-mediated activation of caspase-9 and caspase-8 was inhibited, and followed inhibition of caspase-3 cleavage. The apoptotic analysis showed that Z-LETD-FMK or Z-IETD-FMK partially inhibited rapamycin-mediated apoptosis (Figure 4B). Rapamycin-mediated apoptosis was inhibited by both Z-LETD-FMK and Z-IETD-FMK treatment (Figure 4B).
Figure 4.
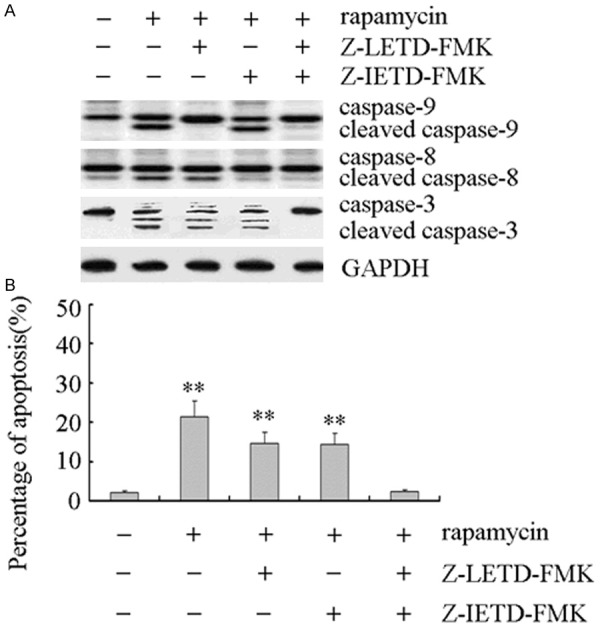
Rapamycin induced apoptosis involvement of both intrinsic and extrinsic signaling in Y79 cells. A. The cleavage of caspase-3 was inhibited by both Z-LETD-FMK and Z-IETD-FMK treatment. Y79 cells were plated in six-well culture plates. After preincubation with 20 μmol/L Z-IETDFMK or Z-LETD-FMK for 12 h, Y79 cells were treated with 0.2 μmol/L rapamycin for 48 h, respectively. Cells were harvested and total protein was extracted as described in “Materials and methods”. The whole-cell lysates were assayed by Western blot and corresponding antibodies. GAPDH protein levels were used as a loading control. All these experiments were replicated at least thrice, and a representative experiment was presented in each panel. B. Rapamycin induced apoptosis was inhibited by both Z-LETD-FMK and Z-IETD-FMK treatment. Y79 cells were plated in six-well culture plates. After pre-incubation with 20 μmol/L Z-IETDFMK or Z-LETD-FMK for 12 h, Y79 cells were treated with 0.2 μmol/L rapamycin for 48 h, respectively. Cells were harvested and stained with Annexin V-FITC and PI as described in “Materials and methods”. Stained apoptotic cells were classified by flow cytometry. The number of apoptotic cells was quantified by CellQuest software. Columns, means of triplicate determinations; bars, SDs; **, P < 0.01 as compared with respective controls.
Discussion
Retinoblastoma is the most common primary intracellular malignancy in childhood with an incidence of 1/15,000 to 1/20,000 births [18]. Untreated retinoblastoma is always fatal and the patients die of intracranial extension and disseminated disease within two years [19]. Primary management of retinoblastoma consists of chemoreduction with local consolidation, although newer techniques include local delivery via intra-arterial chemotherapy, periocular, or intravitreal injection [20,21]. In developing countries, treatment is limited. Long-term survival rates are weak and current chemotherapy causes significant morbidity to pediatric patients and significantly limits dosing [3]. Now, the novel targeted therapeutic strategies are intended to effectively control cancer [22].
Serine-threonine kinase, mTOR, plays a key role in cell growth and angiogenesis and may be dysregulated in tumors [23,24]. For these reasons, mTOR has recently promoted to the rank as a potential target for anti-cancer therapy [25,26]. Rapamycin was recognized as an inhibitor of mTOR pathway [27]. In this study, we found that rapamycin induced apoptosis in human retinoblastoma Y79 cell (Figure 1). Moreover, our further investigation elucidated that both intrinsic and extrinsic apoptosis signaling pathway involved in its action mechanisms.
Apoptosis can be triggered by internal and external signals. The loss of mitochondrial membrane potential (ΔΨm) has been suggested to causing the cytochrome C release [28]. The released cytochrome c was essential to activate caspase-9. Which results in internal damage to cells [29]. Death activator transmitted a signal to the cytoplasm that leads to activation of caspase-8. Which initiated extrinsic pathway leading to phagocytosis of the cells [30]. In this study, we observed cleaved caspase-8 and caspase-9, followed a cascade of caspase activation (Figure 3). It was increasingly believed that crosstalk existed between intrinsic and extrinsic apoptosis signaling pathway [31]. For example, the cleavage of Bid caused by caspase-8 can lead to the release of cytochrome c from mitochondria, which subsequently activated caspase-9 mediated intrinsic apoptosis pathway [32]. Next, we investigated the involved pathways. The result showed that Z-LETD-FMK or Z-IETD-FMK only inhibited rapamycin-mediated caspase-9 or caspase-8 activation and partial apoptosis (Figure 4). However, combined Z-LETD-FMK and Z-IETD-FMK can inhibit rapamycin-mediated cascade of caspase activation and apoptosis (Figure 4). The results suggested that rapamycin can trigger both intrinsic and extrinsic apoptosis pathways in human retinoblastoma Y79 cell.
mTOR assumes a key regulatory role in cell growth and homeostasis. Inhibition of mTOR now uses as a novel treatment strategy for several malignancies, either alone or in combination with strategies [33]. In this study, we concisely evaluate the best ability of rapamycin (an mTOR inhibitor) to induce apoptosis in human retinoblastoma Y79 cells. Our studies showed that rapamycin disturbed mitochondrial membrane potential and subsequently helped releasing cytochrome c from mitochondria to cytosol and activated caspase-8. In addition, we observed that selective caspase-9 or caspase-8 inhibitor cannot inhibit rapamycin-mediated activation of caspases-3 and block apoptosis. Combining all, results suggested that rapamycin induced apoptosis in human retinoblastoma Y79 cells involvement of both intrinsic and extrinsic pathways.
Acknowledgements
This work was supported by grants from Guangdong Province special science and technology (new drug discovery) project (2012A080201002), Guangdong Province Foshan City industry-university-research special project of Gaoming District in 2012 (201211).
Disclosure of conflict of interest
None.
References
- 1.Benavente CA, Dyer MA. Genetics and epigenetics of human retinoblastoma. Annu Rev Pathol. 2015;10:547–562. doi: 10.1146/annurev-pathol-012414-040259. [DOI] [PubMed] [Google Scholar]
- 2.Kim JW, Abramson DH, Dunkel IJ. Current management strategies for intraocular retinoblastoma. Drugs. 2007;67:2173–2185. doi: 10.2165/00003495-200767150-00005. [DOI] [PubMed] [Google Scholar]
- 3.Zheng Q, Zhang Y, Ren Y, Wu Y, Yang S, Zhang Y, Chen H, Li W, Zhu Y. Antiproliferative and apoptotic effects of indomethacin on human retinoblastoma cell line Y79 and the involvement of beta-catenin, nuclear factor-kappaB and Akt signaling pathways. Ophthalmic Res. 2014;51:109–115. doi: 10.1159/000355844. [DOI] [PubMed] [Google Scholar]
- 4.Suesskind D, Schrader M, Foerster MH, Ernemann U, Aisenbrey S. Cataract formation: a possible complication of intra-arterial chemotherapy for retinoblastoma. Eur J Ophthalmol. 2013;24:449–453. doi: 10.5301/ejo.5000393. [DOI] [PubMed] [Google Scholar]
- 5.Hugle M, Fulda S. Dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor nvp-BEZ235 synergizes with chloroquine to induce apoptosis in embryonal rhabdomyosarcoma. Cancer Lett. 2015;360:1–9. doi: 10.1016/j.canlet.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 6.Vezina C, Kudelski A, Sehgal SN. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot (Tokyo) 1975;28:721–726. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]
- 7.Singh K, Sun S, Vezina C. Rapamycin (AY-22,989), a new antifungal antibiotic. IV. Mechanism of action. J Antibiot (Tokyo) 1979;32:630–645. doi: 10.7164/antibiotics.32.630. [DOI] [PubMed] [Google Scholar]
- 8.Chen S, Liu D, Wu J, Xu B, Lu K, Zhu W, Chen M. Effect of inhibiting the signal of Mammalian target of rapamycin on memory T cells. Transplant Proc. 2014;46:1642–1648. doi: 10.1016/j.transproceed.2013.10.063. [DOI] [PubMed] [Google Scholar]
- 9.Milani BY, Milani FY, Park DW, Namavari A, Shah J, Amirjamshidi H, Ying H, Djalilian AR. Rapamycin inhibits the production of myofibroblasts and reduces corneal scarring after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 2013;54:7424–7430. doi: 10.1167/iovs.13-12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibbons JJ, Abraham RT, Yu K. Mammalian target of rapamycin: discovery of rapamycin reveals a signaling pathway important for normal and cancer cell growth. Semin Oncol. 2009;36(Suppl 3):S3–S17. doi: 10.1053/j.seminoncol.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Ocana A, Vera-Badillo F, Al-Mubarak M, Templeton AJ, Corrales-Sanchez V, Diez-Gonzalez L, Cuenca-Lopez MD, Seruga B, Pandiella A, Amir E. Activation of the PI3K/mTOR/AKT pathway and survival in solid tumors: systematic review and meta-analysis. PLoS One. 2014;9:e95219. doi: 10.1371/journal.pone.0095219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moirangthem DS, Laishram S, Rana VS, Borah JC, Talukdar NC. Essential oil of Cephalotaxus griffithii needle inhibits proliferation and migration of human cervical cancer cells: involvement of mitochondria-initiated and death receptor-mediated apoptosis pathways. Nat Prod Res. 2015;29:1161–5. doi: 10.1080/14786419.2014.981540. [DOI] [PubMed] [Google Scholar]
- 13.Tao LY, Li JY, Zhang JY. Brazilein, a compound isolated from Caesalpinia sappan Linn. , induced growth inhibition in breast cancer cells via involvement of GSK-3beta/beta-Catenin/cyclin D1 pathway. Chem Biol Interact. 2013;206:1–5. doi: 10.1016/j.cbi.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 14.Guo Y, Zhang W, Yan YY, Ma CG, Wang X, Wang C, Zhao JL. Triterpenoid pristimerin induced HepG2 cells apoptosis through ROS-mediated mitochondrial dysfunction. J Buon. 2013;18:477–485. [PubMed] [Google Scholar]
- 15.Shao Q, Zhao X, Yao L. Matrine inhibits the growth of retinoblastoma cells (SO-Rb50) by decreasing proliferation and inducing apoptosis in a mitochondrial pathway. Mol Biol Rep. 2014;41:3475–3480. doi: 10.1007/s11033-014-3209-3. [DOI] [PubMed] [Google Scholar]
- 16.Dong XZ, Xie TT, Zhou XJ, Mu LH, Zheng XL, Guo DH, Liu P, Ge XY. AG4, a compound isolated from Radix Ardisiae Gigantifoliae, induces apoptosis in human nasopharyngeal cancer CNE cells through intrinsic and extrinsic apoptosis pathways. Anticancer Drugs. 2015;26:331–342. doi: 10.1097/CAD.0000000000000193. [DOI] [PubMed] [Google Scholar]
- 17.Lombard C, Nagarkatti M, Nagarkatti PS. Targeting cannabinoid receptors to treat leukemia: role of cross-talk between extrinsic and intrinsic pathways in Delta9-tetrahydrocannabinol (THC)-induced apoptosis of Jurkat cells. Leuk Res. 2005;29:915–922. doi: 10.1016/j.leukres.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 18.Villegas VM, Hess DJ, Wildner A, Gold AS, Murray TG. Retinoblastoma. Curr Opin Ophthalmol. 2013;24:581–588. doi: 10.1097/ICU.0000000000000002. [DOI] [PubMed] [Google Scholar]
- 19.Chintagumpala M, Chevez-Barrios P, Paysse EA, Plon SE, Hurwitz R. Retinoblastoma: review of current management. Oncologist. 2007;12:1237–1246. doi: 10.1634/theoncologist.12-10-1237. [DOI] [PubMed] [Google Scholar]
- 20.Houston SK, Lampidis TJ, Murray TG. Models and discovery strategies for new therapies of retinoblastoma. Expert Opin Drug Discov. 2013;8:383–394. doi: 10.1517/17460441.2013.772975. [DOI] [PubMed] [Google Scholar]
- 21.Okimoto S, Nomura K. Clinical Manifestations and Treatment of Retinoblastoma in Kobe Children’s Hospital for 16 Years. J Pediatr Ophthalmol Strabismus. 2014;51:222–229. doi: 10.3928/01913913-20140513-01. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Wang X, Li Z, Liu H, Teng Y. MicroRNA-183 suppresses retinoblastoma cell growth, invasion and migration by targeting LRP6. FEBS J. 2014;281:1355–1365. doi: 10.1111/febs.12659. [DOI] [PubMed] [Google Scholar]
- 23.Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, Schreiber SL. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369:756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- 24.Wander SA, Hennessy BT, Slingerland JM. Next-generation mTOR inhibitors in clinical oncology: how pathway complexity informs therapeutic strategy. J Clin Invest. 2011;121:1231–1241. doi: 10.1172/JCI44145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willems L, Tamburini J, Chapuis N, Lacombe C, Mayeux P, Bouscary D. PI3K and mTOR signaling pathways in cancer: new data on targeted therapies. Curr Oncol Rep. 2012;14:129–138. doi: 10.1007/s11912-012-0227-y. [DOI] [PubMed] [Google Scholar]
- 27.Ng VC, Johnson JJ, Cuellar S. Targeting the mammalian target of rapamycin pathway with everolimus: Implications for the management of metastatic breast cancer. J Oncol Pharm Pract. 2014 doi: 10.1177/1078155214540732. pii: 1078155214540732. [DOI] [PubMed] [Google Scholar]
- 28.Gupta S, Kass GE, Szegezdi E, Joseph B. The mitochondrial death pathway: a promising therapeutic target in diseases. J Cell Mol Med. 2009;13:1004–1033. doi: 10.1111/j.1582-4934.2009.00697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valero T. Mitochondrial biogenesis: pharmacological approaches. Curr Pharm Des. 2014;20:5507–5509. doi: 10.2174/138161282035140911142118. [DOI] [PubMed] [Google Scholar]
- 30.Ashkenazi A. Targeting the extrinsic apoptotic pathway in cancer: lessons learned and future directions. J Clin Invest. 2015;125:487–489. doi: 10.1172/JCI80420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarkar A, Das J, Manna P, Sil PC. Nano-copper induces oxidative stress and apoptosis in kidney via both extrinsic and intrinsic pathways. Toxicology. 2011;290:208–217. doi: 10.1016/j.tox.2011.09.086. [DOI] [PubMed] [Google Scholar]
- 32.Yan Y, Su X, Liang Y, Zhang J, Shi C, Lu Y, Gu L, Fu L. Emodin azide methyl anthraquinone derivative triggers mitochondrial-dependent cell apoptosis involving in caspase-8-mediated Bid cleavage. Mol Cancer Ther. 2008;7:1688–1697. doi: 10.1158/1535-7163.MCT-07-2362. [DOI] [PubMed] [Google Scholar]
- 33.Zhao L, Teng B, Wen L, Feng Q, Wang H, Li N, Wang Y, Liang Z. mTOR inhibitor AZD8055 inhibits proliferation and induces apoptosis in laryngeal carcinoma. Int J Clin Exp Med. 2014;7:337–347. [PMC free article] [PubMed] [Google Scholar]


