Abstract
Matrix metalloproteinases (MMPs) are a family of zinc-dependent endopeptidases responsible for degrading essentially all components of the extracellular matrix (ECM). Accumulating evidence suggests that MMPs might play a critical role in growth, invasion, and metastasis of malignant tumors. A single nucleotide polymorphism (SNP) in the promoter region of MMP-12, MMP-12 82 A/G (rs2276109), has been recognized to play a critical role in regulating the expression of MMP-12, however, its correlation with tumor susceptibility remains controversial. To address this issue, we performed meta-analysis to investigate the association MMP-12 82 A/G polymorphism and susceptibility of nine malignant tumors from 11 studies, including 6153 cancer patients and 6838 controls. Two reviewers independently screened studies for eligibility and extracted data for included studies. While overall no evident association between MMP-12 82 A/G and tumor susceptibility was observed, subgroup analysis revealed a specific role of G allele in increasing the susceptibility for epithelial ovarian carcinoma (EOC) using the allele model (fixed effects OR = 2.45, 95% CI = 1.46-4.10, P = 0.001) and the dominant model (fixed effects OR = 2.52, 95% CI = 1.49-4.24, P = 0.001). We thus suggest that G allele of MMP-12 82 A/G polymorphism is a genetic risk factor for EOC.
Keywords: MMP-12, single nucleotide polymorphism, tumor susceptibility
Introduction
MMPs are a pivotal family of zinc enzymes that are playing critical roles in degradation of ECM components during normal development and disease processes. Based on their structure and substrate specificity, MMPs can be divided into five groups: collagenases, gelatinases, stromelysins, matrilysins and membrane type MMPs (MT-MMPs) [1]. Accumulating evidence MMPs are common characteristics of carcinoma progression and are associated with tumor growth, invasion, and metastasis through regulating remodeling of ECM and modulating tumor microenvironment [2-6].
Matrix Metallopeptidase 12 (MMP-12), also known as macrophage elastase, belongs to the stromelysin-like MMPs subgroup and is predominantly expressed in macrophages. The function of MMP-12 involves elastin degradation and macrophage migration as well as angiostatin production, which could prevent tumour angiogenesis. Previous studies showed that a functional SNP in MMP-12 gene are playing a critical role in regulating MMP-12 expression by affecting the binding site of the transcription factor activator protein 1 (AP1). A allele of MMP-12 82 A/G has a greater affinity for the transcription factor and results in higher MMP-12 promoter activity [7]. Recently, several studies reported that reduced that abnormal expression of MMP-12 are associated with both reduced tumor growth and increased overall survival [8,9]. It has also been shown that G allele of MMP-12 82 A/G increases the risk of bladder cancer [10], epithelial ovarian carcinoma [11] and colorectal cancer [12] studies. In contrast, no significant association was observed between MMP-12 82 A/G polymorphism and non-small cell lung cancer or breast cancer [13]. Accordingly, the association between MMP-12 82 A/G polymorphism and cancer risk remains controversial. To address this issue, we conducted a comprehensive meta-analysis based on 11 studies containing 6153 cancer patients and 6838 control subjects to evaluate the association between MMP-12 82 A/G genetic polymorphism and susceptibility of various malignant tumors, including breast cancer (BRC), bladder cancer (BC), colorectal cancer (CRC), lung cancer (LC), epithelial ovarian carcinoma (EOC), esophageal squamous cell carcinoma (ESCC), esophageal adenocarcinoma (EA), gastric cardia adenocarcinoma (GCA), hepatocellular carcinoma (HCC).
Materials and methods
Search strategy
We searched for all articles that had been published on the association between MMP-12 polymorphisms and cancer risk in Pubmed, Embase, the Cochrane Library, CBM, and CNKI Electronic databases, by using the following keywords: ‘MMP-12’ or ‘metalloproteinase-12’, ‘polymorphism’ and ‘cancer’ (before Nov 20, 2014). The reference lists of all relevant articles were also searched to identify additional studies. Database searching and data extraction were performed by two independent reviewers.
Selection criteria
All eligible studies should meet the following criteria: (1) independent case-control studies that evaluated about MMP-12-82 A-G polymorphism and cancer risk; (2) all patients diagnosed with cancers were confirmed; (3) containing information about available genotype frequency; (4) study published in English; (5) P value on Hardy-Weinberg equilibrium (HWE) of controls must be more than 0.05; (6) only full-text manuscripts were included. Major exclusion criteria included: (1) no control population; (2) duplication of previous publications; (3) no available genotype frequency. The data sources are summarized in Table 1.
Table 1.
Main characteristics and methodological quality of all eligible studies
| First author | Year | Country | Ethnicity | Source | Number | Gender (M/F) | Age (years) | Disease | Sample | Method | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||
| Case | Control | Case | Control | Case | Control | ||||||||
| Aesun Shin [19] | 2005 | China | Asians | PB | 1129 | 1229 | 0/1129 | 0/1229 | 47.6±8.0 | 47.2±8.7 | BRC | blood | Sequencing |
| A.K Kader [20] | 2006 | US | Caucasians | PB | 560 | 560 | 433/127 | 431/129 | 65 (21-88) | 64 (24-89) | BC | blood | TaqMan |
| Li Su [21] | 2006 | US | Caucasians | HB | 2014 | 1323 | 1041/973 | 584/739 | 67 (26-91) | 59 (19-96) | LC | blood | TaqMan |
| MJ Woo [22] | 2007 | Korea | Asians | HB | 185 | 304 | 95/90 | NR | 54 (23-81) | NR | CRC | blood | PCR-RFLP |
| Yun Zhai [23] | 2007 | China | Asians | HB | 433 | 480 | NR | NR | NR | NR | HCC | blood | Sequencing |
| Bradbury [24] | 2009 | US | Mixed | PB | 313 | 455 | 279/34 | 396/59 | 64 (21-91) | 64 (19-96) | EA | blood | TaqMan |
| Yan Li [11] | 2009 | China | Asians | HB | 256 | 329 | 0/256 | 0/329 | 53 (20-79) | 51 (20-75) | EOC | blood | PCR-RFLP |
| Yan Li [26] | 2010 | China | Asians | HB | 335 | 624 | 225/110 | 400/224 | 60.1±9.3 | 60.4±8.4 | ESCC | blood | PCR-RFLP |
| Yan Li [26] | 2010 | China | Asians | HB | 257 | 624 | 168/89 | 400/224 | 60.5±8.3 | 60.4±8.4 | GCA | blood | PCR-RFLP |
| Jia J [25] | 2010 | China | Asians | HB | 300 | 300 | 0/300 | 0/300 | 53 (20-79) | 52 (20-76) | EOC | blood | PCR-RFLP |
Note: HB, hospital-based; PB, population-based; NR, not reported; RFLP, restriction fragment length polymorphism. PCR-RFLP polymerase chain reaction-restriction fragment length polymorphism. BRC, breast cancer; BC, bladder cancer; LC, lung cancer; CRC, colorectal cancer; HCC, hepatocellular carcinoma; EA, esophageal adenocarcinoma; EOC, epithelial ovarian carcinorma; ESCC, esophageal squamous cell carcinoma; GCA, gastric cardia adenocarcinoma.
Data extraction
From each eligible article, two investigators extracted information according to the selection criteria independently and arrived at a final agreement on all the items through discussion and reexamination. Data were collected on the first author’s name, year of publication, country of origin, ethnicity, source of control, genotyping methods, cancer type, sample size in cases and controls and so on.
Statistical analysis
Odds ratios (ORs) corresponding to 95% confidence interval (CI) were applied to measure the strength of the association based on the genotype frequencies in cases and controls. We examined the association between MMP-12-82-A/G polymorphism and cancer risk using five genetic contrasts: allelic contrast (G-allele vs A-allele), homozygote comparison (GG vs AA), heterozygote comparison (A/G vs AA), dominant genetic model (GG+A/G vs AA) and recessive genetic model (GG vs A/G+AA). Different variables were adjusted for different studies, and thereby, only crude ORs were pooled in the meta-analysis. 95% CI was calculated for the summary OR using the Z test. A fixed or random effect model was applied in this meta-analysis. The heterogeneity across the enrolled studies was evaluated by the Cochran’s Q-statistic (P < 0.05 was considered as statistically significant) and I2 test (ranges from 0 to 100%). The random effect model was used when a significant Q test with P < 0.05 or I2 > 50%, which indicates heterogeneity among studies. When there was no statistical heterogeneity, we used a fixed effects model [14-16]. We plotted Begg’s funnel plots and used Egger’s weighted regression method to examine the underlying publication bias, and calculated P for bias [17,18]. For sensitivity analysis, relatively smaller studies were excluded and the summary ORs (95% CIs) were recalculated. All statistical analysis were done with Review Manager 5.0 version and STATA software (version 12.0; Stata Corporation, College Station, TX, USA), using two-sided P values (P ≤ 0.05 was considered significant).
Results
Baseline characteristics of included studies
A total of 101 abstracts that met the inclusion criteria were retrieved by two independent reviewers. After reading the full articles, a total of 11 eligible studies that described the association between the MMP-12 polymorphism and cancer were included in this study, which included 6153 cases and 6838 controls (Figure 1). Baseline characteristics of the studies included in our analysis are shown in Table 1. Studies included in this meta-analysis involve breast cancer (BRC) [19], bladder cancer (BC) [20], lung cancer (LC) [21], colorectal cancer (CRC) [12,22], hepatocellular carcinoma (HCC) [23], esophageal adenocarcinoma (EA) [24], epithelial ovarian carcinoma (EOC) [11,25], esophageal squamous cell carcinoma (ESCC) [26] and gastric cardia adenocarcinoma (GCA) [26]. A total of seven studies were performed in Asians, three studies were in Caucasians descendants, and one was categorized as mixed population. Blood samples were used to determine genetic polymorphisms in all the included studies by various genotyping methods including PCR-RFLP, TaqMan Assay and direct DNA sequencing. No genotype distribution in the control groups deviating from Hardy-Weinberg equilibrium (HWE) was observed in any of the studies included (Table 2), supporting the reliability of our analysis.
Figure 1.
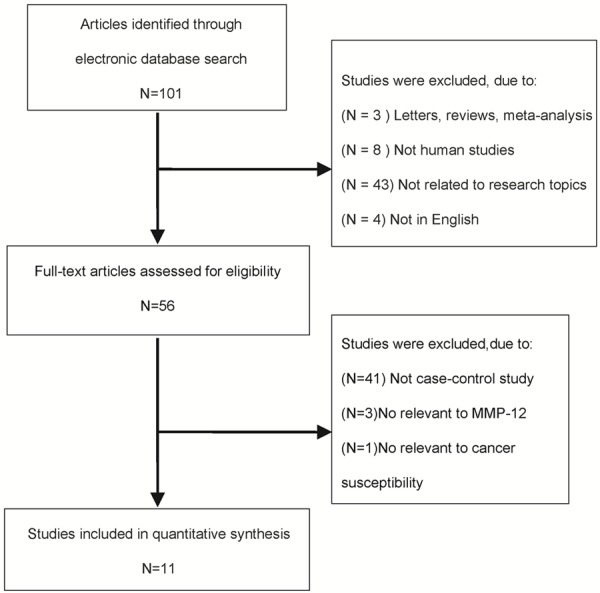
Flow chart explaining the selection of eligible studies included in this study.
Table 2.
The genotype distribution of studies included in this study
| First author | Year | Case | Control | HWE | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| AA | AG | GG | AA | AG | GG | |||
| Aesun Shin [19] | 2005 | 1063 | 51 | 4 | 1153 | 69 | 1 | 0.975 |
| A.K Kader [20] | 2006 | 438 | 110 | 9 | 420 | 133 | 4 | 0.059 |
| Li Su [21] | 2006 | 1535 | 449 | 30 | 1008 | 289 | 26 | 0.324 |
| MJ Woo [22] | 2007 | 180 | 5 | 0 | 295 | 9 | 0 | 0.793 |
| Yun Zhai [23] | 2007 | 404 | 29 | 0 | 449 | 30 | 1 | 0.508 |
| Bradbury [24] | 2009 | 241 | 69 | 3 | 373 | 77 | 5 | 0.648 |
| Yan Li [11] | 2009 | 238 | 18 | 0 | 318 | 11 | 0 | 0.758 |
| Yan Li [26] | 2010 | 322 | 13 | 0 | 588 | 36 | 0 | 0.458 |
| Yan Li [26] | 2010 | 241 | 16 | 0 | 588 | 36 | 0 | 0.458 |
| Jia J [25] | 2010 | 271 | 29 | 0 | 289 | 11 | 0 | 0.746 |
Overall effect of MMP-12 82 A/G polymorphism on cancer risk
We first evaluated the association between MMP-12 82 A/G polymorphism and cancer risk when all the eligible studies were pooled in the overall population. As shown in Table 3 and Figure 2A-E, however, no overall effect was observed when the allele model (G allele vs A allele, fixed-effects OR = 1.00, 95% CI = 0.91-1.10, P-heterogeneity = 0.067 , P = 0.994, I2 = 42.3%) or the dominant model (GG+AG vs AA, random-effects OR = 1.04, 95% CI = 0.87-1.24, P-heterogeneity = 0.028, P = 0.699, I2 = 50.4%) were used. As there was no G/G genotype in some studies, only six case-control studies were included to analysis the other genetic model. No association was found using the recessive model (GG vs AG+AA, fixed-effects OR = 1.03, 95% CI = 0.70-1.52, P-heterogeneity = 0.373, P = 0.877, I2 = 6.8%), the homozygote model (GG vs AA, fixed-effects OR = 1.02, 95% CI = 0.69-1.50, P-heterogeneity = 0.424, P = 0.916, I2 = 0.0%), or the heterozygote model (GG vs AG, fixed-effects OR = 1.06, 95% CI = 0.72-1.58, P-heterogeneity = 0.195, P = 0.754, I2 = 32.0%).
Table 3.
Meta-analysis of the association between MMP-12 82A/G polymorphism and cancer risk
| Subgroup analysis | G allele vs A (Allele model) | GG+A/G vs AA (Dominant model) | GG vs A/G+AA (Recessive model) | GG vs AA (Homozygous model) | GG vs A/G (Heterozygous model) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||||
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| Number of studies | 11 | 11 | 6 | 6 | 6 | ||||||||||
| A>G | 1 | 0.91-1.10 | 0.99 | 1.04 | 0.87-1.24 | 0.69 | 1.03 | 0.70-1.52 | 0.87 | 1.02 | 0.69-1.50 | 0.91 | 1.06 | 0.72-1.58 | 0.75 |
| Ethnicity | |||||||||||||||
| Caucasians | 0.94 | 0.84-1.06 | 0.32 | 0.93 | 0.82-1.06 | 0.28 | 0.99 | 0.66-1.51 | 0.98 | 0.98 | 0.64-1.48 | 0.91 | 1.05 | 0.68-1.60 | 0.83 |
| Asians | 1.09 | 0.89-1.35 | 0.41 | 1.16 | 0.81-1.66 | 0.42 | 1.98 | 0.42-9.32 | 0.38 | 1.97 | 0.42-9.28 | 0.21 | 2.19 | 0.47-10.29 | 0.17 |
| Mixed | 1.29 | 0.93-1.79 | 0.13 | 1.36 | 0.95-1.94 | 0.09 | 0.87 | 0.21-3.67 | 0.85 | 0.93 | 0.22-3.92 | 0.92 | 0.67 | 0.15-2.91 | 0.59 |
| Genotype methods | |||||||||||||||
| PCR-RFLP | 1.33 | 0.76-2.33 | 0.32 | 1.33 | 0.76-2.31 | 0.317 | NA | NA | NA | ||||||
| Non-PCR-RFLP | 0.96 | 0.85-1.09 | 0.55 | 0.96 | 0.85-1.09 | 0.554 | 1.03 | 0.70-1.52 | 0.88 | 1.02 | 0.69-1.50 | 0.92 | 1.06 | 0.72-1.58 | 0.75 |
| Tumor type | |||||||||||||||
| CRC | 0.9 | 0.70-1.15 | 0.41 | 0.86 | 0.65-1.13 | 0.275 | 1.24 | 0.54-2.86 | 0.61 | 1.18 | 0.51-2.74 | 0.69 | 1.43 | 0.60-3.39 | 0.42 |
| LC | 0.98 | 0.84-1.13 | 0.76 | 1 | 0.85-1.18 | 0.99 | 0.75 | 0.44-1.28 | 0.3 | 0.76 | 0.45-1.29 | 0.31 | 0.43-1.28 | 0.29 | |
| EOC | 2.45 | 1.46-4.10 | 0.001 | 2.52 | 1.49-4.24 | 0.001 | NA | NA | 0.74 | NA | |||||
| ESCC | 0.67 | 0.35-1.26 | 0.21 | 0.66 | 0.34-1.26 | 0.21 | NA | NA | NA | ||||||
| EA | 1.29 | 0.93-1.79 | 0.13 | 1.36 | 0.95-1.94 | 0.09 | 0.87 | 0.21-3.67 | 0.85 | 0.93 | 0.22-3.92 | 0.92 | 0.67 | 0.15-2.91 | 0.59 |
| GCA | 1.08 | 0.59-1.97 | 0.79 | 1.08 | 0.59-1.99 | 0.79 | NA | NA | NA | ||||||
| HCC | 1 | 0.60-1.68 | 0.99 | 1.04 | 0.62-1.76 | 0.88 | 0.37 | 0,02-9.08 | 0.54 | 0.37 | 0,02-9.12 | 0.54 | 0.35 | 0.01-8.80 | 0.51 |
| BC | 0.87 | 0.67-1.51 | 0.34 | 0.83 | 0.63-1.10 | 0.21 | 2.27 | 0.70-7.41 | 0.17 | 2.15 | 0.66-7.06 | 0.23 | 2.72 | 0.82-9.07 | 0.1 |
| BRC | 0.86 | 0.60-1.23 | 0.42 | 0.85 | 0.59-1.23 | 0.39 | 4.38 | 0.49-39.31 | 0.18 | 4.34 | 0.48-38.88 | 0.19 | 5.4 | 0.58-49.88 | 0.14 |
Note: W, wild allele; M, mutant allele; WW, wild homozygote; WM, heterozygote; MM, mutant homozygote; SNP, single-nucleotide polymorphism.
Figure 2.

The association between MMP-12 82 A/G polymorphism and cancer risk using different models. A. The Allele model, G allele vs A allele; B. The Dominant model, GG+AG vs AA; C. The Recessive model GG vs AG+AA; D. The Homozygous model, GG vs AA; E. The Heterozygous model, GG vs AG.
G allele of MMP-12 82 A/G polymorphism is associated with enhanced risk for EOC
In the subgroup analysis stratified by ethnicity, no significant association between MMP-12 82 A/G polymorphism and cancer risk was observed in either Asians or Caucasians. Similar results were observed when Non-PCR-RFLP or PCR-RFLP genotype methods were used (Table 3). When stratified by disease types, however, we observed that G allele of MMP-12 82 A/G significantly enhances the risk of epithelial ovarian carcinoma (EOC) using allele model (G allele vs A allele) (fixed effects OR = 2.45, 95% CI = 1.46-4.10, P = 0.001) and dominant model (fixed effects OR = 2.52, 95% CI = 1.49-4.24, P = 0.001) (Table 3 and Figure 3). No significant association could be observed between MMP-12 82 A/G and the risk of other cancers (Table 3, P > 0.05), indicating that the effect is specific to EOC.
Figure 3.
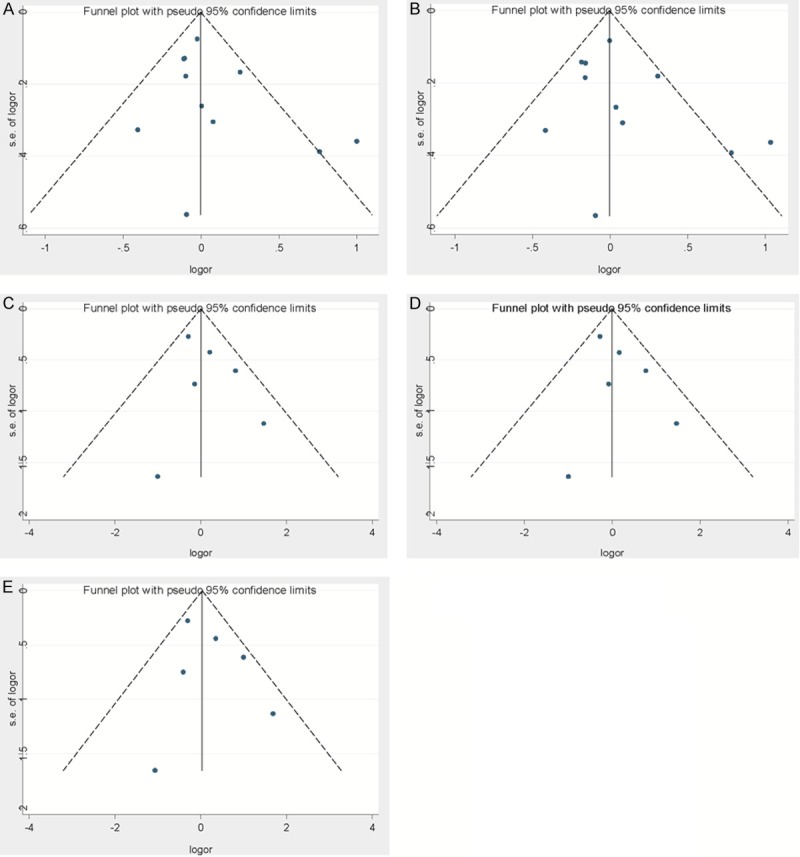
Begg’s funnel plots for bias of studies used in this analysis under different models. A. The Allele model, G allele vs A allele; B. The Dominant model, GG+AG vs AA; C. The Recessive model GG vs AG+AA; D. The Homozygous model, GG vs AA; E. The Heterozygous model, GG vs AG.
Publication bias
Begg’s test and a funnel plot were performed to evaluate the publication bias of the literature used in this analysis. As shown in Figure 3A-E, No significant bias was observed by using any of the models (the Allele model, G allele vs A allele, t = 1.25, P = 0.241; the Dominant model, GG+A/G vs AA, t = 1.09, P = 0.304; the Recessive model, GG vs A/G+AA, t = 1.01, P = 0.369; the Homozygous model, GG vs AA, t = 1.06, P = 0.348; the Heterozygous model, GG vs A/G, t = 0.86, P = 0.441).
Discussion
Meta-analysis is a useful method for investigating the associations of diseases with genetic variations via integrative analysis of different studies on the same topic, thus possibly enhance statistic power and provide more conclusive results. A major advantage of meta-analysis is that it minimises publication bias. Many studies have demonstrated that genetic factors play important roles in the pathogenesis of various tumors. In this study, we performed a meta-analysis was to estimate the relevance of rs2276109 (-82A>G) polymorphism in the MMP-12 gene with cancer susceptibility. With the large number of cases and controls extracted from different studies, overall we did not observed any evidence that MMP-12 82 A/G polymorphism may affect cancer risk. However, based on subgroup analysis based on the type of disease in our finding, it suggested that MMP-12 82 A/G polymorphism may be closely related to increase the risk of EOC (in allelic contrast and dominant model), however, such association was not observed in other cancer types, including CRC, LC,EA, ESCC, GCA, HCC, and BC.
We acknowledge some limitations to our meta-analysis. Firstly, it has been widely accepted that cancer is caused by multitudinous factors, such as, interactions of gene-environment and gene-gene, even various polymorphism loci in the same chromosome (eg.1082A>G variant) could affect the gene expression [27], Sun [28] and Su [21] et al reported that multiple cancer-associated genetic variants suggested that the MMP1-MMP3-MMP12 gene cluster plays important roles in lung cancer progression. Accordingly, the effect of MMP-12 82 A/G polymorphism alone might be compromised without considering the influence of other genetic variations. On the hand, almost all of the studies we included were conducted in Asians and Caucasians ethnic groups, additional studies are needed to be investigated the influence in other populations. In addition, as the selection criteria outlined above, all studies were published in English. Papers published in languages such as Chinese were not included, which might also bring bias to our analysis. Moreover, as cancer is caused by multiple genetic and environmental factors, lacking of environment data limited our further evaluation of gene-environment interaction and whether these SNPs are causal remained uncertain. As gene expression is regulated at multiple levels [6,29-35], further studies are also required to investigate the roles of these SNPs in regulating the expression of MMP-12 gene in vivo.
In conclusion, G allele of MMP-12 82 A/G polymorphism may significantly increase the risk of EOC, while such relationship was not evident for other cancer types. Further association studies with larger sample sizes and functional characterization of are required to reveal the role of MMP-12 82 A/G polymorphism in EOC pathogenesis.
Acknowledgements
This study was funded by National Natural Science Foundation of China (Grant numbers: 81160027) and Natural Science Foundation of Jiang Xi Province (Grant numbers: 20114BAB205001).
Disclosure of conflict of interest
None.
References
- 1.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 2.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 3.Shuman Moss LA, Jensen-Taubman S, Stetler-Stevenson WG. Matrix metalloproteinases: changing roles in tumor progression and metastasis. Am J Pathol. 2012;181:1895–1899. doi: 10.1016/j.ajpath.2012.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yin S, Yang J, Lin B, Deng W, Zhang Y, Yi X, Shi Y, Tao Y, Cai J, Wu CI, Zhao G, Hurst LD, Zhang J, Hu L, Kong X. Exome sequencing identifies frequent mutation of MLL2 in non-small cell lung carcinoma from Chinese patients. Sci Rep. 2014;4:6036. doi: 10.1038/srep06036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng WJ, Nie S, Dai J, Wu JR, Zeng R. Proteome, phosphoproteome, and hydroxyproteome of liver mitochondria in diabetic rats at early pathogenic stages. Mol Cell Proteomics. 2010;9:100–116. doi: 10.1074/mcp.M900020-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan CT, Deng W, Li F, DeMott MS, Babu IR, Begley TJ, Dedon PC. Highly Predictive Reprogramming of tRNA Modifications Is Linked to Selective Expression of Codon-Biased Genes. Chem Res Toxicol. 2015;28:978–88. doi: 10.1021/acs.chemrestox.5b00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jormsjo S, Ye S, Moritz J, Walter DH, Dimmeler S, Zeiher AM, Henney A, Hamsten A, Eriksson P. Allele-specific regulation of matrix metalloproteinase-12 gene activity is associated with coronary artery luminal dimensions in diabetic patients with manifest coronary artery disease. Circ Res. 2000;86:998–1003. doi: 10.1161/01.res.86.9.998. [DOI] [PubMed] [Google Scholar]
- 8.Yang W, Arii S, Gorrin-Rivas MJ, Mori A, Onodera H, Imamura M. Human macrophage metalloelastase gene expression in colorectal carcinoma and its clinicopathologic significance. Cancer. 2001;91:1277–1283. [PubMed] [Google Scholar]
- 9.Houghton AM, Grisolano JL, Baumann ML, Kobayashi DK, Hautamaki RD, Nehring LC, Cornelius LA, Shapiro SD. Macrophage elastase (matrix metalloproteinase-12) suppresses growth of lung metastases. Cancer Res. 2006;66:6149–6155. doi: 10.1158/0008-5472.CAN-04-0297. [DOI] [PubMed] [Google Scholar]
- 10.Kader AK, Liu J, Shao L, Dinney CP, Lin J, Wang Y, Gu J, Grossman HB, Wu X. Matrix metalloproteinase polymorphisms are associated with bladder cancer invasiveness. Clin Cancer Res. 2007;13:2614–2620. doi: 10.1158/1078-0432.CCR-06-1187. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Jia JH, Kang S, Zhang XJ, Zhao J, Wang N, Zhou RM, Sun DL, Duan YN, Wang DJ. The functional polymorphisms on promoter region of matrix metalloproteinase-12, -13 genes may alter the risk of epithelial ovarian carcinoma in Chinese. Int J Gynecol Cancer. 2009;19:129–133. doi: 10.1111/IGC.0b013e31819a1d8e. [DOI] [PubMed] [Google Scholar]
- 12.VAN Nguyen S, Skarstedt M, Löfgren S, Zar N, Andersson RE, Lindh M, Matussek A, Dimberg J. Gene polymorphism of matrix metalloproteinase-12 and -13 and association with colo- rectal cancer in Swedish patients. Anticancer Res. 2013;33:3247–3250. [PubMed] [Google Scholar]
- 13.Hughes S, Agbaje O, Bowen RL, Holliday DL, Shaw JA, Duffy S, Jones JL. Matrix metalloproteinase single-nucleotide polymorphisms and haplotypes predict breast cancer progression. Clin Cancer Res. 2007;13:6673–6680. doi: 10.1158/1078-0432.CCR-07-0884. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 16.Yu Y, Chi B, Xia W, Gangopadhyay J, Yamazaki T, Winkelbauer-Hurt ME, Yin S, Eliasse Y, Adams E, Shaw CE, Reed R. U1 snRNP is mislocalized in ALS patient fibroblasts bearing NLS mutations in FUS and is required for motor neuron outgrowth in zebrafish. Nucleic Acids Res. 2015;43:3208–18. doi: 10.1093/nar/gkv157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stuck AE, Rubenstein LZ, Wieland D. Bias in meta-analysis detected by a simple, graphical test. Asymmetry detected in funnel plot was probably due to true heterogeneity. BMJ. 1998;316:469. author reply 470-461. [PMC free article] [PubMed] [Google Scholar]
- 19.Shin A, Cai Q, Shu XO, Gao YT, Zheng W. Genetic polymorphisms in the matrix metalloproteinase 12 gene (MMP12) and breast cancer risk and survival: the Shanghai Breast Cancer Study. Breast Cancer Res. 2005;7:R506–512. doi: 10.1186/bcr1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kader AK, Shao L, Dinney CP, Schabath MB, Wang Y, Liu J, Gu J, Grossman HB, Wu X. Matrix metalloproteinase polymorphisms and bladder cancer risk. Cancer Res. 2006;66:11644–11648. doi: 10.1158/0008-5472.CAN-06-1212. [DOI] [PubMed] [Google Scholar]
- 21.Su L, Zhou W, Asomaning K, Lin X, Wain JC, Lynch TJ, Liu G, Christiani DC. Genotypes and haplotypes of matrix metalloproteinase 1, 3 and 12 genes and the risk of lung cancer. Carcinogenesis. 2006;27:1024–1029. doi: 10.1093/carcin/bgi283. [DOI] [PubMed] [Google Scholar]
- 22.Woo M, Park K, Nam J, Kim JC. Clinical implications of matrix metalloproteinase-1, -3, -7, -9, -12, and plasminogen activator inhibitor-1 gene polymorphisms in colorectal cancer. J Gastroenterol Hepatol. 2007;22:1064–1070. doi: 10.1111/j.1440-1746.2006.04424.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhai Y, Qiu W, Dong XJ, Zhang XM, Xie WM, Zhang HX, Yuan XY, Zhou GQ, He FC. Functional polymorphisms in the promoters of MMP-1, MMP-2, MMP-3, MMP-9, MMP-12 and MMP-13 are not associated with hepatocellular carcinoma risk. Gut. 2007;56:445–447. doi: 10.1136/gut.2006.112706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradbury PA, Zhai R, Hopkins J, Kulke MH, Heist RS, Singh S, Zhou W, Ma C, Xu W, Asomaning K, Ter-Minassian M, Wang Z, Su L, Christiani DC, Liu G. Matrix metalloproteinase 1, 3 and 12 polymorphisms and esophageal adenocarcinoma risk and prognosis. Carcinogenesis. 2009;30:793–798. doi: 10.1093/carcin/bgp065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jia J, Kang S, Zhao J, Zhang X, Wang N, Zhou R, Li Y. Association of functional polymorphisms on MMP-12 and MMP-13 gene promoter region with epithelial ovarian carcinoma. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2010;27:209–213. doi: 10.3760/cma.j.issn.1003-9406.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Sun DL, Duan YN, Zhang XJ, Wang N, Zhou RM, Chen ZF, Wang SJ. Association of functional polymorphisms in MMPs genes with gastric cardia adenocarcinoma and esophageal squamous cell carcinoma in high incidence region of North China. Mol Biol Rep. 2010;37:197–205. doi: 10.1007/s11033-009-9593-4. [DOI] [PubMed] [Google Scholar]
- 27.Heist RS, Marshall AL, Liu G, Zhou W, Su L, Neuberg D, Lynch TJ, Wain J, Christiani DC. Matrix metalloproteinase polymorphisms and survival in stage I non-small cell lung cancer. Clin Cancer Res. 2006;12:5448–5453. doi: 10.1158/1078-0432.CCR-06-0262. [DOI] [PubMed] [Google Scholar]
- 28.Sun T, Gao Y, Tan W, Ma S, Zhang X, Wang Y, Zhang Q, Guo Y, Zhao D, Zeng C, Lin D. Haplotypes in matrix metalloproteinase gene cluster on chromosome 11q22 contribute to the risk of lung cancer development and progression. Clin Cancer Res. 2006;12:7009–7017. doi: 10.1158/1078-0432.CCR-06-0464. [DOI] [PubMed] [Google Scholar]
- 29.Lei H, Zhai B, Yin S, Gygi S, Reed R. Evidence that a consensus element found in naturally intronless mRNAs promotes mRNA export. Nucleic Acids Res. 2013;41:2517–2525. doi: 10.1093/nar/gks1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang P, Yin S, Zhang Z, Xin D, Hu L, Kong X, Hurst LD. Evidence for common short natural trans sense-antisense pairing between transcripts from protein coding genes. Genome Biol. 2008;9:R169. doi: 10.1186/gb-2008-9-12-r169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yin S, Deng W, Hu L, Kong X. The impact of nucleosome positioning on the organization of replication origins in eukaryotes. Biochem Biophys Res Commun. 2009;385:363–368. doi: 10.1016/j.bbrc.2009.05.072. [DOI] [PubMed] [Google Scholar]
- 32.Yin S, Deng W, Zheng H, Zhang Z, Hu L, Kong X. Evidence that the nonsense-mediated mRNA decay pathway participates in X chromosome dosage compensation in mammals. Biochem Biophys Res Commun. 2009;383:378–382. doi: 10.1016/j.bbrc.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 33.Yin S, Wang P, Deng W, Zheng H, Hu L, Hurst LD, Kong X. Dosage compensation on the active X chromosome minimizes transcriptional noise of X-linked genes in mammals. Genome Biol. 2009;10:R74. doi: 10.1186/gb-2009-10-7-r74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopez MF, Krastins B, Sarracino DA, Byram G, Vogelsang MS, Prakash A, Peterman S, Ahmad S, Vadali G, Deng W, Inglessis I, Wickham T, Feeney K, Dec GW, Palacios I, Buonanno FS, Lo EH, Ning M. Proteomic signatures of serum albumin-bound proteins from stroke patients with and without endovascular closure of PFO are significantly different and suggest a novel mechanism for cholesterol efflux. Clin Proteomics. 2015;12:2. doi: 10.1186/1559-0275-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan CT, Pang YL, Deng W, Babu IR, Dyavaiah M, Begley TJ, Dedon PC. Reprogramming of tRNA modifications controls the oxidative stress response by codon-biased translation of proteins. Nat Commun. 2012;3:937. doi: 10.1038/ncomms1938. [DOI] [PMC free article] [PubMed] [Google Scholar]


