Abstract
Background: Portal hypertension is one of the death reasons for the liver cirrhosis patients. The oxidative stress is related to the occurrence and development of portal hypertension in cirrhosis. Malondialdehyde (MDA), one of the lipid peroxides, increases substantially in cirrhotic patients. Aims: To evaluate the relevance between the MDA level and portal hypertension in cirrhotic patients. Methods: 60 liver cirrhotic patients and 30 healthy controls were enrolled. The plasma MDA level and general blood tests including ALT, AST, ALB, total bilirubin, and platelet were measured. All people enrolled accepted endoscopic examination and B-Ultrasound check to evaluate the severity of portal hypertension. Results: The MDA plasma level of cirrhotic patients was significantly higher than the controls (P<0.001) and increased significantly accompanied by the severity of liver fibrosis and portal hypertension (P<0.01). Further, the plasma MDA level of cirrhotic patients was significantly correlated with Child-Pugh classification of cirrhosis (r=0.820, P<0.001), the degree of esophageal varices (r=0.857, P<0.001) and the width of portal vein (r=0.652, P<0.001). The ROC curve analyses showed that the plasma MDA level is a strong predictor of liver cirrhosis and portal hypertension. Conclusions: Plasma MDA level may correlate with the severity of portal hypertension in cirrhotic patients.
Keywords: Malondialdehyde (MDA), esophageal varices, portal hypertension, liver cirrhosis
Introduction
Portal hypertension is one of the major occurrence and death reasons of patients with liver cirrhosis. The major pathophysiology of portal hypertension is the rising of the portal vein blood flow resistance which is partly caused by the liver structure alteration and the blood structure alteration as well. Increased oxidative stress aggravates liver fibrosis in various pathogenesis during chronic liver injury [1]. In the progress of cell necrosis, inflammation and death, oxidative stress response is integrated with the protein, damaging DNA, reacting with the cell chemical composition to cause the cell damage [2,3]. In addition, the researchers found out that the increased oxidative stress response is due to the angiotensin alteration in hypertension [4,5], atherosis [6] and diabetes [7].
Malondialdehyde (MDA), a typical aldehydic product of lipid peroxidation, results from lipid peroxidation of polyunsaturated fatty acids [8]. The degree of lipid peroxidation can be estimated by the amount of MDA in tissues and it is a marker for oxidative stress. MDA had been proved to be up-expression significantly in cirrhotic patients [9]. At present, the relationship between oxidative stress and portal hypertension in the liver cirrhotic patients is still undefined. The aim of our study was to characterize the relations between liver cirrhosis patients’ oxidative stress response and liver function, portal vein hypertension and esophageal varices and to preliminarily reveal the potential role of MDA as a new non-invasive diagnostic method in cirrhotic patients with portal hypertension.
Methods
Patients selection
The study was performed in the Division of Gastroenterology and Digestive Diseases Institute, Tongji Hospital (Shanghai, China). Between 2009 and 2011, a total of 60 in-hospital patients diagnosed with virus-related chronic liver disease were enrolled consecutively in the study, including 34 male and 26 female patients aged from 34-87 years (average age was 63.22±15.86 years). All patients enrolled were diagnosed with esophageal varices by endoscopic examination previously. Among them, 54 were HBV-related cirrhosis, 6 were HCV-related cirrhosis. Diagnosis of hepatitis B and C virus was made by the laboratory examination and the diagnosis of cirrhosis was made by the medical history, body check, lab and iconography check. 12 of these patients (20%) were confirmed the diagnosis of cirrhosis by liver biopsy. Exclusion criteria included the presence of hepatocellular carcinoma, presence of active infection, presence of esophageal varices bleeding with splenectomy, use of vasoactive and anti-oxygen agent within a week, history of severe heart disease patients and kidney disease, abuse of alcoholic and refusal to participate.
30 healthy controls were age- and sex- matched with the cirrhotic patients who were enrolled for measurement of general blood tests, plasma level of MDA, B-Ultrasound Check and endoscopic examination. None of these subjects had a history of major systemic diseases. All subjects agreed to participate in the study and were aware of its content.
Endoscopic examination
All endoscopic examinations were completed by special experienced endoscope technicians, and the gastroscopic instrument was OLYMPU326O. Esophageal varices endoscopic diagnosis was done per the esophageal varices endoscopic classification criteria, “The Diagnosis and Treatment Solution of Endoscopic Esophageal Varices 2003”, which was worked out by Chinese Medical Association Digestion Endoscopic Branch in October 2003. According to the criteria, the degree of esophageal varices was defined as mild, moderate and severe. Mild: The esophageal varices are a straight line or a little bit curved with no red color. Moderate: the esophageal varices are a straight line or a little bit curved, red color feature, or the esophageal varices is snake-like curved and evection but not red color feature. Severe: esophageal varices has snake-like curve and evection with red color feature or esophageal varices has a ball-like curve, joint--like curve or tumor-like curve (whether red color or not).
B-Ultrasound check
B-Ultrasound Check was completed by assigned experienced ultrasonic technicians. All patients and healthy controls accepted B ultrasonic check after 12 h limosis, regularly scanning the liver, gallbladder, spleen, pancreas and kidney. The ultrasonic technicians determined if the patients have liver cirrhosis or ascites and measured the portal vein inner diameter during the deep breath. The diameter measured wider than 13 mm or above was defined as portal hypertension.
Clinical biochemistry
The test indexes of clinical Biochemistry when the patients and healthy control admitted by hospital contained several parameters including limosis blood alanine aminotransferase (ALT), aspartate aminotransferase (AST), r-glutamylacylase (γ-GT), albumin (ALB), total bilirubin (T-BIL) and platelet (PLT).
Evaluation the severity of liver cirrhosis
The severity of liver cirrhosis was established according to Pugh’s modification of the Child classification [10]. According to the score, the cirrhotic patients were divided into 3 grades: A, B and C.
Plasma MDA level measurement
The plasma MDA concentration was measured by a kit based on thiobarbituric acid (TBA) reactivity (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). After mixing trichloroacetic acid with the sample and centrifuging, a supernatant was obtained, and TBA was added. The absorbance of the sample was measured at 532 nm with a spectrophotometer. MDA concentration was calculated according to the formula provided in the protocols.
Statistical analysis
Statistical analysis was performed by using the SPSS 17.0 statistical package (SPSS Inc., Chicago, IL). All results are expressed as mean ± standard deviation (SD). Statistical significance of differences between groups was analyzed by using one-way ANOVA with an unpaired Student’s t-test. Correlations between continuous parameters were assessed by using Pearson’s correlation analysis and correlations between discontinuous parameters were assessed by using Spearman correlation analysis. Receiver operating characteristic (ROC) curve analyses were used to determine the diagnostic sensitivity and specificity of MDA for distinguishing cirrhotic patients from healthy controls and cirrhotic patients with or without portal vein dilation. Differences of P<0.05 were considered statistically significant.
Results
We used a compound model to evaluate the severity of liver fibrosis through the following parameters: Child-Pugh classification, Clinical Biochemistry, the degree of esophageal varices and the width of portal vein. Demographic and clinical data are summarized in Table 1. The cirrhotic patients had significant higher level of ALT, AST, GGT and total bilirubin than those of the healthy controls (P<0.05) and lower level of PLT as well (P<0.01). The average width of portal vein of cirrhotic patients was 13 mm, much wider than the controls (P<0.01). Meanwhile, the plasma MDA level of cirrhotic patients was significantly higher than the controls as well (P<0.001) (Figure 1A).
Table 1.
Demographic and clinical data
| Control (n=30) | Cirrhosis (n=60) | |
|---|---|---|
| Sex (male:female) | 18:12 | 36:24 |
| Age (years) | 65±10 | 64±12 |
| Child (A:B:C) | ∕ | 21:22:17 |
| Degree of gastro-esophageal varices (mild: moderate: severe) | ∕ | 23:15:22 |
| Width of portal vein (mm) | 8.4±2.3 | 13.0±2.8** |
| Ascites volume (abcent: mild: moderate: severe) | ∕ | 30:16:12:4 |
| ALT (U/L) | 30±8 | 46±20* |
| AST (U/L) | 22±12 | 45±18* |
| GGT (U/L) | 24±8 | 42±16* |
| MDA (nM) | 288±138 | 673±301** |
| Total bilirubin (µmol/L) | 14.2±6.4 | 35.6±10.5** |
| PLT (×109/L) | 202±54 | 76±36** |
All data expressed as mean ± SD.
P<0.05 versus the healthy controls;
P<0.01 versus the healthy controls.
Figure 1.
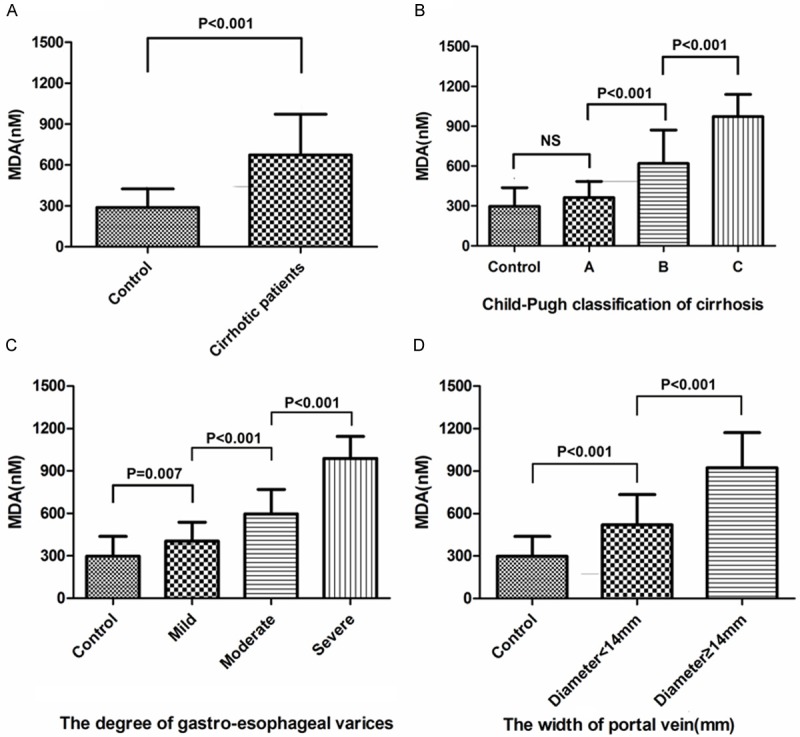
A. The plasma MDA level of cirrhotic patients was significantly higher than the controls as well (P<0.001). B-D. The plasma MDA level increased significantly accompanied by the Child-Pugh classification, the degree of esophageal varices and the width of portal vein (P<0.01). Statistical comparisons were performed using one-way ANOVA with an unpaired Student’s t-test.
We next divided the cirrhotic patients into different subgroups according to the Child-Pugh classification, the degree of esophageal varices and the width of portal vein, and then compared the MDA level. The result showed that plasma MDA level increased significantly accompanied by the severity of liver fibrosis and portal hypertension (P<0.01) (Figure 1B-D).
Further, the correlation of plasma MDA level with the Child-Pugh classification of cirrhosis, the degree of esophageal varices and the width of portal vein was performed to identify which factors had relevance with MDA in cirrhotic patients. The plasma MDA level were significantly correlated with the three factors stated above (r=0.820, P<0.001; r=0.857, P<0.001; r=0.652, P<0.001, respectively) (Figure 2A-C), suggesting strong relevance between them.
Figure 2.
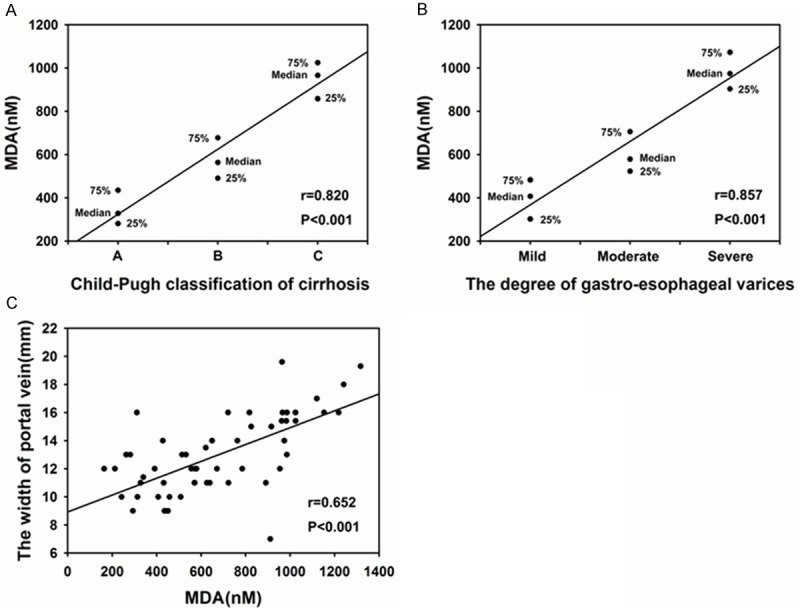
Correlation of plasma MDA level with the Child-Pugh classification (A), the degree of esophageal varices (B) and the width of portal vein (C) in cirrhotic patients. Correlations between continuous parameters were assessed by using Pearson’s correlation analysis and correlations between discontinuous parameters were assessed by using Spearman correlation analysis.
The ROC curve analyses are shown in Figure 3 (sensitivity versus 1-specificity). The cutoff value of plasma MDA to separate cirrhotic patients from the control was 426.5 nM, and the sensitivity and specificity was 78.2%, 86.2%, respectively (Figure 3A). However, the cutoff value of plasma MDA to separate the cirrhotic patients with portal vein dilation (the width of portal vein >13 mm, suggesting portal hypertension) was 696.0 nM and the sensitivity and specificity was 82.4%, 85.8%, respectively (Figure 3B).
Figure 3.
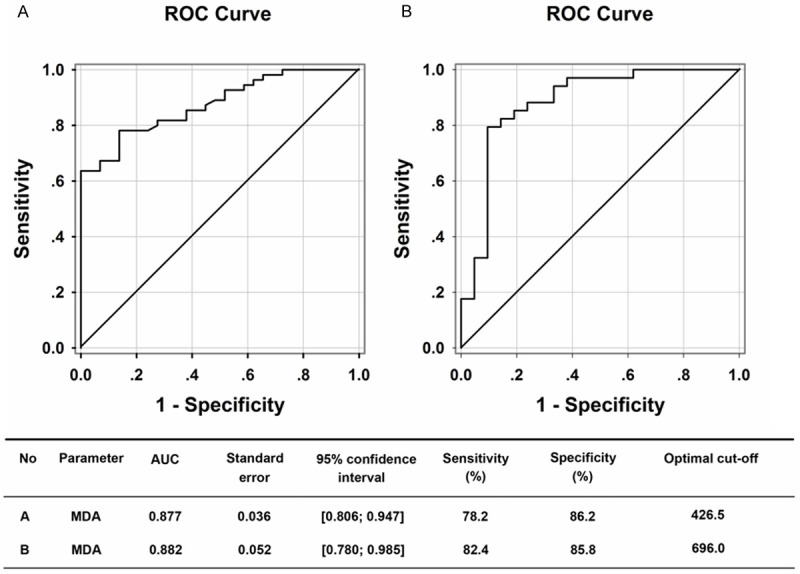
Receiver operating characteristic (ROC) curve and optimal cutoff levels of MDA for distinguishing cirrhotic patients from healthy controls (A) and cirrhotic patients with or without portal vein dilation (the width of portal vein >13 mm, suggesting portal hypertension) (B).
Discussion
Oxidative stress (OS) refers to a condition under which organism or cell reactive oxygen species (ROS) are excessively produced and the antioxidant defense function is weakened, which causes big imbalance and damage the organism cell [11]. Portal hypertension is one of the common and serious complications of chronic liver disease which is caused by intrahepatic vascular resistance and blood increase [1]. It had been proved that oxidative stress response speed up the development of liver fibrosis and lead to the initiation of the hepatic epithelioid malfunction, which is adjusted by the bioavailability of NO in the intrahepatic microcirculation [12]. During the portal hypertension and BDL, antioxidant treatment reduced the portal hypertension and improved the dynamic circulation in the experimental rats, suggesting that liver cirrhotic oxidative stress promotes the development of portal hypertension and dynamic circulation [13,14]. Since no clinical proof support the relationship between oxidative stress and portal hypertension so far, our study was focus on the aspect.
In this study, it was found out that the plasma MDA level of cirrhotic patients is higher than that of the control. The result was accordance with the previous researches that the virus-related cirrhotic patients have higher oxidant MDA level [1,15] and suggested that more oxidants stress and weaker antioxidants protection exist in the cirrhotic patients than the control cohort. We then defined the severity of liver cirrhosis under the criteria of Pugh’s modification of the Child classification as grade A, B and C. The plasma MDA level increased significantly in the patients of grade B and C, suggesting that MDA level may increase in the physiological process of cirrhosis gradually. Hepatic fibrosis oxidative stress is an important signal of HSC activation and disease development, however, oxidative stress is indispensable of external and internal HSC activation [16-18]. Oxidative stress can result in chondriosome and microsome damage [19,20], and block the breath link to pass the electron while any block on the breath link will increase the ROS production and the increased ROS production will further increase oxygenation lipid sediment and block the electron pass, thus forms a vicious circle [19]. The other vicious circle is the consumption of antioxidant materials [21]. The increased plasma MDA level may partly due to the increased oxygenation lipid during the process of liver cirrhosis.
Further, our study discovered that cirrhotic patients’ MDA level have positive relativity with the width of main portal vein and esophageal varices degree. The result demonstrated that the variation of MDA level relate to the severity of portal hypertension synchronously. The vessel diameter width of venous system is positively related to the portal vein pressure in portal hypertension caused by cirrhosis, and the main vessel width represents the portal pressure to some extent. Almost all the cirrhotic patients have side portal vein circulation when portal hypertension appears. MDA can restrain acetylcholine activation of endothelium dependent dilatation [22], meanwhile, NO plays key roles in the high pressure circulation and portal hypertension development in the development of cirrhosis [23]. Plasma MDA level may be considered as one of the independent factors to predict the liver venous vein pressure, due to the interaction NO and MDA in cirrhotic patients.
In addition, the high plasma MDA level of the cirrhotic patients may be caused by the increase of enterogenous endotoxin translocation [24]. Endotoxemia enhances the release of MDA and increases intrahepatic resistance, as a result, increases the portal vein pressure eventually [25,26].
In conclusion, oxidative stress not only promotes the development of liver fibrosis, but also changes the liver cirrhosis patient’s haemodynamics. The increased plasma MDA level may strongly correlate with hemodynamic disorder and portal hypertension in cirrhotic patients. The result can lead MDA into a new non-invasive diagnostic marker of portal hypertension in cirrhotic patients.
Acknowledgements
This work was supported by grants from The National Natural Science Foundation of China (No. 81070343), Shanghai Innovation Program (No. 12431901002) and the liver disease research fundation of Tianqing (No. TQGB20140148).
Disclosure of conflict of interest
None.
References
- 1.Parola M, Robino G. Oxidative stress-related molecules and liver fibrosis. J Hepatol. 2001;35:297–306. doi: 10.1016/s0168-8278(01)00142-8. [DOI] [PubMed] [Google Scholar]
- 2.Boonstra J, Post JA. Molecular events associated with reactive oxygen species and cell cycle progression in mammalian cells. Gene. 2004;337:1–13. doi: 10.1016/j.gene.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 3.Kunsch C, Medford RM. Oxidative stress as a regulator of gene expression in the vasculature. Circ Res. 1999;85:753–66. doi: 10.1161/01.res.85.8.753. [DOI] [PubMed] [Google Scholar]
- 4.Touyz RM. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: what is the clinical significance? Hypertension. 2004;44:248–52. doi: 10.1161/01.HYP.0000138070.47616.9d. [DOI] [PubMed] [Google Scholar]
- 5.Paravicini TM, Touyz RM. Redox signaling in hypertension. Cardiovasc Res. 2006;71:247–58. doi: 10.1016/j.cardiores.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Schulze PC, Lee RT. Oxidative stress and atherosclerosis. Curr Atheroscler Rep. 2005;7:242–8. doi: 10.1007/s11883-005-0013-5. [DOI] [PubMed] [Google Scholar]
- 7.Dixon LJ, Hughes SM, Rooney K, Madden A, Devine A, Leahey W, Henry W, Johnston GD, McVeigh GE. Increased superoxide production in hypertensive patients with diabetes mellitus: role of nitric oxide synthase. Am J Hypertens. 2005;18:839–43. doi: 10.1016/j.amjhyper.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Davey MW, Stals E, Panis B, Keulemans J, Swennen RL. High-throughput determination of malondialdehyde in plant tissues. Anal Biochem. 2005;347:201–7. doi: 10.1016/j.ab.2005.09.041. [DOI] [PubMed] [Google Scholar]
- 9.Jain SK, Pemberton PW, Smith A, McMahon RF, Burrows PC, Aboutwerat A, Warnes TW. Oxidative stress in chronic hepatitis C: not just a feature of late stage disease. J Hepatol. 2002;36:805–11. doi: 10.1016/s0168-8278(02)00060-0. [DOI] [PubMed] [Google Scholar]
- 10.Pagliaro L. MELD: the end of Child-Pugh classification? J Hepatol. 2002;36:141–2. doi: 10.1016/s0168-8278(01)00302-6. [DOI] [PubMed] [Google Scholar]
- 11.Catapano AL, Maggi FM, Tragni E. Low density lipoprotein oxidation, antioxidants, and atherosclerosis. Curr Opin Cardiol. 2000;15:355–63. doi: 10.1097/00001573-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez-Vilarrupla A, Bosch J, Garcia-Pagan JC. Potential role of antioxidants in the treatment of portal hypertension. J Hepatol. 2007;46:193–7. doi: 10.1016/j.jhep.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Yang YY, Lee KC, Huang YT, Wang YW, Hou MC, Lee FY, Lin HC, Lee SD. Effects of N-acetylcysteine administration in hepatic microcirculation of rats with biliary cirrhosis. J Hepatol. 2008;49:25–33. doi: 10.1016/j.jhep.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Paradis V, Mathurin P, Kollinger M, Imbert-Bismut F, Charlotte F, Piton A, Opolon P, Holstege A, Poynard T, Bedossa P. In situ detection of lipid peroxidation in chronic hepatitis C: correlation with pathological features. J Clin Pathol. 1997;50:401–6. doi: 10.1136/jcp.50.5.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trevisani F, Caraceni P, Simoncini M, Micati M, Domenicali M, Dazzani F, Zambruni A, Stefanelli C, Grazi G, Nardo B, Guarnieri C, Bernardi M. Evidence of oxidative imbalance in long-term liver transplant patients. Dig Liver Dis. 2002;34:279–84. doi: 10.1016/s1590-8658(02)80148-7. [DOI] [PubMed] [Google Scholar]
- 16.Kim KY, Choi I, Kim SS. Progression of hepatic stellate cell activation is associated with the level of oxidative stress rather than cytokines during CCl4-induced fibrogenesis. Mol Cells. 2000;10:289–300. [PubMed] [Google Scholar]
- 17.Lee KS, Lee SJ, Park HJ, Chung JP, Han KH, Chon CY, Lee SI, Moon YM. Oxidative stress effect on the activation of hepatic stellate cells. Yonsei Med J. 2001;42:1–8. doi: 10.3349/ymj.2001.42.1.1. [DOI] [PubMed] [Google Scholar]
- 18.Apte M. Oxidative stress: does it ‘initiate’ hepatic stellate cell activation or only ‘perpetuate’ the process? J Gastroenterol Hepatol. 2002;17:1045–8. doi: 10.1046/j.1440-1746.2002.02845.x. [DOI] [PubMed] [Google Scholar]
- 19.Michikawa Y, Mazzucchelli F, Bresolin N, Scarlato G, Attardi G. Aging-dependent large accumulation of point mutations in the human mtDNA control region for replication. Science. 1999;286:774–9. doi: 10.1126/science.286.5440.774. [DOI] [PubMed] [Google Scholar]
- 20.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–92. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 21.Esposito LA, Melov S, Panov A, Cottrell BA, Wallace DC. Mitochondrial disease in mouse results in increased oxidative stress. Proc Natl Acad Sci U S A. 1999;96:4820–5. doi: 10.1073/pnas.96.9.4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez-Martinez MA, Martinez-Orgado J, Salaices M, Marín J. Impairment of acetylcholine relaxations by malondialdehyde, a marker of lipid peroxidation. J Vasc Res. 1996;33:463–70. doi: 10.1159/000159185. [DOI] [PubMed] [Google Scholar]
- 23.Iwakiri Y, Groszmann RJ. The hyperdynamic circulation of chronic liver diseases: from the patient to the molecule. Hepatology. 2006;43:S121–31. doi: 10.1002/hep.20993. [DOI] [PubMed] [Google Scholar]
- 24.Toh Y, Korenaga D, Maekawa S, Matsumata T, Muto Y, Ikeda T, Sugimachi K. Assessing the permeability of the gastrointestinal mucosa after oral administration of phenolsulfonphthalein. Hepatogastroenterology. 1997;44:1147–51. [PubMed] [Google Scholar]
- 25.Ramachandran A, Prabhu R, Thomas S, Reddy JB, Pulimood A, Balasubramanian KA. Intestinal mucosal alterations in experimental cirrhosis in the rat: role of oxygen free radicals. Hepatology. 2002;35:622–9. doi: 10.1053/jhep.2002.31656. [DOI] [PubMed] [Google Scholar]
- 26.Miller AM, Masrorpour M, Klaus C, Zhang JX. LPS exacerbates endothelin-1 induced activation of cytosolic phospholipase A2 and thromboxane A2 production from Kupffer cells of the prefibrotic rat liver. J Hepatol. 2007;46:276–85. doi: 10.1016/j.jhep.2006.08.026. [DOI] [PubMed] [Google Scholar]


