Abstract
Background: Systemic lupus erythematosus (SLE) is a chronic autoimmune disease. Lupus nephritis (LN) is an important cause of morbidity and even mortality in patients with SLE. Some evidences suggest that neutrophil-lymphocyte ratio (NLR) associated with different inflammatory malignancies, ischemic injury and cardiovascular disease. Few scholars have investigated the relationship between NLR and SLE. This study aims to evaluate the role of NLR in SLE without nephritis and LN patients. Methods: A total of 228 subjects were participated in this study. 79 diagnosed with SLE in patients group and 149 healthy age-and sex-matched in control group. In patient team, 20 of them were diagnosed with LN. Results: The SLE without nephritis group showed significantly higher NLR than control group (control=2.00±0.76, SLE=4.26±3.38, P<0.001), and the NLR values of the patients with LN were higher than those of the patients without LN (SLE=4.26±3.38, LN=7.21±6.01, P<0.001). Receiver-operating characteristics analysis (ROC) of NLR to predict SLE showed that the area under the curve (AUC) was 0.757. The cutoff value using the ROC curve was 3.13 (sensitivity, 0.574; specificity, 0.926; 95% confidence interval (CI), 0.668-0.845; P<0.001). While ROC analysis of NLR to predict LN showed that the AUC was 0.828). Logistic regression analysis showed that SLE without nephritis and LN were independently related to NLR. Conclusion: NLR is independently associated with SLE, and it may be a promising marker that reflects renal involvement in patients with SLE.
Keywords: Neutrophil-lymphocyte ratio, systemic lupus erythematosus, lupus nephritis
Introduction
Systemic lupus erythematosus is a chronic autoimmune disease. Most patients with SLE develop kidney disease related to this systemic underlying disease process. Lupus nephritis is the most common and severe clinical manifestation of SLE [1-3], though overall mortality has decreased remarkably in SLE patients over the last decades. Kidney failure is also the leading cause of death in these patients [2]. Thus the early diagnosis of LN is helpful for patients. Immune complex (IC) formation and deposition are the main cause of SLE kidney damage mechanism. IC can activate complement and inflammatory cell infiltration, thus cause kidney damage [4,5].
White blood cell (WBC) count is a serum marker for systemic inflammation [6]. Neutrophil-lymphocyte ratio is easily calculated by dividing the absolute neutrophil count by the absolute lymphocyte count from a complete blood count. It is simple and cheap. Many studies have shown that NLR is positively associated with inflammatory, different malignancies, ischemic injury, cardiovascular disease and diabetic nephropathy [7-13]. However, to our knowledge, the relationship between NLR and SLE as well as LN has not been well studied so far.
In this study, the aim was to evaluate the role of NLR in SLE and whether NLR is an independent predictor of SLE without nephritis and LN.
Material and methods
Study population
We retrospectively analyzed the hospital records of all patients diagnosed as SLE (N=79), 20 of whom have LN, in the Second Xiangya Hospital of central south university between August 2013 and October 2014. All of them were not treated. The control group was composed of 149 healthy age-and gender-matched subjects without any risk factors or chronic diseases. Patients who had active infection, different malignancies, acute poisoning, thrombus formation and ischemic injury, myocardial infarction, heart failure, diabetic nephropathy and renal insufficiency were excluded from the study. What is more, the patients who treated with corticosteroid and mycophenolate did not be recruited. Clinical and laboratory data were collected. Patient characteristics included age and sex, UA (Uric acid), CRE (creatinine), TG (triglycerides), total cholesterol, HDL (high-density lipoprotein cholesterol), LDL (low density lipoprotein cholesterol), WBC, neutrophils, lymphocyte, NLR, CRP (C-reactive protein), ESR (Erythrocyte Sedimentation Rate), 24 h Proteinuria, SLEDAI (Systemic Lupus Erythematosus Disease Activity Index) and RDW (red blood cell distribution width). NLR was calculated by dividing the absolute neutrophil count by the absolute lymphocyte count in a complete blood count taken before treatment.
Definition for lupus nephritis
Lupus nephritis is defined as clinical and laboratory manifestations that meet American College Of Rheumatology criteria (once the SLE diagnosis was established, and clinically persistent proteinuria >0.5 g/d or greater than 3+ by dipstick, and/or cellular casts including red cell, hemoglobin, granular, tubular or mixed). At present, the renal biopsy is the gold standard in confirming the diagnosis of LN [14].
Statistical analysis
All statistical analyses were performed using the SPSS statistical software (SPSS for Windows, version 19.0; SPSS, Inc., Chicago, IL, USA). Con-tinuous variables are presented as mean ± standard deviation (SD), and categorical variables are expressed as percentages. Student t-test or Mann-Whitney U-test was used to compare the two independent groups according to distribution state. The Chi-square test was used to compare proportions in different groups. Least-significant Difference (LSD) was used to multiple comparisons. Logistic regression analysis was also conducted to assess relationships. The utility of the NLR in long-term mortality in critically ill patients was evaluated via receiver operating characteristic curve. P values less than 0.05 were considered statistically significant.
Results
A total of 228 subjects were participated in the study. Of 79 patients diagnosed with SLE, 20 of them were diagnosed with LN. All the subjects were divided into three groups -control, SLE without nephritis and LN. Of the 79 patients were 73 women (92.4%) and 6 men (7.6%), whose median age was 28.49±12.04. Patients group had similar ages and genders with control group (P>0.05).
In Table 1, the patients were divided into two groups (SLE without nephritis and LN) according to the clinical and laboratory evaluation. In the comparison, these two groups had similar ages and genders (P>0.05). There were no differences detected for age, sex, LDL, Lymphocytes, RDW, CRP and ESR (P>0.05). UA, CRE, TG, Total cholesterol, HDL, WBC, Neutrophils, NLR, Proteinuria and SLEDAI were at significantly higher levels in LN group than SLE group (P<0.05). However, only TG, Neutrophils, Lymphocytes and NLR had significant in the control and SLE group (P<0.05). The NLR values of the patients group were significantly higher than control group (P<0.001), and the NLR values of the patients with LN were higher than those of SLE patients without nephritis (P<0.001) (Figure 1). According to the laboratory data, there was not correlation between NLR and SLEDAI (P=0.678).
Table 1.
Laboratory data of the groups
| Variable | Control (n=149) | SLE without nephritis (n=59) | LN (n=20) | P-value |
|---|---|---|---|---|
| Age (years) | 28.44±4.42 | 29.47±12.63 | 25.42±9.67 | 0.150* |
| Sex | 0.472* | |||
| Female | 132 (88.6%) | 55 (93.2%) | 18 (90.0%) | |
| Male | 17 (11.4%) | 4 (6.8%) | 2 (10.0%) | |
| UA (μmol/l) | 258.01±57.50 | 250.56±97.89 | 355.98±102.97 | 0.206∆ |
| <0.001# | ||||
| CRE (μmol/l) | 55.84±10.25 | 49.08±13.89 | 86.55±76.53 | 0.078∆ |
| <0.001# | ||||
| TG (mmol/l) | 0.84±0.38 | 1.39±0.72 | 2.54±1.70 | <0.001* |
| Total cholesterol (mmol/l) | 4.36±0.66 | 4.10±0.84 | 5.09±1.54 | 0.078∆ |
| <0.001# | ||||
| HDL (mmol/l) | 1.50±0.27 | 1.00±0.31 | 1.10±0.42 | 0.209∆ |
| <0.001# | ||||
| LDL (mmol/l) | 2.34±0.59 | 2.55±0.68 | 2.51±1.09 | 0.148* |
| WBC (109/l) | 6.01±1.33 | 5.96±2.69 | 8.27±3.20 | 0.876∆ |
| <0.001# | ||||
| Neutrophils (109/l) | 3.49±1.10 | 4.26±2.35 | 6.30±2.56 | <0.001* |
| Lymphocytes (109/l) | 1.84±0.48 | 1.29±0.71 | 1.42±0.81 | 0.005∆ |
| 0.406# | ||||
| NLR | 2.00±0.76 | 4.26±3.38 | 7.21±6.01 | <0.001* |
| RDW (%) | 13.71±0.98 | 14.00±3.39 | 14.81±2.14 | 0.357∆ |
| 0.158# | ||||
| CRP (mg/l) | - | 10.50±19.81 | 17.79±32.22 | 0.144# |
| ESR (mm/h) | - | 43.81±34.71 | 51.40±26.09 | 0.449# |
| Proteinuria (mg/day) | - | 268.60±295.08 | 18888.46±1687.95 | <0.001# |
| SLEDAI | - | 5.51±3.76 | 19.05±6.10 | <0.001# |
Significance level was set at P<0.05. Values are expressed as mean ± SD, or frequency (percent).
P-value among the three groups;
P-value between control group and SLE group;
P-value between LN group and SLE group.
UA-Uric acid, CRE-creatinine, TG-triglycerides, HDL-high-density lipoprotein cholesterol, LDL-low density lipoprotein cholesterol, WBC-white blood cells, NLR-neutrophil-lymphocyte ratio, RDW-red blood cell distribution width, CRP-C-reactive protein, ESR -Erythrocyte Sedimentation Rate, SLEDAI-systemic Lupus Erythematosus Disease Activity Index.
Figure 1.
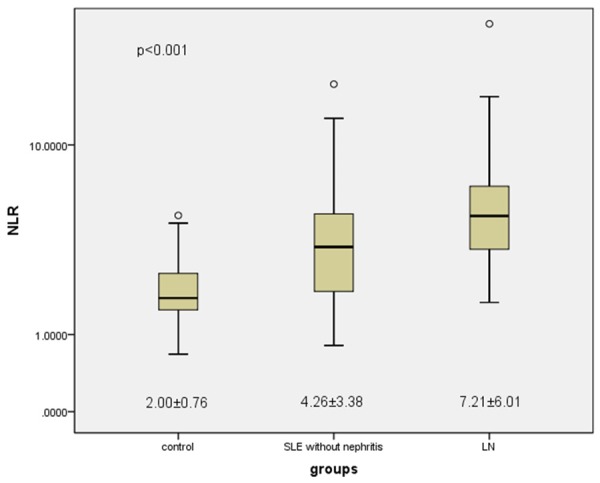
Mean NLR values of the groups. The SLE without nephritis group showed significantly higher NLR than control group (control=2.00±0.76, SLE=4.26±3.38, P<0.001), and the NLR values of the patients with LN were higher than those of the patients without LN (SLE=4.26±3.38, LN=7.21±6.01, P<0.001).
The risk factors were participated in a logistic regression analysis. Results showed that SLE without nephritis was independently related to NLR [EXP (B)=1.001, 95% CI (0.991-1.011), P<0.001], CRE (P=0.007) and TG (P<0.001) (Table 2). LN was independently related to NLR [EXP (B) =1.488, 95% CI (1.021-2.171), P=0.039] and Proteinuria (P=0.022) (Table 3). Receiver-operating characteristics analysis of NLR to predict SLE without nephritis showed that the AUC was 0.757. The cutoff value using the ROC curve was 3.13 (sensitivity, 0.574; specificity, 0.926; 95% CI, 0.668-0.845; P<0.001) (Figure 2). While ROC analysis of NLR to predict LN showed that the AUC was 0.828. The cutoff value using the ROC curve was 4.40 (sensitivity, 0.647; specificity, 0.916; 95% CI, 0.723-0.932; P<0.001) (Figure 3).
Table 2.
Logistic regression analysis of factors independently associated with SLE without nephritis
| Variable | p | EXP (B) | 95% CI of EXP (B) |
|---|---|---|---|
| UA | 0.900 | 1.001 | 0.991-1.011 |
| CRE | 0.007 | 0.915 | 0.857-0.976 |
| TG | <0.001 | 22.834 | 5.395-96.650 |
| WBC | 0.200 | 0.778 | 0.531-1.141 |
| NLR | <0.001 | 4.464 | 2.210-9.020 |
| Age | 0.055 | 1.074 | 0.998-1.156 |
Significance level was set at P<0.05. CI (confidence interval).
Table 3.
Logistic regression analysis of factors independently associated with LN
| Variable | P | EXP (B) | 95% CI of EXP (B) |
|---|---|---|---|
| NLR | 0.039 | 1.488 | 1.021-2.171 |
| Proteinuria | 0.022 | 1.003 | 1.000-1.006 |
| Lymphocytes | 0.089 | 5.328 | 0.777-36.549 |
| Age | 0.148 | 0.914 | 0.809-1.033 |
| UA | 0.207 | 1.009 | 0.995-1.024 |
| CRE | 0.614 | 0.979 | 0.899-1.065 |
Significance level was set at P<0.05.
Figure 2.
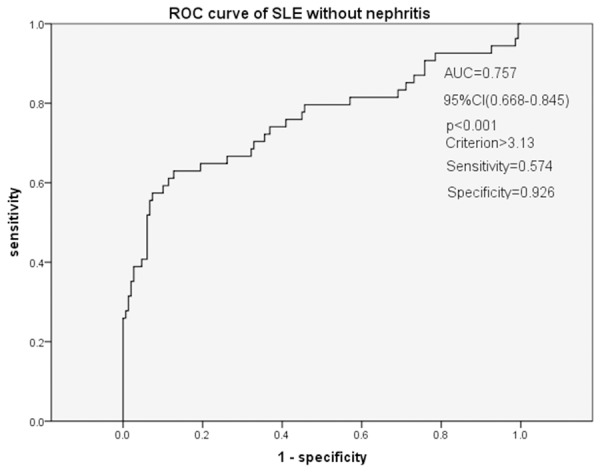
Receiver-operating characteristics (ROC) analysis of NLR to predict SLE. Receiver-operating characteristics analysis of NLR to predict SLE without nephritis showed that the AUC was 0.757. The cutoff value using the ROC curve was 3.13 (sensitivity, 0.574; specificity, 0.926; 95% CI, 0.668-0.845; P<0.001).
Figure 3.
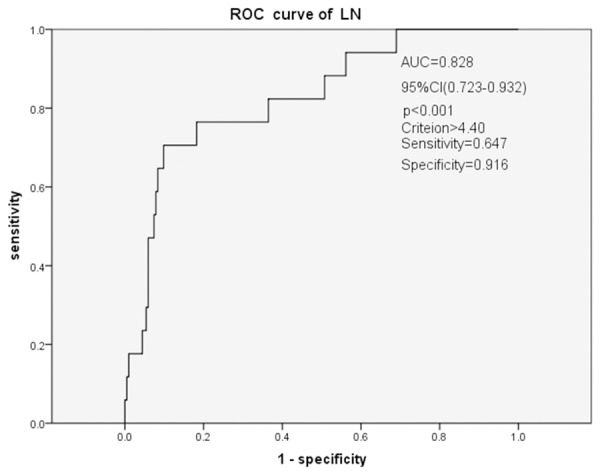
Receiver-operating characteristics (ROC) analysis of NLR to predict LNROC analysis of NLR to predict LN showed that the AUC was 0.828. The cutoff value using the ROC curve was 4.40 (sensitivity, 0.647; specificity, 0.916; 95% CI, 0.723-0.932; P<0.001).
Discussion
This study was a retrospective study evaluating the predictive value of the NLR in the SLE without nephritis and LN patients. The present study, to our knowledge, is the first to analyze the independent relationship between NLR and SLE without nephritis. According to the data we obtained from this study, NLR values were significantly higher in LN patients than in SLE patients without nephritis and the healthy controls. Furthermore, NLR is a risk factor for SLE without nephritis and LN. NLR (>3.13) was a significant predictor of SLE without nephritis progression, and NLR (>4.40) was useful for predicting LN disease progression.
Lupus nephritis is characterized by renal deposition of immune complexes [2]. Immune complexes can activate complements, which are powerful chemotactic agents that can recruit of inflammatory cells such as neutrophils, monocytes, eosinophils and T lymphocytes, and it can stimulate phagocytosis of cells and release of reactive oxygen species that can damage host tissue [4,5,15]. Some evidences suggest that the inflammasome machinery is dysregulated in SLE, and it plays an important role in promotion of organ damage [16]. Immune complex formation and complement activation may make the kidney in a state of chronic damage and chronic inflammation.
White blood cell count and its classification have been reported as inflammatory markers in chronic inflammatory diseases [6,7]. NLR is the ratio of neutrophils to lymphocytes. Its stability is less influenced by physiological, pathological and physical factors [11,12]. Additionally, NLR is cost-effective and can easily be calculated, since NLR has been used frequently to predict outcomes in patients with cancer and systemic inflammatory diseases such as colorectal cancer, diabetes, cardiovascular diseases, and familial mediterranean fever [7-13]. In this study, we excluded the patients with active infection, different malignancies, acute poisoning, thrombus formation and ischemic injury, myocardial infarction, heart failure, diabetic nephropathy and renal insufficiency. Recently several studies have explored the significance of NLR in kidney disease patients [13,17,18]. Gu et al found that increased NLR was significantly associated with in chronic kidney disease (CKD) including predialysis and dialysis patients [17]. Huang et al. detected that NLR might provide significant information regarding inflammation in diabetic nephropathy, and high NLR values may be a reliable predictive marker of early-stage diabetic nephropathy (DN) [13]. Yilmaz et al. suggested that NLR is superior to CRP and WBC for predicting the development of acute kidney injury in patients with severe sepsis [18]. However, to date, the studies on NLR and its association with the lupus nephritis have not been published. This study is the first to explore the relationship between NLR values and SLE patients with lupus nephritis. The result showed that there is a significant increase in NLR in LN patients compared to SLE patients without nephritis and healthy controls. We suggested that lupus is a process of chronic inflammation. Immune complexes can recruit inflammatory cell such as neutrophils, monocytes and T lymphocyte, what is more, infiltration and complement may make patients in a state of chronic inflammation.
Results showed that SLE without nephritis was also independently related to NRL and CRE. And LN was independently related to NLR and proteinuria. Most patients with SLE develop kidney disease related to this systemic underlying disease process [1,2]. CRE is an indicator of renal function [19]. Proteinuria is also a conventional indicator of renal function, clinically persistent proteinuria >0.5 g/d is an indicator for LN [14,20].
However, there are several limitations in our study. One of them was its sample size, which was relatively small for generalizing our findings in patients with LN. Another limitation was its retrospective design. Thus, a prospective study is needed to validate our results.
In conclusion, NLR is independently associated with SLE. And NLR is independently associated with LN as well. Because compared with the traditional indicators, such as CRE and 24 h proteinuria, NLR is more cheap, quick and easily measurable; it may be a promising marker that reflects renal involvement in patients with SLE.
Acknowledgements
Many thanks to Ping Cheng and Canhui Peng for their help in data collection and Chunmei Chen for providing language help.
Disclosure of conflict of interest
None.
References
- 1.Dooley MA, Aranow C, Ginzler EM. Review of ACR renal criteria in systemic lupus erythematosus. Lupus. 2004;13:857–860. doi: 10.1191/0961203304lu2023oa. [DOI] [PubMed] [Google Scholar]
- 2.Grande JP. Experimental models of lupus nephritis. Contrib Nephrol. 2011;169:183–197. doi: 10.1159/000319241. [DOI] [PubMed] [Google Scholar]
- 3.Borchers AT, Leibushor N, Naguwa SM, Cheema GS, Shoenfeld Y, Gershwin ME. Lupus nephritis: a critical review. Autoimmun Rev. 2012;12:174–194. doi: 10.1016/j.autrev.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 4.Cook HT, Botto M. Mechanisms of Disease: the complement system and the pathogenesis of systemic lupus erythematosus. Nat Clin Pract Rheumatol. 2006;2:330–337. doi: 10.1038/ncprheum0191. [DOI] [PubMed] [Google Scholar]
- 5.Koscielska-Kasprzak K, Bartoszek D, Myszka M, Zabińska M, Klinger M. The complement cascade and renal disease. Arch Immunol Ther Exp (Warsz) 2014;62:47–57. doi: 10.1007/s00005-013-0254-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zahorec R. Ratio neutrophil to lymphocyte counts-rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102:5–14. [PubMed] [Google Scholar]
- 7.Ahsen A, Ulu MS, Yuksel S. As a new inflammatory marker for familial Mediterranean fever: neutrophil-to-lymphocyte ratio. Inflammation. 2013;36:1357–1362. doi: 10.1007/s10753-013-9675-2. [DOI] [PubMed] [Google Scholar]
- 8.Li MX, Liu XM, Zhang XF, Zhang JF, Wang WL, Zhu Y, Dong J, Cheng JW, Liu ZW, Ma L, Lv Y. Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: A systematic review and meta-analysis. Int J Cancer. 2014;134:2403–2413. doi: 10.1002/ijc.28536. [DOI] [PubMed] [Google Scholar]
- 9.Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G, Knox JJ, Tran B, Tannock IF, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 10.Tamhane UU, Aneja S, Montgomery D, Rogers EK, Eagle KA, Gurm HS. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol. 2008;102:653–660. doi: 10.1016/j.amjcard.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Núñez J, Núñez E, Bodí V, Sanchis J, Miñana G, Mainar L, Santas E, Merlos P, Rumiz E, Darmofal H, Heatta AM, Llàcer A. Usefulness of the neutrophil to lymphocyte ratio in predicting long-term mortality in ST segment elevation myocardial infarction. Am J Cardiol. 2008;101:747–752. doi: 10.1016/j.amjcard.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Gibson PH, Croal BL, Cuthbertson BH, Small GR, Ifezulike AI, Gibson G, Jeffrey RR, Buchan KG, El-Shafei H, Hillis GS. Preoperative neutrophil-lymphocyte ratio and outcome from coronary artery bypass grafting. Am Heart J. 2007;154:995–1002. doi: 10.1016/j.ahj.2007.06.043. [DOI] [PubMed] [Google Scholar]
- 13.Huang W, Huang J, Liu Q, Lin F, He Z, Zeng Z, He L. Neutrophil-lymphocyte ratio is a reliable predictive marker for early-stage diabetic nephropathy. Clin Endocrinol (Oxf) 2014;82:229–33. doi: 10.1111/cen.12576. [DOI] [PubMed] [Google Scholar]
- 14.Hahn BH, McMahon MA, Wilkinson A, Wallace WD, Daikh DI, Fitzgerald JD, Karpouzas GA, Merrill JT, Wallace DJ, Yazdany J, Ramsey-Goldman R, Singh K, Khalighi M, Choi SI, Gogia M, Kafaja S, Kamgar M, Lau C, Martin WJ, Parikh S, Peng J, Rastogi A, Chen W, Grossman JM American College of Rheumatology. American College of rheumatology guidelines for screening, case definition, treatment and management of Lupus nephritis. Arthritis Care Res (Hoboken) 2012;64:797–808. doi: 10.1002/acr.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo RF, Ward PA. Role of C5a in inflammatory responses. Annu Rev Immunol. 2005;23:821–852. doi: 10.1146/annurev.immunol.23.021704.115835. [DOI] [PubMed] [Google Scholar]
- 16.Kahlenberg JM, Kaplan MJ. The inflammasome and lupus: another innate immune mechanism contributing to disease pathogenesis? Curr Opin Rheumatol. 2014;26:475–481. doi: 10.1097/BOR.0000000000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okyay GU, Inal S, Oneç K, Er RE, Paşaoğlu O, Paşaoğlu H, Paşaoğlu H, Derici U, Erten Y. Neutrophil to lymphocyte ratio in evaluation of inflammation in patients with chronic kidney disease. Ren Fail. 2013;35:29–36. doi: 10.3109/0886022X.2012.734429. [DOI] [PubMed] [Google Scholar]
- 18.Hakki Yilmaz, Muzaffer Cakmak, Osman Inan, Tahir Darcin, Ali Akcay. Can neutrophil-lymphocyte ratio be independent risk factor for predicting acute kidney injury in patients with severe sepsis? Ren Fail. 2015;37:225–229. doi: 10.3109/0886022X.2014.982477. [DOI] [PubMed] [Google Scholar]
- 19.Queiroz AL, Barreto DM, Silva Junior GB, Tavares Neto JE, Costa FI, Patrocínio RM, Daher Ede F, Almeida PR. Pattern, clinical features and response to corticoids of glomerular diseases in a Brazilian population, An analytical cross-sectional study. Sao Paulo Med J. 2015;133:43–50. doi: 10.1590/1516-3180.2013.7360006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El Minshawy O, El-Bassuoni E. Albuminuria prediction of kidney function outcome in kidney transplant recipients. Saudi J Kidney Dis Transpl. 2015;26:227–231. doi: 10.4103/1319-2442.152397. [DOI] [PubMed] [Google Scholar]


