Abstract
As one of the limited reference parameters for the appropriate timing of human chorionic gonadotropin (hCG) priming and embryo transfer (ET), peak serum estradiol (E2) level and related parameters have been considered to be a possible marker of in vitro fertilization (IVF) outcomes. To our knowledge, few reports have investigated the correlation between the ratio of peak serum E2 to the number of follicles ≥ 14 mm on the day of hCG administration (i.e., the E2/fol. ratio) and the miscarriage rate (MR) in assisted reproductive cycles. In this study, a total of 1376 cycles were examined and grouped into quartiles by E2/fol. ratio. The patient characteristics, controlled ovarian hyperstimulation (COH) performance, and IVF/ICSI results were compared between the four groups. Patients were further categorized as younger than 35 years of age or 35 years of age and older. The association between the E2/fol. ratio and the implantation rate (IR) or MR was examined using the Mantel-Haenszel test for each group. We found that the E2/fol. ratio correlated with the IR and MR for women younger than 35 years of age. There was a statistically significant increase in the IR with E2/fol. ratio between 279.83 and 552.28 pg/ml, and women with an E2/fol. ratio > 552.28 pg/ml were more likely to suffer miscarriages. Our data support a role for cryopreservation of all embryos when E2/fol. ratio exceeds 552.28 pg/ml for women younger than 35 years of age.
Keywords: E2/fol. ratio, miscarriage rate, implantation rate, ART
Introduction
Controlled ovarian hyperstimulation (COH) is important to the success of in vitro fertilization and embryo transfer (IVF-ET). In addition to the number and diameter of the developing follicles estimated with transvaginal ultrasound scan, the serum estradiol (E2) level is important to consider when adjusting the gonadotropin (Gn) dose and deciding the appropriate timing of human chorionic gonadotropin (hCG) administration. It is generally accepted that serum E2 is necessary for follicle/oocyte maturation and endometrial receptivity, but as multiple follicles develop during COH and the number of follicles varies between individuals, using serum E2 alone to evaluate the effect of COH or to predict IVF outcomes has limitations.
Because of this limitation, the ratio of peak serum E2 to the number of follicles ≥ 14 mm on the day of hCG administration (E2/fol. ratio) and the ratio of serum E2 to oocytes retrieved (E2/oocyte ratio) have received more attention in recent years. Findings have varied regarding the predictive value of E2/fol. or E2/oocyte ratio in assisted reproductive technology (ART), without resulting consensus. Orvieto et al. have found that the E2/fol. and E2/oocyte ratios could not predict IVF outcomes for normal-to-high responder patients undergoing long GnRH-agonist protocols [1], while Loumaye et al. have reported that the E2/oocyte ratio was a powerful predictor of pregnancy outcomes, specifically finding that the pregnancy rate (PR) was highest when the E2/oocyte ratio was between 70 and 140 pg/ml [2]. However, past literature has adopted implantation rate (IR) and PR as the main indices of ART outcome, while the miscarriage rate (MR) has generally not been considered.
Building on these findings, this study sought to evaluate the probable influence of E2/fol. on pregnancy outcomes, particularly the MR and IR, and to establish an optimal range of E2/fol. levels without compromising outcomes.
Materials and methods
Patients
This retrospective, non-interventional, single-center cohort study evaluated patients undergoing long gonadotropin-releasing hormone agonist (GnRH-a) protocols with IVF/intracytoplasmic sperm injection (ICSI) treatment at a reproductive medicine center in Tongji hospital between July 2011 and July 2012. A total of 1376 IVF/ICSI cycles were examined. Patients using antagonist, short, or prolonged protocols were excluded. All semen analyses of the spouses were normal. Institutional review board approval was not necessary because subjects underwent routine IVF/ICSI treatment in our center, and no additional intervention was applied.
COH protocol
All patients underwent COH using the GnRH-a long protocol. Briefly, patients received a subcutaneous injection of 0.1 mg GnRH-a (Decapeptyl, Ferring, Switzerland, or Diphereline, Ipsen, Australia) daily starting from the midluteal phase of the preceding cycle and reduced to 0.05 mg after adequate pituitary down-regulation was achieved. Serum E2 measurement and ultrasound monitoring were initiated after 14 days of downregulation. Complete pituitary suppression was confirmed by serum E2 level < 30 pg/ml and serum LH level < 3 mIU/ml. Ovarian stimulation was initiated by intramuscularly administering 150-300 IU/d recombinant follicle-stimulating hormone (FSH) (Gonal-F, Serono, Switzerland, or Puregon, Organon, Netherlands). The FSH dosage was adjusted according to the ovarian response, which was assessed by the transvaginal ultrasound and serum E2 level. Recombinant hCG (Serono, Switzerland) was administered to trigger follicle maturation when at least 2-3 follicles ≥ 18 mm in size were observed. Vaginal oocyte retrieval was performed 34-36 hours after administering hCG. ICSI was performed if the sperm quality was unexpectedly low on the day of oocyte retrieval, or if low or no fertilization had occurred in the previous cycles.
The embryos were scored according to the cleavage stage, blastomere size and shape, and fragmentation. Embryos were categorized in 4 classes. Class 1 and Class 2 embryos were considered to be high-quality embryos. Fewer than 3 embryos were transferred on day 2 or 3 after the oocyte retrieval, and the remaining high-quality embryos were cryopreserved for subsequent frozen-thawed embryo transfer (FET) cycles. Luteal phase support was provided by injecting 60 mg progesterone daily beginning the day after the oocyte retrieval.
Patient groupings
Patients in the study were grouped according to E2/fol. ratio quartiles. The patient characteristics, COH performance, and IVF/ICSI results were compared between the 4 groups.
Patients were further categorized into 2 age groups: (i) younger than 35 years of age and (ii) 35 years of age or older. The association between E2/fol. and IR or MR was examined for each group.
Pregnancy outcomes
The IR was defined as the number of gestational sacs observed on ultrasound scan 5-7 weeks after transfer divided by the number of embryos transferred. An ongoing pregnancy was defined as a pregnancy with a fetal heartbeat observed on ultrasound after 12 weeks of gestation. A miscarriage was defined as a clinical pregnancy that did not result in a delivery.
Statistical analysis
The Shapiro-Wilks test was used to evaluate the distribution of the data. Continuous data with a normal distribution was reported as the mean and standard deviation (SD). Data with non-normal distribution was presented as the median and range. Differences between the mean values were tested using a one-way analysis of variance (ANOVA). Differences between proportions were evaluated with the chi-square test and Fisher’s exact test.
To avoid introducing bias by assuming that any correlation between the serum E2/fol. ratio and IR or MR may be linear, patients were divided into 8 distinct groups according to serum E2/fol. ratio on the day of the hCG administration: < 279.83, 279.83-337.23, 337.23-381.26, 381.26-426.08, 426.08-483.59, 48-3.59-552.28, 552.28-636.58, and > 636.58 pg/ml. The IR and MR were calculated for each E2/fol. ratio interval group. The trend analyses of the data were assessed using the Mantel-Haenszel test which can be used to estimate the common odds ratio (OR) and to test whether the overall degree of association is significant. To identify any detrimental effect of the E2/fol. ratio threshold on pregnancy outcomes, the OR value and 95% confidence interval (CI) for the IR and MR in each E2/fol. ratio interval were calculated using the lowest ratio group as a comparison.
A P value < 0.05 was considered to be statistically significant. All analyses were conducted using SPSS (Statistical Package for the Social Sciences) version 13.0 software (SPSS Inc.).
Results
Of the 1376 IVF/ICSI cycles included in the analysis (IVF; n = 1253; ICSI; n = 123), 292 cycles were cancelled (cancellation rate, 21.2%) either because no embryo was available for transfer (29 cycles), the number of retrieved oocytes was larger than or equal to 20 (170 cycles), high serum progesterone levels (87 cycles), or for other reasons (6 cycles). The average patient age was 30.9 years (range, 20-49 years).
The clinical characteristics and outcomes of the four groups are presented in Table 1, which demonstrates that the IR for cycles with an E2/fol. ratio < 337.23 pg/ml was lower than those with an E2/fol. ratio between 337.23 and 426.08 pg/ml, between 426.08 and 552.28 pg/ml, or ≥ 552.28 pg/ml (25.86%, vs. 31.47%, 33.5-8%, and 27.58%, respectively, P = 0.022). Concurrently, the MR for cycles with an E2/fol. ratio ≥ 552.28 pg/ml was higher than those with an E2/fol. ratio < 337.23 pg/ml, between 337.23 and 426.08 pg/ml, or between 426.08 and 552.28 pg/ml (22.90% vs. 12.59%, 7.10%, and 13.41%, respectively, P = 0.001). The groups did not differ significantly with regards to the patient age, duration of infertility, cycle D3 FSH, number of follicles ≥ 14 mm, and number of available embryos, number of D3 high-quality embryos, ongoing pregnancy rate, or ectopic pregnancy rate.
Table 1.
Cycle parameter outcomes of variables, by quartile of E2/fol levels
| Parameter | Quartile of E2/fol levels | P value | |||
|---|---|---|---|---|---|
|
| |||||
| 1 (Lowest) | 2 | 3 | 4 (Highest) | ||
| E2/fol (pg/ml) | < 337.23 | 337.23-426.08 | 426.08-552.28 | ≥ 552.28 | / |
| No. of cycles | 305 | 357 | 356 | 358 | / |
| Mean age (years) | 30.7 ± 4.9 | 31.0 ± 4.7 | 31.1 ± 4.7 | 30.8 ± 4.5 | NS |
| BMI (kg/m2) | 22.12 ± 3.09 | 21.44 ± 2.81* | 21.19 ± 2.75* | 20.69 ± 2.44* | < 0.001 |
| Duration of infertility (years) | 4.93 ± 3.78 | 4.63 ± 3.70 | 4.57 ± 3.43 | 4.67 ± 3.45 | NS |
| Cycle D3 FSH (mIU/mL) | 7.02 ± 2.51 | 6.94 ± 2.92 | 6.92 ± 2.37 | 6.70 ± 2.18* | NS |
| E2 (pg/mL) | 3049 ± 1541 | 4232 ± 1825* | 5135 ± 2270* | 7061 ± 3246* | < 0.001 |
| No. of follicles with diameter > 14 mm | 11.27 ± 4.95 | 11.12 ± 4.76 | 10.64 ± 4.61 | 10.49 ± 4.76 | NS |
| No. of oocytes retrieved | 12.19 ± 6.73 | 12.50 ± 6.36 | 12.67 ± 6.39 | 13.86 ± 7.94* | 0.01 |
| No. of fertilised oocytes | 7.75 ± 4.88 | 8.01 ± 4.81 | 7.90 ± 4.47 | 8.79 ± 5.74* | 0.033 |
| No. of cleavage embryos | 7.73 ± 4.89 | 8.01 ± 4.81 | 7.90 ± 4.48 | 8.78 ± 5.75* | 0.032 |
| No. of available embryos | 4.43 ± 2.83 | 4.46 ± 2.79 | 4.32 ± 2.68 | 4.77 ± 3.11 | NS |
| No. of D3 high-quality embryos | 6.00 ± 4.47 | 5.83 ± 4.36 | 5.99 ± 4.11 | 6.63 ± 5.13 | NS |
| No. of transferred embryos | 1.63 ± 0.78 | 1.58 ± 0.80 | 1.57 ± 0.85 | 1.40 ± 0.94 | 0.002 |
| No. of implantation embryos | 0.68 ± 0.42 | 0.72 ± 0.50 | 0.74 ± 0.53 | 0.64 ± 0.39 | 0.029 |
| Implantation rate (%) | 25.86 | 31.47 | 33.58 | 27.58 | 0.022 |
| Miscarriage rate (%) | 12.59 | 7.10 | 13.41 | 22.90 | 0.001 |
| Ectopic pregnancy rate (%) | 3.50 | 4.14 | 1.68 | 2.29 | NS |
Data are presented as number (percentage) for categorical data, and mean ± standard deviation for parametrically distributed data. Differences between mean values were tested with one-way ANOVA. Differences between proportions were evaluated with the chi-square test.
The mean difference is significant (P < 0.05) when using the lowest quartile group as comparison.
The overall trends of positive correlation between IR or MR and E2/fol. ratio were further illustrated in Figure 1. As shown in the figure, the MR decreased with an elevated E2/fol. ratio when the E2/fol. ratio value was < 381.26 pg/ml and sharply increased with an elevated ratio when its value was > 552.28 pg/ml. IR increased with elevated E2/fol. ratio when its value was > 279.83 pg/ml, plateaued when the E2/fol. ratio ranged from 337.23 to 552.28 pg/ml, and then declined when the E2/fol. ratio was > 552.28 pg/ml.
Figure 1.
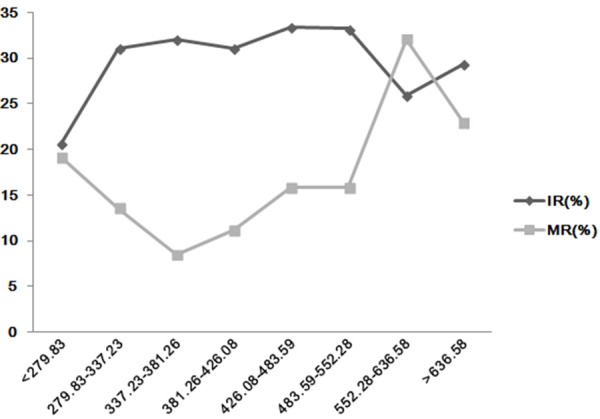
The Relationship between the IR or MR and peak E2/fol. ratio of the ART cycles.
To find the specific range of E2/fol. ratio which could be detrimental to IVF outcomes, the patients were divided into 2 age groups: younger than 35 years of age and 35 years of age and older. Figure 2A and 2B displayed the ORs for the IR and MR, respectively, for each of the serum E2/fol. ratio groups, using the lowest ratio group among patients younger than 35 years of age (n = 1082) as a comparison. As shown in Figure 2A, when the E2/fol. ratio was between 279.83 and 552.28 pg/ml, there was a statistically significant increase in the IR. When the E2/fol. ratio was > 552.28 pg/ml, there was a reduced IR; however, this difference was not statistically significant. Figure 2B displays an increased MR when the E2/fol. ratio was > 552.28 pg/ml. Therefore, an E2/fol. ratio of 552.28 pg/ml may represent the threshold concentration, above which there was a negative effect of the E2/fol. ratio on pregnancy outcomes for patients younger than 35 years of age. Patients with low (< 552.28 pg/ml) or high (> 552.28 pg/ml) ratio levels were similar in terms of age, infertility type, duration of infertility, cycle D3 FSH, and number of embryos transferred. However, compared with the high E2/fol. ratio group, the low ratio group had more oocytes retrieved, more available embryos, and more D3 high-quality embryos (Table S1).
Figure 2.
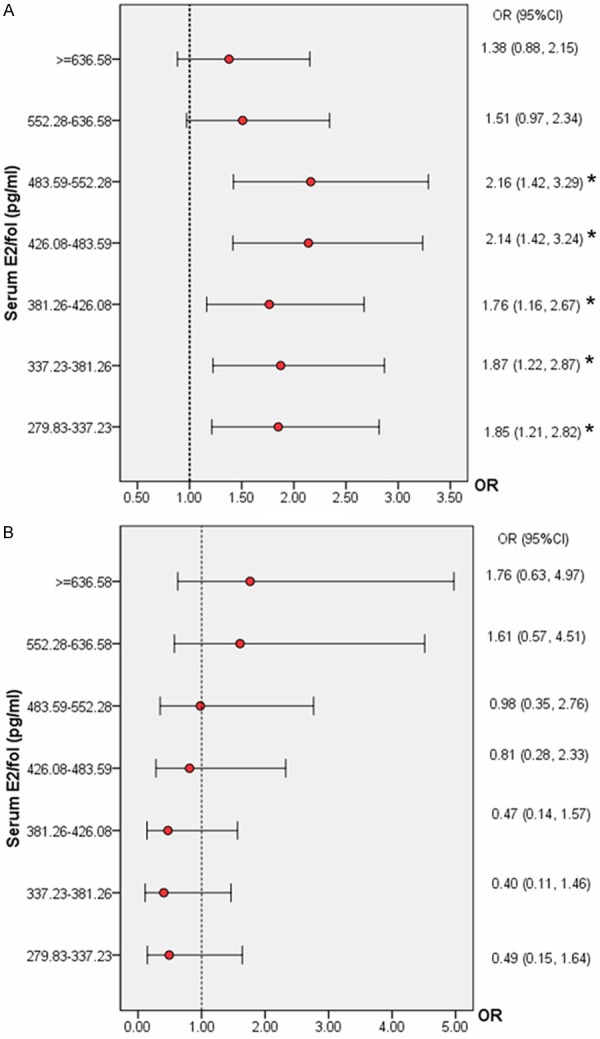
A. The IR according to the serum E2/fol. ratio levels in women younger than 35 years of age. Data are expressed as the OR (95% CI) for each interval using the lowest ratio group (< 279.83 pg/ml) as a comparison. *P < 0.05 for comparison with the lowest E2/fol. ratio interval. B. The MR according to the serum E2/fol. ratio levels in women younger than 35 years of age. Data are expressed as the OR (95% CI) for each interval using the lowest ratio group (< 279.83 pg/ml) as a comparison. *P < 0.05 for comparison with the lowest E2/fol. ratio interval.
Figure 3A and 3B displayed the ORs for the IR and MR, respectively, in each of the serum E2/fol. ratio groups using the lowest E2/fol. ratio group among patients 35 years of age and older (n = 294) as a comparison. There was no similar threshold concentration with significance for IVF outcomes. Patients with either low (< 552.28 pg/ml) or high (> 552.28 pg/ml) ratio levels were similar with regards to age, infertility type, duration of infertility, cycle D3 FSH, duration of gonadotropin administration, number of follicles with diameter > 14 mm, and embryos transferred. Additionally, compared with the high E2/fol. ratio group, the low ratio group had more oocytes retrieved, more available embryos, and more day 3 high-quality embryos (Table S2).
Figure 3.
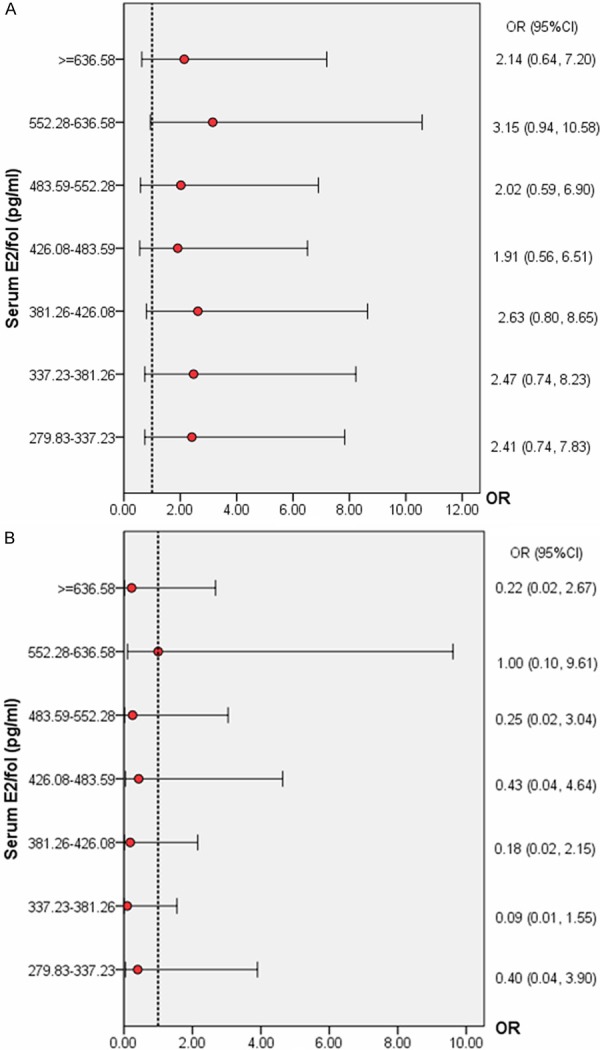
A. IR according to serum E2/fol. ratio levels in women older than 35 years of age. Data are expressed as the OR (95% CI) for each interval, using the lowest ratio group (< 279.83 pg/ml) as a comparison. *P < 0.05 for comparison with the lowest E2/fol. ratio interval. B. MR according to serum E2/fol. ratio levels in women older than 35 years of age. Data are expressed as the OR (95% CI) for each interval, using the lowest ratio group (< 279.83 pg/ml) as a comparison. *P < 0.05 for comparison with the lowest E2/fol. ratio interval.
Discussion
In this cohort study, the E2/fol. ratio correlated with the IR and MR for patients under the age of 35 years. Although it is generally accepted that a live birth was the ultimate aim for patients seeking fertility treatments, miscarriage has become a major challenge to positive IVF outcomes, and the live birth rate remained around 30% [3,4]. In our study, the MR following ART treatment was 17.11%, similar to previous findings. As a pilot study, we found a statistically significant increase in the IR with an E2/fol. ratio between 279.83 and 552.28 pg/ml (Figure 2A). Additionally, the E2/fol. ratio was strongly correlated with the risk of miscarriage, and patients younger than 35 years of age with cycles with an E2/fol. ratio > 552.28 pg/ml were more likely to suffer miscarriages (Figure 2B).
This study has some major strength. Compared with ultrasound examination, the use of the serum E2 level in COH to predict impending ovulation has been debated. The conflict stems from the fact that the recruitment of various cohorts of follicles at different developmental stages in the same cycle, with various degrees of steroidogenic capacity may result in asynchrony of follicle growth and estrogen secretion. Despite this fact, as the reference parameters for appropriate timing of hCG priming and ET are limited, researchers have sought to use serum E2 level and related parameters as a predictor of IVF/ICSI outcomes. To our knowledge, few reports have demonstrated the correlation between E2/fol. ratio and MR, which ranged from 10% to 20% [3]. Moreover, to remove the variable of partner fertility, we only included the cases with normal spousal semen analysis. In addition, as a lower PR and a higher MR most commonly occurred in IVF cycles in patients older than 35 years, with outcomes worsening in patients older than 40 years [5,6], the cases were further divided into patients younger than 35 years of age, and patients 35 years of age and older. In this pilot study, the small sample size of patients aged 35 years and older is the limitation of our findings. Thus, further study is warranted to examine the association between the E2/fol. ratio and the IR or MR.
An elevated E2/fol. ratio was associated with a higher serum E2 level, which may have affected oocyte maturation and compromised endometrial receptivity. As demonstrated in Tables S1, S2, for the different age groups, the low E2/fol. ratio group had more oocytes retrieved, more available embryos and more D3 high-quality embryos when compared with the high ratio group. This result demonstrates that pregnancy outcomes do not improve with unlimited increases in serum E2 and that control of serum E2 levels may decrease the occurrence of COH complications, such as OHSS. However, FET has been highly praised in recent years. Ubaldi et al [7] and Fauque et al [8] have suggested that FET may increase the cumulative pregnancy rate per patient, a finding that is supported by Lundin et al [9], who reported that FET improved the cumulative live birth rate per patient.
These reports suggest that FET cycles achieved better embryo-endometrial synchrony when compared with fresh ET cycles. In recent years, researchers have also reported that neonatal outcomes and birth defects after cryopreservation were similar or even improved when compared to fresh ET [10-12]. Moreover, our large cohort study demonstrated that FET cycles were associated with a statistically significantly lower risk of ectopic pregnancy when compared with fresh cycles [13].
In conclusion, our data suggest that for patients younger than 35 years of age, the cryopreservation of all embryos when the E2/fol. ratio > 552.28 pg/ml may be warranted. Our findings may be most helpful to clinicians counseling patients regarding the influence of serum E2 level on the timing of ET and pregnancy outcomes. For patients older than 35 years of age, however, we did not find a similar threshold value for the E2/fol. ratio and maximal IR or MR optimization, which may have been caused by our limited sample size of patients older than 35 years of age.
Acknowledgements
The authors thank H.G. (School of Public Health, Tongji Medicine College, Huazhong University of Science and Technology, China) for the suggestion of statistical methods.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Orvieto R, Zohav E, Scharf S, Rabinson J, Meltcer S, Anteby EY, Homburg R. The influence of estradiol/follicle and estradiol/oocyte ratios on the outcome of controlled ovarian stimulation for in vitro fertilization. Gynecol Endocrinol. 2007;23:72–5. doi: 10.1080/09513590601137137. [DOI] [PubMed] [Google Scholar]
- 2.Loumaye E, Engrand P, Howles CM, O’Dea L. Assessment of the role of serum luteinizing hormone and estradiol response to follicle-stimulating hormone on in vitro fertilization treatment outcome. Fertil Steril. 1997;67:889–99. doi: 10.1016/s0015-0282(97)81402-1. [DOI] [PubMed] [Google Scholar]
- 3.Assisted reproductive technology in the United States: 2000 results generated from the American Society for Reproductive Medicine/Society for Assisted Reproductive Technology Registry. Fertil Steril. 2004;81:1207–20. doi: 10.1016/j.fertnstert.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Andersen AN, Gianaroli L, Felberbaum R, de Mouzon J, Nygren KG. Assisted reproductive technology in Europe, 2002. Results generated from European registers by ESHRE. Hum Reprod. 2006;21:1680–97. doi: 10.1093/humrep/del075. [DOI] [PubMed] [Google Scholar]
- 5.Assisted reproductive technology in the United States: 1999 results generated from the American Society for Reproductive Medicine/Society for Assisted Reproductive Technology Registry. Fertil Steril. 2002;78:918–31. doi: 10.1016/s0015-0282(02)04198-5. [DOI] [PubMed] [Google Scholar]
- 6.Chuang CC, Chen CD, Chao KH, Chen SU, Ho HN, Yang YS. Age is a better predictor of pregnancy potential than basal follicle-stimulating hormone levels in women undergoing in vitro fertilization. Fertil Steril. 2003;79:63–8. doi: 10.1016/s0015-0282(02)04562-4. [DOI] [PubMed] [Google Scholar]
- 7.Ubaldi F, Rienzi L, Baroni E, Ferrero S, Iacobelli M, Minasi MG, Sapienza F, Martinez F, Anniballo R, Cobellis L, Tesarik J, Greco E. Cumulative pregnancy rates after transfer of fresh and thawed embryos. Eur J Obstet Gynecol Reprod Biol. 2004;115(Suppl 1):S106–9. doi: 10.1016/j.ejogrb.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 8.Fauque P, Jouannet P, Davy C, Guibert J, Viall- on V, Epelboin S, Kunstmann JM, Patrat C. Cumulative results including obstetrical and neonatal outcome of fresh and frozen-thawed cycles in elective single versus double fresh embryo transfers. Fertil Steril. 2010;94:927–35. doi: 10.1016/j.fertnstert.2009.03.105. [DOI] [PubMed] [Google Scholar]
- 9.Lundin K, Bergh C. Cumulative impact of adding frozen-thawed cycles to single versus double fresh embryo transfers. Reprod Biomed Online. 2007;15:76–82. doi: 10.1016/s1472-6483(10)60695-5. [DOI] [PubMed] [Google Scholar]
- 10.Wennerholm UB, Soderstrom-Anttila V, Bergh C, Aittomaki K, Hazekamp J, Nygren KG, Selbing A, Loft A. Children born after cryopreservation of embryos or oocytes: a systematic review of outcome data. Hum Reprod. 2009;24:2158–72. doi: 10.1093/humrep/dep125. [DOI] [PubMed] [Google Scholar]
- 11.Kato O, Kawasaki N, Bodri D, Kuroda T, Kawachiya S, Kato K, Takehara Y. Neonatal outcome and birth defects in 6623 singletons born following minimal ovarian stimulation and vitrified versus fresh single embryo transfer. Eur J Obstet Gynecol Reprod Biol. 2012;161:46–50. doi: 10.1016/j.ejogrb.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Maheshwari A, Kalampokas T, Davidson J, Bhattacharya S. Obstetric and perinatal outcomes in singleton pregnancies resulting from the transfer of blastocyst-stage versus cleavage-stage embryos generated through in vitro fertilization treatment: a systematic review and meta-analysis. Fertil Steril. 2013;100:1615–21. doi: 10.1016/j.fertnstert.2013.08.044. [DOI] [PubMed] [Google Scholar]
- 13.Huang B, Hu D, Qian K, Li Y, Jin L, Zhu G, Zhang H. Is frozen embryo transfer cycle associated with significantly lower incidence of ectopic pregnancy? An analysis of more than 30,000 cycles. Fertil Steril. 2014;102:1345–9. doi: 10.1016/j.fertnstert.2014.07.1245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


