Abstract
Bladder cancer (BC) is the most common urological malignancy. Early diagnosis of BC is crucial to improve patient outcomes. Currently, metabolomics is a potential technique that can be used to detect BC. We reviewed current publications and synthesised the findings on BC and metabolomics, i.e. metabolite upregulation and downregulation. Fourteen metabolites (lactic acid, leucine, valine, phenylalanine, glutamate, histidine, aspartic acid, tyrosine, serine, uracil, hypoxanthine, carnitine, pyruvic acid and citric acid) were identified as potential biomarkers for BC. In conclusion, this systematic review presents new opportunities for the diagnosis of BC.
Keywords: Bladder cancer, metabolomics, mass spectrometry, systematic review
Introduction
Bladder cancer (BC) is among the most common cancers worldwide: with more than 330,000 new cases of BC and more than 130,000 deaths because of BC occur each year [1]. Thus, early detection of BC can reduce mortality and improve the patients’ quality of life. The early symptom of BC is gross or microscopic blood in the urine, but hematuria only has a 5% specificity in BC diagnosis [2]. The standard for the initial diagnosis of BC is cystoscopy and histopathological analysis of biopsy specimens. However, these procedures are invasive, uncomfortable and costly [3,4]. Furthermore, cystoscopy may omit certain lesions, particularly small areas of carcinoma in situ [5]. Urine cytology has a sensitivity of only 35% and specificity of 94% [6], but it can be used to detect high-grade BC. Therefore, the development of new biomarkers to detect of BC would benefit patients. Recently, evidence suggested that metabolomics is useful for this purpose.
Metabolomics is the quantitative description of all endogenous low-molecular-weight components (<1 kDa) of biological samples, such as plasma, tissue or urine [7]. Metabolomics is a potential technique for effectively diagnosing BC by identifying one or more biomarkers in biological fluids and tissues. These low molecules are involved in the whole of metabolic processes including anabolism and catabolism as well as all the related cellular processes such as absorption, distribution, and energy utilization [8,9]. These endogenous compounds are highly susceptible to various factors, such as hormones, age, lifestyle, physical activities, medication and underlying disease. Metabolomics aims to elucidate potential changes in biochemical processes in various disease states, recovery and so on [10]. In oncology, tumor cells are known to have abundant biomarkers that function in specific metabolic processes, and the profiles of these biomarkers are possibly useful to assess the pathology, progression and prognosis of cancer.
Predominantly, two analytic platforms including mass spectrometry (MS) and nuclear magnetic resonance (NMR) spectrometry were used to analyse metabolomic profiling, which are based on spectroscopic techniques. MS and NMR have become crucial steps in quantitative and qualitative metabolic analyses. Both techniques allow extensive and rapid analysis of small molecule metabolites [11]. The choice of either one of these technologies depends on the aims and sensitivity and selectivity requirements of the study. Advantages and limitations of each of these technologies have been presented [8,10,12-15].
In recent years, metabolomic studies primarily aimed to identify tumour-specific biomarkers for cancers, including colorectal cancer, breast cancer, pancreatic cancer, lung cancer, gastric cancer, oesophageal cancer, cervical cancer and cancer cells [16]. As expected, an increasing amount of evidence revealed the metabolomic profiles of biological samples from BC patients [17-27]. In this study, we systematically evaluated and synthesized the validating data from various metabolomic studies that have attempted to identify various metabolites as potential biomarkers for BC.
Methods
Literature search
An initial literature search was performed in Pubmed, Medline, Embase and Web of science (the last search was updated on 31 December 2014). We used the following search strings: metabolo*, metabolic profiling, mass spectrometry, nuclear magnetic resonance spectrometry, and bladder cancer. The search was limited to English-language articles. The reviewer also hand-searched all included studies to ensure that a comprehensive search strategy was implemented.
Inclusion and exclusion criteria
Inclusion criteria
(1) Profiling studies on BC tissues, serum and urine. (2) Profiling studies on BC cell lines. (3) Profiling studies using the metabolomic techniques, such as MS and NMR.
Exclusion criteria
(1) Studies on genomics, transcriptomics and proteomics. (2) Studies on benign bladder disease. (3) Studies without detailed metabolites. (4) Animal studies.
Quality assessment
To ensure that all relevant studies included in our review were of good quality and should be included, three independent assessors conducted a critical assessment using a Quality Assessment of Diagnostic Accuracy Studies (QUADAS) tool [28]. The QUADAS-2 tool comprises four key domains namely, patient selection, index test, reference standard, and flow and timing. Seven questions were used to evaluate the quality of diagnostic accuracy studies. Each question can be answered with “yes”, “no”, or “unclear”. The answer “yes” means that the risk of bias is low, whereas the answer “no” or “unclear” means the risk of bias is high. The individual quality assessments of QUADAS-2 were provided in Supplementary Table 1.
Results
A total of 488 potentially relevant publications were identified during the literature search. A total of 477 non-relevant publications for this systematic review were excluded after reviewing the titles, abstracts and the full text. Finally, 11 original articles met the inclusion criteria and were eligible for systematic review (Table 1). Figure 1 shows the selection process. The biological samples used in metabolomic analyses included urine (n = 6), serum (n = 3), cell (n = 1) and a combination of urine, tissue and cell (n = 1). Results of the QUADAS-2 study quality assessment are shown in a bar graph in Figure 2. The majority of all included studies in this review met most items in QUADAS-2, thereby indicating that the overall quality of the included studies was generally good.
Table 1.
Summary of included studies. All comparisons are determined at a statically significant level
| Author (year) | Analytical platform | Sample type | NCa | Control | NC | Metabolites of cancer compared to control | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Up-Regulated | Down-regulated | ||||||
| Pasikanti et al. (2010) | GC/TOF-MS | Urine | 24 | non-BC patients | 51 | Uridine | Senecioic acid, Citric acid |
| Melibiose | 2-Butenedioic acid, Ribitol | ||||||
| Valine | Ribonic acid, Sumiki’s acid | ||||||
| 2,5-Furandicarboxylic acid | |||||||
| 2-Propenoic acid | |||||||
| Srivastava et al. (2010) | 1H NMR | Urine | 33 | HC, n = 37 | 70 | Taurine | Citrate, Phenylalanine |
| UTI, n = 31 | Hippurate | ||||||
| BS, n = 2 | |||||||
| Putluri et al. (2011) | LC-MS | Urine Tissues BC cell lines | 83 | Benign adjacent tissues | 51 | Spermindine, NI-acetylspermine | Aniline |
| Serine, Uracil, Thymine, Urocanic acid | Creatine | ||||||
| N8- acetylspermine, Tyrosine, Histidine | 2-Hydroxykynurenine acid | ||||||
| S-Adenosylmethionine, Oleic acid | Histamine | ||||||
| Isoleucine, Homoserine, Kynurenine | Giyceraldhyde-3-PO4 | ||||||
| Guanine, Phenylalanine, Palmitic acid | Hippuric acid | ||||||
| Lysine, Hypoxanthine, Valine, Leucine | 4-pyridoxic acid | ||||||
| D-Ribonolactone, Pipecolic acid | Coumarin | ||||||
| Cytidine monophosphate, Homocysteine | Taurine | ||||||
| Niacinamine , Asparagines, Carnitine | Lauric acid | ||||||
| 3-Hydroxybutyic acid, Retinol | 2-hydroxykynurenine | ||||||
| Niacinamide, 3-Hydroxykynurenie | Giyceraldhyde-3-po4 | ||||||
| Lin et al. (2012) | RPLC-MS | Serum | 13 | HC, n = 20 | 38 | Eicosatrienol, Phosphatidylcholine | |
| HILIC-MS | Nephrolithiasis, n = 8 | Azaprostanoic acid, Docosatrienol | |||||
| BPH, n = 10 | 14’-apo-beta-carotenal, Acetylphenylalanine | ||||||
| Methyl hippuric acid | |||||||
| Cao et al. (2012) | 1H NMR | Serum | 37 | HC, n = 25 | 73 | Glucose, Acetoacetate | Tyrosine, Phenylalanine |
| CP, n = 28 | Isoleucine, Leucine | ||||||
| Post-TURBT, n = 20 | Lactate, Glycine | ||||||
| Alberice et al. (2013) | LC-MS | Urine | 48 | Histidine, Phenylalanine, Hypoxanthine | |||
| CE-MS | Tyrosine, Tryptophan, Leucine | ||||||
| N-acetyltryptophan, Carnitine | |||||||
| Bansal et al. (2013) | 1H NMR | Serum | 67 | HC | 32 | DMA, Malonate, Histidine, | |
| Lactate, Glutamine, Valine | |||||||
| Dettmer et al. (2013) | LC-MS | BC cell lines | Alanine, Arginine, Histidine, Fructose | Cystine, Glycine | |||
| CE-MS | Asparagines, Aspartate, Ornithine | Glucose, Pyruvate | |||||
| Citruline, Glutamine, Leucine | Glycerate, Fumarate | ||||||
| Methionine, Myoinositol, Threonine | Citrate, Indole 3 lactate | ||||||
| Phenylalanine, Serine, Tryptophan | Hydroxy indole 3 acetate | ||||||
| Valine, Lactate, Kynurenine | |||||||
| Pasikanti et al. (2013) | GC×GC-TOFMS | Urine | 38 | non-BC controls | 61 | Adipic acid, Erythritol, Pseudouridine | Citric acid |
| Anthranilic acid, Talonic acid | Dihydroxyacetone | ||||||
| Coumaric acid, Lactic acid | Ethyl tartrate | ||||||
| Cyclopentane-1, 2-diamine | Gluconic acid, Glycerol | ||||||
| Erythropentonic acid, 2-pentadecanol | Levulinic acid | ||||||
| Ethylmalonic acid, Melibiose | Pinene, Ribitol | ||||||
| Gluconic acid, Itaconic acid | Ribonic acid, Sebacic acid | ||||||
| Heptadecanoic acid, Uridine | Sumiki’s acid | ||||||
| Hydroxybutyric acid | 2,5-furandicarboxylic acid | ||||||
| N-acetyl-anthranilic acid | 2-butanedioic acid | ||||||
| Vanillylmandelic acid | 2-hydroxyglutaric acid | ||||||
| 2-aminoisobutyric acid | |||||||
| 2-hydroxymalonic acid | |||||||
| 3,4-dihydroxyphenylpyruvate | |||||||
| 3-hydroxysebacic acid | |||||||
| 4-methoxycinnamic acid | |||||||
| Tripathi et al. (2013) | HR-MAS-NMR | Tissue | 33 | Benign disease | 26 | Triglycerides , Leucine, Aspartate | |
| GC-MS | Isoleucine, Valine, Lactate, Creatine | ||||||
| Alanine, Glutamine, Acetate, Uridine | |||||||
| Lysine, Glutamate, Choline, Taurine | |||||||
| Glutathione, Phosphocholine, Glycine | |||||||
| Glycerophosphocholine, Myoinositol | |||||||
| Tyrosine, Phenylalanine | |||||||
| Diphosphate sugars | |||||||
| Jin et al. (2014) | HPLC-QTOFMS | Urine | 138 | HC | 121 | Pyruvate, Phosphoenolpyruvate | Melatonin, Glutarylcarnitine, Decanoylcarnitine |
| Carnitine, Trimethyllysine, | |||||||
| Isovalerylcarnitine, Oxoglutarate, | |||||||
| palmitoyltransferase | |||||||
GC/TOFMS, Gas chromatography-time-of-flight mass spectrometry; GC-MS, Gas chromatography-mass spectrometry; LC-MS, Liquid chromatography-mass spectrometry; CE-MS, Capillary electrophoresis-mass spectrometry; HR-MAS-NMR, High resolution-magic angle spinning-nuclear magnetic resonance spectroscopy; 1H NMR, Proton nuclear magnetic resonance; BC, Bladder cancer; RPLC-MS, Reversed phase liquid chromatography-mass spectrometry; HILIC-MS, Hydrophilic interaction chromatography-mass spectrometry; GC×GC-TOFMS, Two-dimensional gas chromatography time-of-flight mass spectrometry; BPH, Benign prostatic hyperplasia; UTI, Urinary tract infection; BS, Bladder stone; HC, Healthy controls; Nc, Number of control patients; NCa, Number of cancer patients; CP, Calculi patients; HPLC-QTOFMS, High-performance liquid chromatography-quadrupole time-of-flight mass spectrometry.
Figure 1.
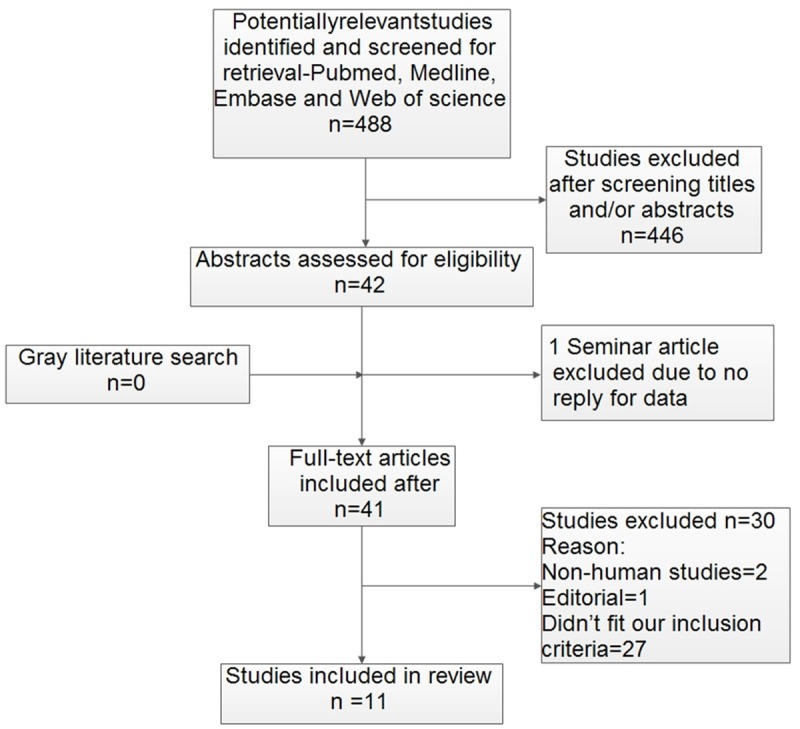
Study flow diagram.
Figure 2.

Overall quality assessment of included articles using the QUADAS-2 tool. A. Risk of bias. B. Applicability concerns.
Glucose metabolites
Glucose metabolism includes glycolysis and aerobic oxidation. The metabolite intermediates that were found to be significantly different in cancer samples are shown in Table 2. Data revealed that lactic and citric acids were the most represented metabolites, and these were upregulated and downregulated, respectively. Results for glucose and fructose have been inconsistent in different articles. Pyruvic acid and fumarate, which are the metabolic intermediates of glycolysis and tricarboxylic acid (TCA) cycle, respectively, were downregulated in BC cell lines [24].
Table 2.
Altered glucose metabolites expression in bladder cancer biological samples
| Author | Sample type | Analytical platform | Anaerobic oxidation (Glycolysis) | Aerobic oxidation (The TCA cycle) | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Glucose | Fructose | Pyruvic acid | Lactic acid | Citric acid | Fumaric acid | |||
| Pasikanti et al. (2010) | Urine | GC-MS | ↓ | ↓ | ||||
| Shatakshi et al. (2010) | Urine | 1H NMR | ↓ | |||||
| Cao et al. (2012) | Serum | 1H NMR | ↑ | ↓ | ||||
| Bansal et al. (2013) | Serum | 1H NMR | ↑ | |||||
| Dettmer et al. (2013) | Cell | LC-MS | ↓ | ↑ | ↓ | ↑ | ↓ | ↓ |
| CE-MS | ||||||||
| Pasikanti et al. (2013) | Urine | GC×GC-TOFMS | ↑ | ↓ | ||||
| Tripathi et al. (2013) | Tissue | HR-MAS-NMR | ↑ | |||||
| GC-MS | ||||||||
Amino acid metabolites
Table 3 shows that the metabolic intermediates of amino acids (both essential and non-essential) in cancer samples were significantly different from those of the control. Thirteen amino acid metabolites are the most consistently upregulated biomarker in the urine, serum and tumor tissues of patients with BC. A similar trend was observed in BC cell lines as well [19,24]. Meanwhile, upregulation of amino acids, especially leucine, valine, phenylalanine, glutamate, histidine, aspartic acid, tyrosine and serine, was generally observed in the majority of studies.
Table 3.
Altered amino acid metabolites expression in bladder cancer biological samples
| Author | Sample type | Analytical platform | Essential amino acid | Non-essential amino acid | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||||||||||||
| Leu | Met | Lys | Iie | Val | Phe | Trp | Thr | Glu | Gly | His | Ala | Arg | Cys | Asp | Pro | Tyr | Asn | Gln | Ser | |||
| Pasikanti et al. (2010) | Urine | GC/TOF-MS | ↑ | |||||||||||||||||||
| Shatakshi et al. (2013) | Urine | 1H NMR | ↑ | |||||||||||||||||||
| Putluri et al. (2011) | Urine Tissue Cell | LC-MS | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ||||||||||||
| Cao et al. (2012) | Serum | 1H NMR | ↓ | ↓ | ↓ | ↓ | ↓ | |||||||||||||||
| Alberice et al. (2013) | Urine | LC-MS | ↑ | ↑ | ↑ | ↑ | ↑ | |||||||||||||||
| CE-MS | ||||||||||||||||||||||
| Bansal et al. (2013) | Serum | 1H NMR | ↑ | ↑ | ↑ | |||||||||||||||||
| Dettmer et al. (2013) | Cell | LC-MS | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |||||||
| CE-MS | ||||||||||||||||||||||
| Tripathi et al. (2013) | Tissue | HR-MAS-NMR | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |||||||||
| GC-MS | ||||||||||||||||||||||
Leu, Leucine; Met, Methionine; Lys, Lysine; Ile, Isoleucine; Val, Valine; Phe, Phenylalanine; Trp, Tryptophan; Thr, Threonine; Glu, Glutamate; Gly, Glycine; His, Histidine; Ala, Alanine; Arg, Arginine; Cys, Cysteine; Asp, Aspartic acid; Pro, Proline; Tyr, Tyrosine; Asn, Asparagine; Gln, Glutamine; Ser, Serine.
Lipid metabolites
A study on saturated and unsaturated free fatty acids (FFA) has shown upregulation of oleic and palmitic acids and downregulation of lauric acid in BC samples using LC-MS [19]. In addition to FFA, several other metabolites related to lipid metabolism were upregulated in BC (Table 4). A study also demonstrated that demonstrates up-regulation of choline-containing compounds (choline, phosphocholine and glycerophosphocholine) were upregulated in BC patients compared with benign controls [26]. Meanwhile, Cao et al. reported the increase in acetoacetate amount in cancer patients compared with the control [20]. Carnitine was also upregulated in the different BC specimens. In addition, Pasikanti et al. and Lin et al. demonstrated upregulation of phosphatidylcholine and downregulation of glycerol in patients with BC, respectively [21,25].
Table 4.
Altered lipid metabolites expression in bladder cancer biological samples
| Author | Sample type | Analytical platform | Un-saturated/saturated fatty acid | PC | Glycerol | TG | Aceto-acetate | Inositol | Carnitine | Choline-containing compounds | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||||
| Oleic acid | Lauric acid | Palmitic acid | Phospho-choline | Choline | Glyceropho-sphocholine | |||||||||
| Putluri et al. (2011) | Urine Tissue Cell | LC-MS | ↑ | ↓ | ↑ | ↑ | ||||||||
| Lin et al. (2012) | Serum | RPLC-MS | ↑ | |||||||||||
| HILIC-MS | ||||||||||||||
| Cao et al. (2012) | Serum | 1H NMR | ↑ | |||||||||||
| Bansal et al. (2013) | Serum | 1H NMR | ↑ | |||||||||||
| Pasikanti et al. (2013) | Urine | GC-MS | ↓ | |||||||||||
| Tripathi et al. (2013) | Tissue | HR-MAS-NMR | ↑ | ↑ | ↑ | ↑ | ||||||||
| GC-MS | ||||||||||||||
| Dettmer et al. (2013) | Cell | LC-MS | ↑ | |||||||||||
| CE-MS | ||||||||||||||
| Jin et al. (2014) | Urine | HPLC-QTOFMS | ↑ | |||||||||||
PC, Phosphatidylcholine; TG, Triglycerides.
Nucleotide metabolites
Table 5 shows that purine, pyrimidine and ribose in cancer samples were significantly different from those in the control. Two studies found that hypoxanthine levels in urine significantly increased in BC patients versus normal individuals [19,22]. In addition, mass spectrometry revealed that uracil was significantly higher in urine samples of BC patients. Pentose is another essential component of nucleotide, which comprises ribose and deoxyribose. It has shown that pentose was upregulated in the urine of patients with BC using GC×GC-TOFMS.
Table 5.
Altered nucleotide metabolites expression in bladder cancer biological samples
| Author | Sample type | Analytical platform | Purine | Pyrimidine | Hypoxanthine | Ribose | |||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Adenine | Guanine | Cytosine | Thymine | Uracil | |||||
| Pasikanti et al. (2010) | Urine | GC-MS | ↑ | ||||||
| Putluri et al. (2011) | Urine Tissue Cell | LC-MS | ↑ | ↑ | ↑ | ↑ | |||
| Pasikanti et al. (2013) | Urine | GC×GC-TOFMS | ↑ | ||||||
| Alberice et al. (2013) | Urine | LC-MS | ↑ | ||||||
| CE-MS | |||||||||
Discussion
This study is the first systematic review on the current status of metabolomic profiling as a diagnostic tool for BC. The main aim of this review was to summarise the use of non-invasive urine and serum based tests for detecting BC during diagnosis. A total of 11 studies were included. These studies reported that discriminatory metabolomics is a potential biomarker for BC detection (Table 1). High sensitivity and specificity biomarkers should be investigated by non-invasion method. In the present systematic review, we founded that out of 148 differentially expressed metabolites, 1 glucose metabolite, 12 amino acid metabolites, 10 lipid metabolites and 5 nucleotide metabolites were highly expressed, whereas, 3 glucose metabolites and 2 lipid metabolites were obtained at low concentrations. Metabolites whose expression levels have obviously changed may be identified as potential biomarkers in BC patients.
The essential hallmarks of cancer are associated with an altered cancer cell-intrinsic metabolism, either as a consequence or as a cause [9]. Six essential characteristics are acquired by a cell during its progression into malignancy: limitless replicative potential, sustained angiogenesis, avoidance of apoptosis, self-sufficiency in growth signals, insensitivity to anti-growth signals and tissue invasion and metastasis. These hallmarks have been studied extensively [29,30]. Energy metabolism, an obvious hallmark of cancer, has been added to the list [9,31,32]. The incremental rate of cell proliferation in cancer requires the reprogramming of metabolic pathways to satisfy large demands for ATP, NADPH, NADH and carbon skeletons [33]. In other words, the metabolic pathways associated with BC cells are complex.
The studies revealed that lactic acid is upregulated in BC sample [23-26]. This phenomenon may be attributed to the Warburg effect [34], which states that cancer cells exhibit increased dependence on glycolytic pathway for ATP generation and gives rise to enhanced lactic acid production [35]. Such acidic condition in tumor microenvironment promotes tumor invasion and suppresses anticancer immune effectors [36-38]. Pyruvic acid is mainly transformed into lactic acid by LDH enzymes. Dettmer et al. found that pyruvate is downregulated in BC cell lines [24]. Several other lactic acid precursor metabolites related to glycolysis metabolism are downregulated. In addition, low level of pyruvic acid is believed to be related to a high synthetic rate of lactic acid. These observations suggest that increased lactic acid level in biological samples of BC is useful in BC diagnosis.
Decreased levels of citric acid and fumarate, which are the metabolic intermediates of aerobic oxidation (especially in the TCA cycle), were observed in BC samples [17,24,25]. The downregulated metabolite intermediates of Krebs cycle may explain cancer cell preference for glycolysis over oxidative phosphorylation in mitochondria. This observation, as discussed above, implies that reduced citric acid concentration resulted from its active uptake from the extracellular matrix into the tumor cell [39]. Some studies confirmed that citric acid has been used as an antineoplastic drug in cancer treatment [40,41]. Thus, reduced citric acid concentration can be regarded as a specific marker of BC.
In this review, increased amino acids levels (Table 3) have been demonstrated in the urine, serum and tissue samples of BC patients. The availability of amino acids in the tumor microenvironment is necessary for cell proliferation and as a metabolic substrate. The source of these amino acids has not been determined, but amino source is likely a combination of systemic protein catabolism [42], tumor microenvironment degradation of extracellular matrix and de novo biosynthesis [43]. Increased levels of leucine, valine, phenylalanine, histidine and serine resulted from the increased rates of amino acid metabolism in BC patients. These findings corroborate that amino acids are important biomarkers of BC and provide a promising diagnostic standard between patients and healthy controls. Thus, amino acid metabolism, particularly the increased levels of leucine, valine, phenylalanine, glutamate, histidine, aspartic acid, tyrosine and serine, could be used for monitoring tumor occurrence in BC patients.
Increased levels of the majority of lipid metabolic intermediates are shown in our review. Fatty acids are the major building blocks for the synthesis of triglycerides (TG), which are mainly used for energy storage [44]. Several studies have demonstrated that tumor cells reactivate de novo lipid synthesis [45-47], which may suggest that lipid synthesis plays an important role in tumor pathogenesis [48]. Meanwhile, some studies confirmed that fatty acid ß-oxidation is a dominant pathway for energy production in prostate [49] and pancreatic cancers [50]. Rapid tumor cells proliferation relies on large amounts of lipids as building blocks of cell membranes. The level of choline, a vital precursor of cell membrane components, was observed to be significantly higher in human and animal studies [26,51]. Higher concentrations of choline in the extracellular fluid are positively correlated with cancer cell proliferation [52]. Meanwhile, acetoacetate is among the components of ketone bodies to be upregulated in the serum of BC patients, as revealed by Cao et al. using 1H NMR [20]. Acetoacetate is being evaluated as an intermediate to measure ketone body utilization of the tumors [53,54]. Jobard et al., reported that acetoacetate plays an important role in the diagnosis, prognosis and management of cancer [55]. Interestingly, upregulation of carnitine is also presented in this review, which might be an important factor in determining the cancer status [27]. Results showed that using lipid metabolism profile for BC diagnosis is relatively promising.
Nucleotide metabolism imbalance plays an important role in a variety of human diseases, including cancer [56]. Increased levels of guanine, cytosine, thymine, hypoxanthine, uracil and ribose are found in the urine of BC patients [17,19,22,25]. Concentrations of these metabolites were significantly higher in BC patients than in the controls. Hypoxanthine is an upstream metabolite in the nucleotide biosynthetic pathway and is possibly a biomarker in BC diagnosis. Purine and pyrimidine nucleotides play an essential role in a large number of cellular processes involving DNA and RNA synthesis, biosynthesis, energy supply and regulatory mechanisms. These nucleotides are synthesised in mammalian cells by de novo pathways, which are energy consuming, or by conserve energy salvage pathways [57]. These studies confirmed that a high level of nucleotide metabolism may be considered as a novel biomarker for BC.
Our systemic review has several limitations. The possibility of publication bias exists, and eligible studies may have been omitted despite a comprehensive and systematic literature search. In fact, the majority of papers included in this review were published within the last three years. In some of these studies, tumor stages and grades were not elaborated. Thus, evaluating the diagnostic effects of these potential biomarkers was difficult. The specificity and sensitivity for BC diagnosis using metabolites are still unclear. Furthermore, analogous results possibly do not represent identical metabolites that result from the diverse samples and analysis techniques used in different studies. Because of the lack of abundant homogenous data, we were not able to perform a diagnostic meta-analysis.
A systematic review on metabolomic profiling studies on BC was presented. Although a few conflicting results may have interfered with our judgment, the majority of consistent results are useful in BC diagnosis. A few metabolites, namely, lactic acid, leucine, valine, phenylalanine, glutamate, histidine, aspartic acid, tyrosine, serine, uracil, hypoxanthine and carnitine are considered novel potential biomarkers for BC, and these potential biomarkers may have diagnostic value and may even indicate the risk of cancer recurrence. However, the clinical application of metabolomic profiling for BC detection still requires further validation in future studies.
Acknowledgements
The work was supported by the Provincial Initiative Program for Excellency Disciplines of Jiangsu Province, the National Natural Science Foundation of China (grants No. 81272832 and 81201997), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and Jiangsu Provincial Special Program of Medical Science (BL2012027).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Ploeg M, Aben KK, Kiemeney LA. The present and future burden of urinary bladder cancer in the world. World J Urol. 2009;27:289–293. doi: 10.1007/s00345-009-0383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosser CJ, Urquidi V, Goodison S. Urinary biomarkers of bladder cancer: an update and future perspectives. Biomark Med. 2013;7:779–790. doi: 10.2217/bmm.13.73. [DOI] [PubMed] [Google Scholar]
- 3.Lotan Y, Svatek RS, Sagalowsky AI. Should we screen for bladder cancer in a high-risk population? A cost per life-year saved analysis. Cancer. 2006;107:982–990. doi: 10.1002/cncr.22084. [DOI] [PubMed] [Google Scholar]
- 4.Avritscher EB, Cooksley CD, Grossman HB, Sabichi AL, Hamblin L, Dinney CP, Elting LS. Clinical model of lifetime cost of treating bladder cancer and associated complications. Urology. 2006;68:549–553. doi: 10.1016/j.urology.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 5.Vrooman OP, Witjes JA. Urinary markers in bladder cancer. Eur Urol. 2008;53:909–916. doi: 10.1016/j.eururo.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 6.van Rhijn BW, van der Poel HG, van der Kwast TH. Urine markers for bladder cancer surveillance: a systematic review. Eur Urol. 2005;47:736–748. doi: 10.1016/j.eururo.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Holmes E, Wilson ID, Nicholson JK. Metabolic phenotyping in health and disease. Cell. 2008;134:714–717. doi: 10.1016/j.cell.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 8.Griffin JL, Shockcor JP. Metabolic profiles of cancer cells. Nat Rev Cancer. 2004;4:551–561. doi: 10.1038/nrc1390. [DOI] [PubMed] [Google Scholar]
- 9.Kroemer G, Pouyssegur J. Tumor cell metabolism: Cancer’s Achilles’ heel. Cancer Cell. 2008;13:472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Van QN, Veenstra TD. How close is the bench to the bedside? Metabolic profiling in cancer research. Genome Med. 2009;1:5. doi: 10.1186/gm5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicholson JK, Connelly J, Lindon JC, Holmes E. Metabonomics: a platform for studying drug toxicity and gene function. Nat Rev Drug Discov. 2002;1:153–161. doi: 10.1038/nrd728. [DOI] [PubMed] [Google Scholar]
- 12.Dettmer K, Hammock BD. Metabolomics--a new exciting field within the “omics” sciences. Environ Health Perspect. 2004;112:A396–397. doi: 10.1289/ehp.112-1241997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robertson DG, Watkins PB, Reily MD. Metabolomics in toxicology: preclinical and clinical applications. Toxicol Sci. 2011;120(Suppl 1):S146–170. doi: 10.1093/toxsci/kfq358. [DOI] [PubMed] [Google Scholar]
- 14.Malet-Martino M, Holzgrabe U. NMR techniques in biomedical and pharmaceutical analysis. J Pharm Biomed Anal. 2011;55:1–15. doi: 10.1016/j.jpba.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 15.Claudino WM, Quattrone A, Biganzoli L, Pestrin M, Bertini I, Di Leo A. Metabolomics: available results, current research projects in breast cancer, and future applications. J. Clin. Oncol. 2007;25:2840–2846. doi: 10.1200/JCO.2006.09.7550. [DOI] [PubMed] [Google Scholar]
- 16.Van QN, Veenstra TD, Issaq HJ. Metabolic profiling for the detection of bladder cancer. Curr Urol Rep. 2011;12:34–40. doi: 10.1007/s11934-010-0151-3. [DOI] [PubMed] [Google Scholar]
- 17.Pasikanti KK, Esuvaranathan K, Ho PC, Mahendran R, Kamaraj R, Wu QH, Chiong E, Chan EC. Noninvasive urinary metabonomic diagnosis of human bladder cancer. J Proteome Res. 2010;9:2988–2995. doi: 10.1021/pr901173v. [DOI] [PubMed] [Google Scholar]
- 18.Srivastava S, Roy R, Singh S, Kumar P, Dalela D, Sankhwar SN, Goel A, Sonkar AA. Taurine-a possible fingerprint biomarker in non-muscle invasive bladder cancer: A pilot study by 1H NMR spectroscopy. Cancer Biomark. 2010;6:11–20. doi: 10.3233/CBM-2009-0115. [DOI] [PubMed] [Google Scholar]
- 19.Putluri N, Shojaie A, Vasu VT, Vareed SK, Nalluri S, Putluri V, Thangjam GS, Panzitt K, Tallman CT, Butler C, Sana TR, Fischer SM, Sica G, Brat DJ, Shi H, Palapattu GS, Lotan Y, Weizer AZ, Terris MK, Shariat SF, Michailidis G, Sreekumar A. Metabolomic profiling reveals potential markers and bioprocesses altered in bladder cancer progression. Cancer Res. 2011;71:7376–7386. doi: 10.1158/0008-5472.CAN-11-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao M, Zhao L, Chen H, Xue W, Lin D. NMR-based Metabolomic Analysis of Human Bladder Cancer. Anal Sci. 2012;28:451–6. doi: 10.2116/analsci.28.451. [DOI] [PubMed] [Google Scholar]
- 21.Lin L, Huang Z, Gao Y, Chen Y, Hang W, Xing J, Yan X. LC-MS-based serum metabolic profiling for genitourinary cancer classification and cancer type-specific biomarker discovery. Proteomics. 2012;12:2238–2246. doi: 10.1002/pmic.201200016. [DOI] [PubMed] [Google Scholar]
- 22.Alberice JV, Amaral AF, Armitage EG, Lorente JA, Algaba F, Carrilho E, Marquez M, Garcia A, Malats N, Barbas C. Searching for urine biomarkers of bladder cancer recurrence using a liquid chromatography-mass spectrometry and capillary electrophoresis-mass spectrometry metabolomics approach. J Chromatogr A. 2013;1318:163–170. doi: 10.1016/j.chroma.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Bansal N, Gupta A, Mitash N, Shakya PS, Mandhani A, Mahdi AA, Sankhwar SN, Mandal SK. Low- and high-grade bladder cancer determination via human serum-based metabolomics approach. J Proteome Res. 2013;12:5839–5850. doi: 10.1021/pr400859w. [DOI] [PubMed] [Google Scholar]
- 24.Dettmer K, Vogl FC, Ritter AP, Zhu W, Nurnberger N, Kreutz M, Oefner PJ, Gronwald W, Gottfried E. Distinct metabolic differences between various human cancer and primary cells. Electrophoresis. 2013;34:2836–2847. doi: 10.1002/elps.201300228. [DOI] [PubMed] [Google Scholar]
- 25.Pasikanti KK, Esuvaranathan K, Hong Y, Ho PC, Mahendran R, Raman Nee Mani L, Chiong E, Chan EC. Urinary metabotyping of bladder cancer using two-dimensional gas chromatography time-of-flight mass spectrometry. J Proteome Res. 2013;12:3865–3873. doi: 10.1021/pr4000448. [DOI] [PubMed] [Google Scholar]
- 26.Tripathi P, Somashekar BS, Ponnusamy M, Gursky A, Dailey S, Kunju P, Lee CT, Chinnaiyan AM, Rajendiran TM, Ramamoorthy A. HR-MAS NMR tissue metabolomic signatures cross-validated by mass spectrometry distinguish bladder cancer from benign disease. J Proteome Res. 2013;12:3519–3528. doi: 10.1021/pr4004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin X, Yun SJ, Jeong P, Kim IY, Kim WJ, Park S. Diagnosis of bladder cancer and prediction of survival by urinary metabolomics. Oncotarget. 2014;5:1635–1645. doi: 10.18632/oncotarget.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whiting PF, Weswood ME, Rutjes AW, Reitsma JB, Bossuyt PN, Kleijnen J. Evaluation of QUADAS, a tool for the quality assessment of diagnostic accuracy studies. BMC Med Res Methodol. 2006;6:9. doi: 10.1186/1471-2288-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 30.Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Gillies RJ, Robey I, Gatenby RA. Causes and consequences of increased glucose metabolism of cancers. J Nucl Med. 2008;49:24S–42S. doi: 10.2967/jnumed.107.047258. [DOI] [PubMed] [Google Scholar]
- 32.Ortega AD, Sanchez-Arago M, Giner-Sanchez D, Sanchez-Cenizo L, Willers I, Cuezva JM. Glucose avidity of carcinomas. Cancer Lett. 2009;276:125–135. doi: 10.1016/j.canlet.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Romero-Garcia S, Sullivan Lopez-Gonzalez J, Luis Baez-Viveros J, Aguilar-Cazares D, Prado-Garcia H. Tumor cell metabolism An integral view. Cancer Biol Ther. 2011;12:939–948. doi: 10.4161/cbt.12.11.18140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 35.Brahimi-Horn MC, Chiche J, Pouyssegur J. Hypoxia signalling controls metabolic demand. Curr Opin Cell Biol. 2007;19:223–229. doi: 10.1016/j.ceb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Koukourakis MI, Giatromanolaki A, Harris AL, Sivridis E. Comparison of metabolic pathways between cancer cells and stromal cells in colorectal carcinomas: a metabolic survival role for tumor-associated stroma. Cancer Res. 2006;66:632–637. doi: 10.1158/0008-5472.CAN-05-3260. [DOI] [PubMed] [Google Scholar]
- 37.Swietach P, Vaughan-Jones RD, Harris AL. Regulation of tumor pH and the role of carbonic anhydrase 9. Cancer Metastasis Rev. 2007;26:299–310. doi: 10.1007/s10555-007-9064-0. [DOI] [PubMed] [Google Scholar]
- 38.Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, Gottfried E, Schwarz S, Rothe G, Hoves S, Renner K, Timischl B, Mackensen A, Kunz-Schughart L, Andreesen R, Krause SW, Kreutz M. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109:3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- 39.Anghileri LJ, Crone-Escanye MC, Thouvenot P, Brunotte F, Robert J. Mechanisms of gallium-67 accumulation by tumors: role of cell membrane permeability. J Nucl Med. 1988;29:663–668. [PubMed] [Google Scholar]
- 40.Bucay AH. Clinical report: A patient with primary peritoneal mesothelioma that has improved after taking citric acid orally. Clin Res Hepatol Gastroenterol. 2011;35:241–241. doi: 10.1016/j.clinre.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 41.Halabe Bucay A. Hypothesis proved citric acid (citrate) does improve cancer: a case of a patient suffering from medullary thyroid cancer. Med Hypotheses. 2009;73:271. doi: 10.1016/j.mehy.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 42.Argiles JM, Azcon-Bieto J. The metabolic environment of cancer. Mol Cell Biochem. 1988;81:3–17. doi: 10.1007/BF00225648. [DOI] [PubMed] [Google Scholar]
- 43.Hirayama A, Kami K, Sugimoto M, Sugawara M, Toki N, Onozuka H, Kinoshita T, Saito N, Ochiai A, Tomita M, Esumi H, Soga T. Quantitative Metabolome Profiling of Colon and Stomach Cancer Microenvironment by Capillary Electrophoresis Time-of-Flight Mass Spectrometry. Cancer Res. 2009;69:4918–4925. doi: 10.1158/0008-5472.CAN-08-4806. [DOI] [PubMed] [Google Scholar]
- 44.Santos CR, Schulze A. Lipid metabolism in cancer. FEBS J. 2012;279:2610–2623. doi: 10.1111/j.1742-4658.2012.08644.x. [DOI] [PubMed] [Google Scholar]
- 45.Swinnen JV, Vanderhoydonc F, Elgamal AA, Eelen M, Vercaeren I, Joniau S, Van Poppel H, Baert L, Goossens K, Heyns W, Verhoeven G. Selective activation of the fatty acid synthesis pathway in human prostate cancer. Int J Cancer. 2000;88:176–179. doi: 10.1002/1097-0215(20001015)88:2<176::aid-ijc5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 46.Li JN, Mahmoud MA, Han WF, Ripple M, Pizer ES. Sterol regulatory element-binding protein-1 participates in the regulation of fatty acid synthase expression in colorectal neoplasia. Exp Cell Res. 2000;261:159–165. doi: 10.1006/excr.2000.5054. [DOI] [PubMed] [Google Scholar]
- 47.Yoon S, Lee MY, Park SW, Moon JS, Koh YK, Ahn YH, Park BW, Kim KS. Up-regulation of acetyl-CoA carboxylase alpha and fatty acid synthase by human epidermal growth factor receptor 2 at the translational level in breast cancer cells. J Biol Chem. 2007;282:26122–26131. doi: 10.1074/jbc.M702854200. [DOI] [PubMed] [Google Scholar]
- 48.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 49.Liu Y. Fatty acid oxidation is a dominant bioenergetic pathway in prostate cancer. Prostate Cancer Prostatic Dis. 2006;9:230–234. doi: 10.1038/sj.pcan.4500879. [DOI] [PubMed] [Google Scholar]
- 50.Khasawneh J, Schulz MD, Walch A, Rozman J, Hrabe de Angelis M, Klingenspor M, Buck A, Schwaiger M, Saur D, Schmid RM, Kloppel G, Sipos B, Greten FR, Arkan MC. Inflammation and mitochondrial fatty acid beta-oxidation link obesity to early tumor promotion. Proc Natl Acad Sci U S A. 2009;106:3354–3359. doi: 10.1073/pnas.0802864106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang J, Wei S, Liu L, Nagana Gowda GA, Bonney P, Stewart J, Knapp DW, Raftery D. NMR-based metabolomics study of canine bladder cancer. Biochim Biophys Acta. 2012;1822:1807–1814. doi: 10.1016/j.bbadis.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 52.Bianchi L, De Micheli E, Bricolo A, Ballini C, Fattori M, Venturi C, Pedata F, Tipton KF, Della Corte L. Extracellular levels of amino acids and choline in human high grade gliomas: an intraoperative microdialysis study. Neurochem Res. 2004;29:325–334. doi: 10.1023/b:nere.0000010462.72557.6d. [DOI] [PubMed] [Google Scholar]
- 53.Prenen GH, Go KG, Zuiderveen F, Paans AM, Vaalburg W. An improved synthesis of carbon-11 labeled acetoacetic acid and an evaluation of its potential for the investigation of cerebral pathology by positron emission tomography. Int J Rad Appl Instrum A. 1990;41:1209–1216. doi: 10.1016/0883-2889(90)90208-x. [DOI] [PubMed] [Google Scholar]
- 54.Authier S, Tremblay S, Dumulon V, Dubuc C, Ouellet R, Lecomte R, Cunnane SC, Benard F. [11C] acetoacetate utilization by breast and prostate tumors: A PET and biodistribution study in mice. Mol Imaging Biol. 2008;10:217–223. doi: 10.1007/s11307-008-0143-6. [DOI] [PubMed] [Google Scholar]
- 55.Jobard E, Pontoizeau C, Blaise BJ, Bachelot T, Elena-Herrmann B, Tredan O. A serum nuclear magnetic resonance-based metabolomic signature of advanced metastatic human breast cancer. Cancer Lett. 2014;343:33–41. doi: 10.1016/j.canlet.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 56.Aird KM, Zhang R. Nucleotide metabolism, oncogene-induced senescence and cancer. Cancer Lett. 2015;356:204–10. doi: 10.1016/j.canlet.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jurecka A. Inborn errors of purine and pyrimidine metabolism. J Inherit Metab Dis. 2009;32:247–263. doi: 10.1007/s10545-009-1094-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


