Abstract
Pulmonary function is significantly reduced in the acute phase after coronary artery bypass graft (CABG) surgery. Because pulmonary function partly depends on respiratory muscle strength, we studied whether reductions in pulmonary function are related to postoperative alterations in circulatory factors that affect muscle protein synthesis. Methods: Slow vital capacity (SVC) was assessed in 22 subjects before and 9 ± 3 days after CABG surgery. Blood testosterone, cortisol, insulin-like growth factor-1 (IGF-1), growth hormone, sex-hormone binding globulin (SHBG), glucose, insulin, c-peptide, c-reactive protein (CRP) content, and free androgen index, cortisol/testosterone ratio, HOMA-IR index were assessed before surgery and during the first three days after surgery. Intubation, surgery time and cumulative chest tube drainage were measured. Correlations between changes in SVC and blood parameters after surgery or subject characteristics were studied. This was a prospective observational study. Results: After CABG surgery SVC decreased by 37 ± 18% (P < 0.01). Free androgen index, blood SHBG, testosterone and IGF-1 content decreased, while HOMA-IR index, cortisol/testosterone ratio, blood growth hormone, insulin and CRP content increased (P < 0.0025) in the first three days after surgery. Decrease in SVC was independently (P < 0.05) related to higher preoperative SVC (SC β = 0.66), and greater increase in blood cortisol (SC β = 0.54) and CRP (SC β = 0.37) content after surgery. Conclusions: Larger reductions in pulmonary function after CABG surgery are present in patients experiencing greater postoperative increases in blood CRP and cortisol levels. Decrements in pulmonary function after CABG surgery are, at least in part, thus related to alterations in circulatory factors that affect muscle protein synthesis.
Keywords: Coronary artery bypass grafting, slow vital capacity, pulmonary function, cortisol, c-reactive protein
Introduction
Approximately 641.470 coronary artery bypass graft (CABG) surgery procedures are performed in Europe and the United States each year to restore or optimize myocardial perfusion in coronary artery disease [1,2]. Worldwide there are approximately over 2 million open heart surgeries per year [3]. In this surgical intervention, venous and/or arterial grafts are used to bypass the coronary occlusion or stenosis. Because cardioplegia (induction of temporary cardiac arrest) is routinely used, the patient’s circulation is coupled to a cardiopulmonary bypass and access to the heart is most often achieved by median sternotomy. After CABG surgery, a hospital stay of one to two weeks is generally required.
Although CABG surgery is an effective coronary revascularization technique, it is often associated with a decrease in pulmonary function. During the first week after CABG surgery slow vital capacity decreases by 30-60% [4-7] and even up to one year remains reduced by 12% [8,9].
Pulmonary complications are a leading cause of morbidity and mortality after CABG surgery [7]. A deeper understanding of the mechanisms leading to worse pulmonary function after CABG surgery could lead to optimized postoperative care and thus a reduced morbidity and mortality.
The aetiology of worsening in pulmonary function after CABG surgery is multifactorial: reduced rib cage expansion and uncoordinated chest wall motion [6,10], diaphragmatic dysfunction due to phrenic nerve injury [11,12], pleural fluid accumulation and basal atelectasis [13]. However, reduction in pulmonary function could also be due to dysfunction of respiratory muscles. Respiratory muscles are skeletal muscles and thus vulnerable to alterations in circulatory factors that affect muscle mass and strength. Peripheral skeletal muscle protein synthesis is significantly reduced immediately after CABG surgery, resulting in a significant atrophy and muscle weakness in the first weeks after surgery [14-16]. The relations between changes in pulmonary function after CABG surgery and changes in circulatory factors that alter (respiratory) muscle protein synthesis are unknown.
CABG surgery is associated with short-term lowering in blood anabolic hormone content and increasing blood catabolic hormone content, reduction in insulin sensitivity and an inflammatory reaction [17-22]. These postoperative changes may provoke muscle wasting. Indeed, in a recent study we found that the magnitude of peripheral skeletal muscle wasting was related to changes in blood cortisol/testosterone ratio after CABG surgery [23]. The aim of this study was to examine whether changes in blood parameters, that affect muscle protein synthesis, after CABG surgery are related to decrements in pulmonary function. Such study may lead to the examination and development of new treatment strategies for the preservation of pulmonary function after CABG surgery.
Materials and methods
Subjects and design
After inviting 30 eligible candidates (from March 2011 up to October 2013), 25 subjects agreed to participate in this study (Figure 1). The study sample size (n = 25) was based on previous studies assessing changes in pulmonary function after CABG surgery [4,6,8]. Due to post-operative complications and resultant prolonged hospitalization, three subjects were excluded. Data from 22 subjects were analysed. As a result, data from the present study reflect changes in blood parameters and pulmonary function after CABG surgery in subjects with a normal postoperative course. Subjects were admitted to the hospital for elective CABG surgery. Subjects were excluded when pulmonary, neurologic, and/or nephrologic disease was present. All CABG surgery procedures were performed by the same surgical team, with similar methodology. This study was approved by the local medical ethical committee (Jessa Hospital, Hasselt, Belgium), and written informed consents were obtained from all subjects. This was a prospective observational study.
Figure 1.
Study flowchart.
On-pump CABG surgery through median sternotomy was performed on all subjects. During this procedure, patients were connected to an open circuit extracorporeal circulation (cardio-pulmonary bypass), the aorta was cross-clamped and the heart arrested using blood cardioplegia. Systemic temperature was lowered to 34°C. The average duration of the procedure (start median incision up to end of closing of sternotomy) was 232 ± 43 minutes. During hospital stay (9 ± 3 days) subjects received physical therapy (daily breathing exercises for a duration of 15 min/day and endurance exercises (walking and cycling) up to 30 min/day at a low intensity (exercise heart rate < 120 bts/min).
Measurements
The observed parameters were slow vital capacity (SVC), blood cortisol, c-reactive protein (CRP), insulin-like growth factor 1 (IGF-1), growth hormone, sex-hormone binding globulin (SHBG), insulin, c-peptide, testosterone, glycosylated haemoglobin content, cortisol/testosterone ratio, homeostatic model assessment-insulin resistance index (HOMA-IR index), free androgen index, cumulative chest tube drainage, length of hospitalization, surgery duration and intubation time.
SVC was measured before surgery and at the day of discharge from the hospital (9 ± 3 days postoperative) (Pocket-Spiro USB100, Medical Electronic Construction & Logistic nv, Belgium). SVC can be derived in two ways. The expiratory vital capacity (EVC) is the maximal volume of air exhaled after maximal inhalation. The inspiratory vital capacity (IVC) is the maximal volume of air inhaled after maximal exhalation. SVC is a reliable measurement of restrictive pulmonary function [24] and was used instead of forced vital capacity (FVC) because it is less painful and easier to perform for the patient in the early postoperative phase.
Blood samples were collected between 07.00 and 09.00 AM after an overnight fast, and processed and stored at the University Biobank Limburg (UBiLim). Blood parameter analysis was performed by standard diagnostic tests at the clinical laboratory of the Jessa Hospital. Blood glycosylated haemoglobin content (HbA1c) was determined in whole EDTA (ethylene-diamine-tetra-acetic) blood via a commercial HPLC (high-performance liquid chromatography) based method (Hi-Auto A1C Analyser, Menarini Diagnostics). Serum tubes were left to clot for 30 min and subsequently centrifuged for 5 min at 2500 g. These serum samples were used to asses blood hormones affecting skeletal muscle protein synthesis such as total testosterone (by chemiluminescent ELISA on an automated ELISA device Architect i2000SR, Abbott), total cortisol (by direct chemiluminescence sandwich ELISA on ADVIA Centaur XP analyser, Siemens), total IGF-1 (by ImmuneRadioMetric Assay [IMRA] IGF-I kit, Beckman Coulter) and total growth hormone (by hGH-IRMA kit, DiaSource), as well as for SHBG (by 2-step Chemiflex ImmunoAssay on automated ELISA device Architect i2000SR, Abbott), insulin, c-peptide content (both by direct chemiluminescence sandwich ELISA on ADVIA Centaur XP, Siemens) and blood glucose level (by colorimetric Hexokinase-Glucose-6-fosfaat dehydrogenase method on a Beckman Coulter AU 2700 chemical analyser, Beckman Coulter). Lithium heparin plasma was obtained by spinning blood samples for 5 min at 2500 g. In these samples the lipid profile (total cholesterol, triglycerides, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol) was measured by enzymatic colorimetry, together with the inflammatory status based on blood CRP content by antibody based turbidimetric method, on a Beckman Coulter AU 2700 chemical analyser. HOMA-IR index was calculated to estimate whole-body insulin sensitivity: [(insulin (mU/L) × glucose (mmol/L)]/22.5. Free androgen index was calculated by: (blood total testosterone content (nmol/l) × 100)/blood sexhormone binding globulin content (nmol/l). Catabolic-anabolic hormone balance was calculated by: blood total cortisol content/blood total testosterone content.
Statistical analysis and calculations
All data are expressed as means ± SD. Statistical analyses were executed by use of SPSS version 22.0. Shapiro-Wilk tests indicated that most data were not normally distributed. Therefore, non-parametric tests were applied. Changes in blood parameters and pulmonary function during follow-up were analysed by related-sample Friedman variance of ranks tests, in which Bonferroni corrections for multiple comparisons (pre-operative vs. post-operative, n = 22) were applied (statistical significance was set at P < 0.0025, 2-tailed). Relations between individual parameters were analysed by Spearman’s correlations. A forward stepwise multivariate regression model was constructed to examine which factors were related to a decrease in pulmonary function. From this model, age, gender, body mass index (BMI), preoperative pulmonary function, preoperative glycaemic control (glucose, insulin, HOMA-IR, glycated haemoglobin (HbA1c)), cortisol/testosterone ratio, free androgen index, CRP, cumulative chest tube drainage, length of hospitalization, surgery duration and intubation time came out as possible predicting factors. In final, a multivariate linear regression model was created in which relations between changes pulmonary function and detected significant independent predictors from the forward stepwise multivariate regression model were examined. In these regression models statistical significance was set at P < 0.05, 2-tailed.
Results
Subject characteristics
Twenty-two subjects were examined (3 females, average age 62 ± 9 years, Table 1). Two subjects were revascularised by CABG surgery after acute myocardial infarction, the others were revascularised for stable angina with a positive stress test. Subjects were intubated during 704 ± 590 minutes after CABG surgery and were hospitalized for 9 ± 3 days.
Table 1.
Subject characteristics
| Age (yrs) | 62 ± 9 |
| Gender (n males/females) | 19/3 |
| Body mass index (kg/m2) | 29.5 ± 5.4 |
| Revascularized coronary arteries | |
| Left descending artery (n) | 19 |
| Right coronary artery (n) | 14 |
| Circumflex artery (n) | 11 |
| Acute myocardial infarction (n) | 2 |
| Duration of intubation (min) | 704 ± 590 |
| Duration of hospitalization (days) | 9 ± 3 |
| Interval between pre-operative vs. post-operative SVC (days) | 9 ± 3 |
| Blood glycosylated hemoglobin content (%) | 5.9 ± 0.7 |
| Blood total cholesterol content (mg/dl) | 156 ± 27 |
| Blood LDL cholesterol content (mg/dl) | 83 ± 18 |
| Blood HDL cholesterol content (mg/dl) | 41 ± 13 |
| Blood triglyceride content (mg/dl) | 157 ± 90 |
Data are expressed as means ± SD. Abbreviations: SVC, slow vital capacity; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Changes in pulmonary function
SVC decreased significantly (from 3.9 ± 0.7 L to 2.5 ± 0.7 L, from 100 ± 18% to 63 ± 18% of predicted SVC, P < 0.001), together with IVC (from 3.7 ± 0.7 L to 2.3 ± 0.6 L, from 95 ± 15% to 60 ± 17% of predicted IVC, P < 0.001) and EVC (from 3.9 ± 0.7 L to 2.4 ± 0.7 L, from 100 ± 18% to 62 ± 18% of predicted EVC, P < 0.001) after CABG surgery (Figure 2).
Figure 2.
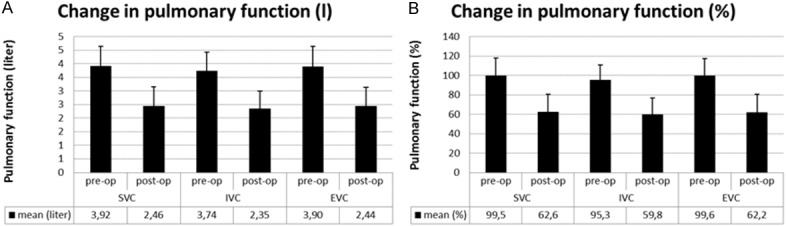
Changes in pulmonary function after CABG surgery. A: Change in litres ± SD. SVC from 3.9 ± 0.7 L to 2.5 ± 0.7 L, P < 0.001, IVC from 3.7 ± 0.7 L to 2.3 ± 0.6 L, P < 0.001 and EVC from 3.9 ± 0.7 L to 2.4 ± 0.7 L, P < 0.001. B: Change in percentages ± SD. SVC from 100 ± 18% to 63 ± 18% of predicted SVC, P < 0.001), ICV from 95 ± 15% to 60 ± 17% of predicted IVC, P < 0.001 and EVC, from 100 ± 18% to 62 ± 18% of predicted EVC, P < 0.001.
Changes in blood parameters
During the first three days after CABG surgery, a significant inflammatory reaction and worsening in insulin sensitivity occurred, as can be observed by significant increases in blood CRP content and HOMA-IR index (P < 0.0025, Table 2). In addition, significant decreases in blood testosterone, IGF-1 and SHBG content, free androgen index, and increases in blood growth hormone levels and cortisol/testosterone ratio, were observed (P < 0.0025, Table 2).
Table 2.
-Blood parameters and impact of CABG surgery
| pre-operative | Day 1 after CABG | Day 2 after CABG | Day 3 after CABG | |
|---|---|---|---|---|
| glucose (mg/dl) | 5.7 ± 2.3 | 6.9 ± 1.6 | 6.8 ± 1.6 | 7.0 ± 2.3 |
| c-peptide (ng/ml) | 1.9 ± 1.0 | 1.0 ± 1.0 | 1.8 ± 1.8 | 2.7 ± 3.0 |
| insulin (mU/l) | 10.7 ± 6.5* | 59 ± 51 | 33 ± 18 | 36 ± 36 |
| HOMA-IR index | 2.7 ± 2.0* | 19.7 ± 18.7 | 9.8 ± 6.8 | 11.9 ± 17.7 |
| c-reactive protein (mg/dl) | 0.5 ± 0.8* | 9.5 ± 3.5 | 21.0 ± 5.8 | 22.1 ± 6.6 |
| cortisol (total, µg/dl) | 18 ± 5 | 21 ± 6 | 18 ± 6 | 19 ± 6 |
| testosterone (total, ng/ml) | 3.7 ± 1.9* | 0.8 ± 0.6 | 0.8 ± 0.5 | 1.0 ± 0.7 |
| sex-hormone binding globulin (nmol/l) | 43 ± 16* | 27 ± 11 | 28 ± 9 | 29 ± 9 |
| insulin-like growth factor 1 (µg/l) | 138 ± 46* | 102 ± 27 | 97 ± 20 | 90 ± 23 |
| growth hormone (µg/l) | 0.2 ± 0.1* | 1.7 ± 1.3 | 1.1 ± 0.7 | 0.6 ± 0.4 |
| free androgen index (%) | 31 ± 16* | 11 ± 7 | 9 ± 6 | 12 ± 7 |
| cortisol/testosterone ratio | 21 ± 51* | 66 ± 136 | 38 ± 41 | 29 ± 26 |
Data are expressed as means ± SD.
Significantly different as opposed to post-operative values in the first three days only (P < 0.0025).
Abbreviations: HOMA-IR, homeostatic model assessment-insulin resistance.
Regression analysis
A higher preoperative IVC (SC β = 0657, P = 0.001), a larger increase in blood cortisol (SC β = 0.536, P = 0.004) and CRP (SC β = 0.37, P = 0.019) content after CABG surgery were independently related to a larger decrease in IVC after CABG surgery (model adjusted r2 = 0.64, P < 0.001) (see Figure 3A-C for univariate correlations).
Figure 3.
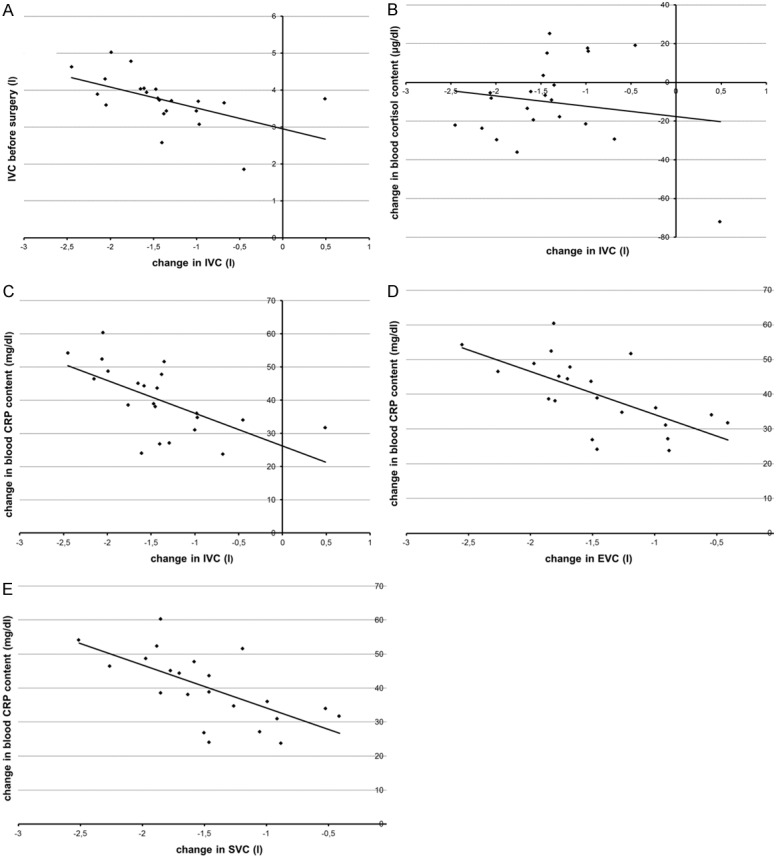
Relations between changes in pulmonary function and subject features. A: Relation between preoperative IVC and change in IVC (SC β = 0.657, P = 0.001). B: Relation between change in blood cortisol and change in IVC (SC β = 0.536, P = 0.004). C: Relation between change in CRP and change in IVC (SC β = 0.37, P = 0.019). D: Relation between change in CRP and change in EVC (SC β = 0.67, P < 0.001). E: Relation between change in CRP and change in SVC (SC β = 0.669, P < 0.001).
A larger increase in blood CRP content (SC β = 0.67, P < 0.001) after CABG surgery was independently related to a larger decrease in EVC after CABG surgery (model adjusted r2 = 0.60, P = 0.001) (see Figure 3D for univariate correlations).
A larger increase in blood CRP content (SC β = 0.669, P < 0.001) after CABG surgery was independently related to a larger decrease in SVC after CABG surgery (model adjusted r2 = 0.53, P = 0.001) (see Figure 3E for univariate correlations).
Discussion
In this study pulmonary function was decreased significantly on the 9th day after CABG surgery and the magnitude of reduction in pulmonary function was related to pre-operative pulmonary function and changes in blood cortisol and CRP concentrations during the first 3 days after surgery.
A significantly impaired pulmonary function (IVC, EVC and SVC) was found within nine days after CABG surgery. This decrease in pulmonary function is in line with previous observations and was thus expected [4-7]. Because respiratory muscles, and hence pulmonary function, are affected by circulatory factors that coordinate skeletal muscle mass and strength, we therefore studied the relations between changes in these blood parameters after CABG surgery and changes in pulmonary function. We observed that a larger postoperative increase in blood CRP and cortisol concentration was independently associated with larger reductions in pulmonary function. These data indicate, to our knowledge for the first time, that changes in circulatory factors after CABG surgery may also mediate changes in pulmonary function. However, it should be mentioned that correlations do not indicate causality.
Data are in support of the hypothesis that inflammatory markers are related to pulmonary function. For example, Aronson [25] found an inverse relation between blood CRP level and pulmonary function in healthy subjects in a cross-sectional observation, while Jiang et al. [26] found that greater circulatory fibrinogen levels predict larger declines in pulmonary function in the elderly during follow-up.
It should be explored in greater detail how short-term changes in blood CRP and cortisol concentrations after CABG surgery are related to decrements in pulmonary function. Such knowledge may lead to improvements in postoperative respiratory care. Based on current literature, hypercortisolaemia leads to skeletal muscle wasting by a combination of an increase in protein breakdown (by activation of cellular proteolytic systems such as ubiquitin-proteasome system (UPS), lysosomal system (cathepsins) and calcium-dependent system (calpains) and lowering in protein synthesis (by lowered transport of amino acids into the muscle, inhibition of insulin and IGF-1 action, and/or inhibition of phosphorylation of eIF4E-binding protein-1 and ribosomal protein S6 kinase-1) [27]. Elevated circulatory CRP levels impair skeletal muscle protein synthesis by various mechanisms [28]. Inflammation leads to activation of intramuscular caspase-3 that breaks down the complex protein structure of muscle. Moreover, inflammation leads to a lowering in phosphorylation of Akt (p-AKT) which decreases phosphorylation of the forkhead transcription factor and permits its translocation into the nucleus, where it stimulates the expression of atrogin-1/muscle atrophy F-box (MAFbx) and muscle ring finger 1 (MuRF1). These enzymes are specific E3 ubiquitin-conjugating enzymes that recognize specific muscle proteins and increase their degradation in the UPS by promoting ubiquitin conjugation to the proteins. The increase in these E3 enzymes leads to loss of muscle proteins and thus muscle wasting [29]. It remains uncertain whether similar intramuscular biochemical changes are present in respiratory muscles during episodes of hypercortisolaemia and/or inflammation.
Whether different catabolic effects of CRP and cortisol on different muscle fibre types occur in humans is also unknown. During normal breathing mainly slow fibres are activated while fast muscle fibres are activated when breathing faster and deeper. When performing a SVC test, as executed in the present study, the diaphragm, intercostal muscles and accessory respiratory muscles are important contributors to pulmonary function [30]. The adult human diaphragm consists of 55% slow muscle fibres (type 1), 21% fast oxidative muscle fibres (type 2A) and 24% fast glycolytic muscle fibres (type 2X). The intercostal muscles consist of 60% slow fibres and 40% fast fibres [30,31]. In animal studies inflammation has a more pronounced catabolic effect on type 2 fibres [32], but the impact of hypercortisolemia on different muscle fibres types remains unknown. It thus remains speculative whether a reduction in pulmonary function after CABG surgery in the present study is related to predominantly respiratory type 1 or type 2 muscle fibre atrophy.
Based on the findings from this study, novel therapies for the preservation of pulmonary function after CABG surgery can be tested or implemented. In this regard, it should be studied whether a less invasive surgical technique (minimally invasive CABG surgery and/or off-pump CABG surgery) indeed leads to a lesser postoperative inflammation and hypercortisolemia and hence preserved pulmonary function.
Because of the significant reductions in pulmonary function after CABG surgery, early physical therapy with or without nutritional support is mandatory. Early deep breathing exercises after CABG surgery contribute to a better preservation of pulmonary function [33]. Nutritional support, such as protein supplementation, in combination with resistance-type exercises significantly augments type 1 and type 2 muscle fibre cross-sectional area (CSA), at least in peripheral muscles [34]. The impact of such intervention on respiratory muscles and respiratory function should be studied as well. Whole-body physical activity leads to a decrease in blood CRP content [35]. It should be examined whether early ambulation after CABG surgery contributes to better preservation in pulmonary function via a suppression of blood CRP content.
Anabolic supplementation could lead to a better preservation of muscle mass [21], but further research is needed because of the side effects, especially in women [29].
In conclusion, higher preoperative IVC and greater postoperative increases in blood CRP and cortisol concentrations are related to a greater decrease in pulmonary function after CABG surgery. Decrements in pulmonary function after CABG surgery are, at least in part, thus related to alterations in circulatory factors that affect muscle protein synthesis. However, it should be mentioned that correlations do not indicate causality and that he aetiology of worsening in pulmonary function after CABG surgery is multifactorial.
Acknowledgements
The authors would like to thank Dr. Mees, Mr. Bonne, Mr. Cleeren, Ms. Vandormael and Ms. Loysch for their appreciated contribution in the acquisition of data in this study. Dr. Linsen is part of the ‘Limburg Clinical Research Program (LCRP) UHasselt-ZOL-Jessa’, supported by the foundation Limburg Sterk Merk, province of Limburg, Flemish government, Hasselt University, Ziekenhuis Oost-Limburg and Jessa Hospital. This study was supported by an unrestricted research grant from Hartcentrum Hasselt vzw.
Disclosure of conflict of interest
None.
References
- 1.Lafortune G, Balestat G, Durand A. Comparing activities and performance of the hospital sector in Europe: how many surgical procedures performed as inpatient and day cases? 2012 [Google Scholar]
- 2.National Hospital Discharge Survey 2010. 2012
- 3.Pezzella AT. Global aspects of cardiothoracic surgery with focus on developing countries. Asian Cardiovasc Thorac Ann. 2010;18:299–310. doi: 10.1177/0218492310370060. [DOI] [PubMed] [Google Scholar]
- 4.Westerdahl E, Lindmark B, Bryngelsson I, Tenling A. Pulmonary function 4 months after coronary artery bypass graft surgery. Respir Med. 2003;97:317–322. doi: 10.1053/rmed.2002.1424. [DOI] [PubMed] [Google Scholar]
- 5.Baumgarten MC, Garcia GK, Frantzeski MH, Giacomazzi CM, Lagni VB, Dias AS, Monteiro MB. Pain and pulmonary function in patients submitted to heart surgery via sternotomy. Rev Bras Cir Cardiovasc. 2009;24:497–505. doi: 10.1590/s0102-76382009000500011. [DOI] [PubMed] [Google Scholar]
- 6.Ragnarsdottir M, KristjAnsdottir A, Ingvarsdottir I, Hannesson P, Torfason B, Cahalin L. Short-term changes in pulmonary function and respiratory movements after cardiac surgery via median sternotomy. Scand Cardiovasc J. 2004;38:46–52. doi: 10.1080/14017430310016658. [DOI] [PubMed] [Google Scholar]
- 7.Morsch KT, Leguisamo CP, Camargo MD, Coronel CC, Mattos W, Ortiz LD, Lima GG. Ventilatory profile of patients undergoing CABG surgery. Rev Bras Cir Cardiovasc. 2009;24:180–187. doi: 10.1590/s0102-76382009000200014. [DOI] [PubMed] [Google Scholar]
- 8.Kristjansdottir A, Ragnarsdottir M, Hannesson P, Beck HJ, Torfason B. Chest wall motion and pulmonary function are more diminished following cardiac surgery when the internal mammary artery retractor is used. Scand Cardiovasc J. 2004;38:369–374. doi: 10.1080/14017430410016396. [DOI] [PubMed] [Google Scholar]
- 9.Kristjansdottir A, Ragnarsdottir M, Hannesson P, Beck HJ, Torfason B. Respiratory movements are altered three months and one year following cardiac surgery. Scand Cardiovasc J. 2004;38:98–103. doi: 10.1080/14017430410028492. [DOI] [PubMed] [Google Scholar]
- 10.Locke TJ, Griffiths TL, Mould H, Gibson GJ. Rib cage mechanics after median sternotomy. Thorax. 1990;45:465–468. doi: 10.1136/thx.45.6.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tripp HF, Bolton JW. Phrenic nerve injury following cardiac surgery: a review. J Card Surg. 1998;13:218–223. doi: 10.1111/j.1540-8191.1998.tb01265.x. [DOI] [PubMed] [Google Scholar]
- 12.Laub GW, Muralidharan S, Chen C, Perritt A, Adkins M, Pollock S, Bailey B, McGrath LB. Phrenic nerve injury. A prospective study. Chest. 1991;100:376–379. doi: 10.1378/chest.100.2.376. [DOI] [PubMed] [Google Scholar]
- 13.Lundquist H, Hedenstierna G, Strandberg A, Tokics L, Brismar B. CT-assessment of dependent lung densities in man during general anaesthesia. Acta Radiol. 1995;36:626–632. [PubMed] [Google Scholar]
- 14.Caso G, Vosswinkel JA, Garlick PJ, Barry MK, Bilfinger TV, McNurlan MA. Altered protein metabolism following coronary artery bypass graft (CABG) surgery. Clin Sci (Lond) 2008;114:339–346. doi: 10.1042/CS20070278. [DOI] [PubMed] [Google Scholar]
- 15.Brodsky IG, Balagopal P, Nair KS. Effects of testosterone replacement on muscle mass and muscle protein synthesis in hypogonadal men--a clinical research center study. J Clin Endocrinol Metab. 1996;81:3469–3475. doi: 10.1210/jcem.81.10.8855787. [DOI] [PubMed] [Google Scholar]
- 16.Kortebein P, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA. 2007;297:1772–1774. doi: 10.1001/jama.297.16.1772-b. [DOI] [PubMed] [Google Scholar]
- 17.Lehot JJ, Piriz H, Villard J, Cohen R, Guidollet J. Glucose homeostasis. Comparison between hypothermic and normothermic cardiopulmonary bypass. Chest. 1992;102:106–111. doi: 10.1378/chest.102.1.106. [DOI] [PubMed] [Google Scholar]
- 18.Roth-Isigkeit A, Dibbelt L, Schmucker P, Seyfarth M. The immune-endocrine interaction varies with the duration of the inflammatory process in cardiac surgery patients. J Neuroendocrinol. 2000;12:546–552. doi: 10.1046/j.1365-2826.2000.00484.x. [DOI] [PubMed] [Google Scholar]
- 19.Roth-Isigkeit AK, Schmucker P. Postoperative dissociation of blood levels of cortisol and adrenocorticotropin after coronary artery bypass grafting surgery. Steroids. 1997;62:695–699. doi: 10.1016/s0039-128x(97)00069-x. [DOI] [PubMed] [Google Scholar]
- 20.Henzen C, Kobza R, Schwaller-Protzmann B, Stulz P, Briner VA. Adrenal function during coronary artery bypass grafting. Eur J Endocrinol. 2003;148:663–668. doi: 10.1530/eje.0.1480663. [DOI] [PubMed] [Google Scholar]
- 21.Maggio M, Ceda GP, De Cicco G, Cattadori E, Visioli S, Ablondi F, Beghi C, Gherli T, Basaria S, Ceresini G, Valenti G, Ferrucci L. Acute changes in circulating hormones in older patients with impaired ventricular function undergoing on-pump coronary artery bypass grafting. J Endocrinol Invest. 2005;28:711–719. doi: 10.1007/BF03347554. [DOI] [PubMed] [Google Scholar]
- 22.Velissaris T, Tang AT, Murray M, Mehta RL, Wood PJ, Hett DA, Ohri SK. A prospective randomized study to evaluate stress response during beating-heart and conventional coronary revascularization. Ann Thorac Surg. 2004;78:506–512. doi: 10.1016/S0003-4975(03)01360-2. discussion 506-512. [DOI] [PubMed] [Google Scholar]
- 23.Hansen D, Linsen L, Verboven K, Hendrikx M, Rummens JL, van Erum M, Eijnde BO, Dendale P. Magnitude of muscle wasting early after on-pump coronary artery bypass graft surgery and exploration of etiology. Exp Physiol. 2015;100:818–28. doi: 10.1113/EP085053. [DOI] [PubMed] [Google Scholar]
- 24.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CPM, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 25.Aronson D, Roterman I, Yigla M, Kerner A, Avizohar O, Sella R, Bartha P, Levy Y, Markiewicz W. Inverse association between pulmonary function and C-reactive protein in apparently healthy subjects. Am J Respir Crit Care Med. 2006;174:626–632. doi: 10.1164/rccm.200602-243OC. [DOI] [PubMed] [Google Scholar]
- 26.Jiang R, Burke GL, Enright PL, Newman AB, Margolis HG, Cushman M, Tracy RP, Wang Y, Kronmal RA, Barr RG. Inflammatory markers and longitudinal lung function decline in the elderly. Am J Epidemiol. 2008;168:602–610. doi: 10.1093/aje/kwn174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schakman O, Gilson H, Thissen JP. Mechanisms of glucocorticoid-induced myopathy. J Endocrinol. 2008;197:1–10. doi: 10.1677/JOE-07-0606. [DOI] [PubMed] [Google Scholar]
- 28.Tanigaki K, Vongpatanasin W, Barrera JA, Atochin DN, Huang PL, Bonvini E, Shaul PW, Mineo C. C-reactive protein causes insulin resistance in mice through Fcgamma receptor IIB-mediated inhibition of skeletal muscle glucose delivery. Diabetes. 2013;62:721–731. doi: 10.2337/db12-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Workeneh BT, Mitch WE. Review of muscle wasting associated with chronic kidney disease. Am J Clin Nutr. 2010;91:1128S–1132S. doi: 10.3945/ajcn.2010.28608B. [DOI] [PubMed] [Google Scholar]
- 30.Polla B, D’Antona G, Bottinelli R, Reggiani C. Respiratory muscle fibres: specialisation and plasticity. Thorax. 2004;59:808–817. doi: 10.1136/thx.2003.009894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sauleda J, Gea J, Orozco-Levi M, Corominas J, Minguella J, Aguar C, Broquetas J, Agusti AG. Structure and function relationships of the respiratory muscles. Eur Respir J. 1998;11:906–911. doi: 10.1183/09031936.98.11040906. [DOI] [PubMed] [Google Scholar]
- 32.Haegens A, Schols AM, Gorissen SH, van Essen AL, Snepvangers F, Gray DA, Shoelson SE, Langen RC. NF-kappaB activation and polyubiquitin conjugation are required for pulmonary inflammation-induced diaphragm atrophy. Am J Physiol Lung Cell Mol Physiol. 2012;302:L103–110. doi: 10.1152/ajplung.00084.2011. [DOI] [PubMed] [Google Scholar]
- 33.Westerdahl E, Lindmark B, Eriksson T, Hedenstierna G, Tenling A. The immediate effects of deep breathing exercises on atelectasis and oxygenation after cardiac surgery. Scand Cardiovasc J. 2003;37:363–367. doi: 10.1080/14017430310014984. [DOI] [PubMed] [Google Scholar]
- 34.Cermak NM, Res PT, de Groot LC, Saris WH, van Loon LJ. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr. 2012;96:1454–1464. doi: 10.3945/ajcn.112.037556. [DOI] [PubMed] [Google Scholar]
- 35.Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. J Am Coll Cardiol. 2005;45:1563–1569. doi: 10.1016/j.jacc.2004.12.077. [DOI] [PubMed] [Google Scholar]


