Abstract
Background: XRCC1 is a multi-domain protein associated with bladder cancer. We investigated the relationship between the distribution of XRCC1 polymorphisms (rs915927 and rs2854501) and clinical outcomes following intravesical instillation with epirubicin (EPI) or mitomycin C (MMC). Methods: A TaqMan assay was performed to determine genotypes of 240 individuals diagnosed with bladder cancer. Logistic regression was used to assess the association between polymorphisms and relapse-free survival (RFS) of patients. Quantitative real-time polymerase chain reaction was performed to determine expression of XRCC1 polymorphisms. Survival curves were generated using the Kaplan-Meier method. Results: Risk of bladder cancer recurrence was significantly reduced in patients receiving EPI who had higher incidences of XRCC1 polymorphisms (P=0.009 for rs915927, P=0.001 for rs2854501). In participants administered MMC, results were not statistically significant. Conclusions: Polymorphisms in XRCC1 SNP variants (rs915927 and rs2854501) were associated with improved clinical outcomes following EPI treatment.
Keywords: Bladder cancer, polymorphisms, XRCC1, instillation, epirubicin
Introduction
Bladder cancer was the fourth most commonly diagnosed cancer in males in the United States in 2013 and the most common cause of urogenital malignant tumors. In 2013, there were 74,690 cases of bladder cancer in the United States, and 15,580 patients died from the disease [1]. Approximately 80% of patients with bladder cancer initially present with superficial disease (stages Ta, T1 or carcinoma in situ), whereas the remaining has invasive muscle and advanced/metastatic disease [2,3]. Cystoscopy and transurethral resection (TUR) are the primary methods for both treatment and diagnosis of superficial cancers. However, tumor recurrence may be a major problem for patients with higher grade Ta and T1 lesions. Twenty percent of patients with low risk and 40% with medium risk experienced tumor recurrence one year following TUR. Furthermore, 90% of patients with high-risk experienced recurrence 2 years after TUR treatment [4].
Intravesical therapy following TUR has been the most commonly used therapeutic approach to decrease the risk of recurrence and progression in bladder cancer patients. Although several chemotherapeutic agents have been administered intravesically to manage superficial bladder cancer, (e.g. thiotepa, doxorubicin, epirubicin (EPI), mitomycin C (MMC), and Bacillus Calmette-Guerin (BCG)), a standard prophylactic treatment has not yet been established.
At present, MMC is one of the standard chemotherapy agents for treating superficial bladder cancer [5]. As an anti-tumor antibiotic, the recommended dose of MMC for intravesical infusion is 20 to 40 mg, according to the manufacturer’s labeling [6]. Given in multiple infusions, MMC has been effective in treating non-muscle-invasive bladder tumors with response rates ranging from 40 to 50% [7].
Epirubicin (EPI), a derivative of doxorubicin, has also been proven effective in patients with superficial carcinoma [8]. The preventive effect of intravesical administration of EPI was similar to that of MMC and doxorubicin. Additionally, EPI also reduced side effects in patients with superficial cancers (stages Ta, T1 or carcinoma in situ) [8,9].
Clinical response to chemotherapy is influenced by both genetic and environmental factors. Genetic factors play a role in controlling drug absorption, distribution, metabolism and excretion. Studies have demonstrated a relationship between response to chemo-radiotherapy and expression of certain genes. For example, X-ray repair cross-complementing gene 1 (XRCC1) was associated with response to chemotherapy in patients with lung cancer [10]. Polymorphisms in the XRCC1 gene have also been shown to play a role in improving survival of bladder cancer patients receiving chemotherapy [11].
XRCC1 encodes a protein involved in the DNA repair pathway. XRCC1 is a multi-domain protein interacting with three other proteins (poly-ADP-ribose polymerase, DNA ligase III, and DNA polymerase β) to repair single-strand breaks in DNA and was associated with repair function in X-irradiated cells [12]. The human XRCC1 gene consists of 17 exons, mapped to chromosome 19q13, 2, and spans approximately 31.9 kb [13]. Genetic variants, such as single nucleotide polymorphisms (SNPs), have been extensively studied in relation to cancer incidence, including urothelial carcinoma [14].
In the present study, XRCC1 polymorphisms may be associated with improved outcomes in bladder cancer patients receiving intravesical instillation. To validate this hypothesis, we investigated XRCC1 rs915927 and XRCC1 rs2854501 polymorphisms in Chinese patients who received EPI and MMC as intravesical instillation agents for treatment of bladder cancer with the aim of assessing the influence of these polymorphisms on clinical outcomes.
Methods and materials
Patient selection and classification
The Institutional Review Board of Nanjing Medical University approved this study. From June 2007 to September 2012, 240 patients diagnosed with bladder cancer were recruited from the First Affiliated Hospital of Nanjing Medical University. Written informed consent was obtained from all patients involved in the present study. To prevent recurrence of cancer, 129 patients had received EPI instillation and 111 had been treated with MMC. Patients were excluded from the study if they previously had cancer or had been subjected to radiotherapy or chemotherapy, or if they currently had metastasized cancer from other or unknown origins.
Two pathologists independently reviewed histopathology slides from core biopsy to confirm diagnosis of bladder cancer. Prior to recruitment, all subjects were personally interviewed to collect demographic data and clinical characteristics, including age, gender, tobacco use, alcohol use, and self-reported family history of cancer. Clinical stage at the time of diagnosis was determined according to tumor, node, and metastasis classifications of cancer stages (2002 International Union against Cancer). All patients were non-muscle invasive (pTa-pT1). According to histopathological grading (WHO 2004, grading of urothelial papilloma), patients were also classified as low risk or high risk. Individuals who smoked daily for >1 yr were defined as smokers and the rest were considered nonsmokers. Individuals who drank alcohol ≥3 times per wk for >6 mos were defined as drinkers and the rest were considered nondrinkers.
EPI (50 mg/wk) and MMC (30 mg/wk) were instilled into the bladder 24 h after trans-urethral resection of the bladder tumor (TURBT). Weekly dosing was maintained for 8 wks followed by monthly dosing (50 mg EPI/mo; 30 mg MMC/mo) maintained for 12 months or more. Survival time was calculated as time between confirmed diagnosis and last follow-up or recurrence. The date of recurrence was obtained from inpatient and outpatient records or from patients’ families via follow-up phone calls. Patients who had not experienced recurrence by the last follow-up date were considered non-recurrent.
Single nucleotide polymorphism selection and genotyping
SNPs (XRCC1 rs915927 and XRCC1 rs2854501) were selected based on previous studies that demonstrated an association with pharmaco sensitivity. Genomic DNA from each patient was obtained from a 150 μL EDTA-anticoagulated peripheral blood sample and extracted according to the manufacturer’s instructions using a DNA extraction kit (Tiangen Biotech, Beijing, China). Polymorphisms were determined using a TaqMan SNP Genotyping Assay (Applied Biosystems, Foster City, CA, USA). The primers, probes and reaction conditions for each SNP are available upon request (Table 3). For quality control, four negative controls were included on each plate, and 5% of the samples were randomly selected for repeated genotyping to verify the results; all of the results were 100% consistent. SDS 2.4 software was used for allelic discrimination.
Table 3.
XRCC1 primers and PCR product length of TaqMan genotyping assay
| Loci | SNP | Forward | Reverse | PCR product length (bp) |
|---|---|---|---|---|
| rs915927 | CCC[A/G]GCA | ATAGGAGTGAAAGGGTCTTGGG | TGATTTCACCTTGGAGGTGCTG | 183 bp |
| rs2854501 | CCA[C/T]GAC | TTATTTGCTATCTGGGATCAGC | GTTGCTTCTCCCTCATATCTTA | 174 bp |
Statistical analysis
Relapse-free survival (RFS) was defined as time from first instillation of EPI or MMC to time of first recurrence of bladder cancer. RFS was estimated by the Kaplan-Meier method, and a log-rank test was used to compare different survival curves. Hazard ratios (HRs) and 95% confidence intervals (CIs) of the HRs were derived from univariate and multivariate Cox proportional hazard models. All analyses were carried out using SPSS 20.0. A two-sided p value <0.05 represented a statistically significant result.
Results
Characteristics of the study population
Frequency distributions for selected characteristics are shown in Table 1. The median period of instillation treatment was 18.3 months for patients instilled with EPI and 17.6 months for patients receiving MMC. Of the 240 patients studied, 87 experienced cancer recurrence and 5 died due to bladder cancer.
Table 1.
Characteristics of the patients
| Variables | n | % |
|---|---|---|
| Age (years) | 67±12 | |
| Gender | ||
| Male | 186 | 77.5 |
| Female | 54 | 22.5 |
| Smoking status | ||
| Never | 187 | 77.9 |
| Ever | 53 | 22.1 |
| Drinking status | ||
| No | 222 | 92.5 |
| Yes | 18 | 7.5 |
| Family history of cancer | ||
| No | 235 | 97.9 |
| Yes | 5 | 2.1 |
| Tumor number | ||
| 1 | 171 | 71.3 |
| ≥2 | 69 | 28.7 |
| Tumor diameter | ||
| <3 cm | 207 | 86.3 |
| ≥3 cm | 33 | 13.8 |
| Tumor grade | ||
| Low risk | 141 | 58.8 |
| High risk | 99 | 41.2 |
RFS of patients receiving EPI treatment
Mean survival time (MST) of patients was 29.3 months in the EPI treatment group. The two polymorphisms (XRCC1 rs915927 and XRCC1 rs2854501) were significantly associated with RFS of patients treated with EPI (Table 2). The MST of patients with the rs915927 AA genotype was 24.6 months, while those with AG and GG genotypes had MSTs of 44.5 and 15.8 months, respectively (P=0.020) (Figure 1A). Furthermore, patients with AG/GG genotypes also had significantly longer MSTs (MST=41.3, P=0.009) (Figure 1B). Compared to AA genotypes with XRCC1 rs915927, risk of bladder cancer recurrence decreased significantly in patients with AG (HR=0.21, 95% CI=0.08-0.53) and AG/GG genotypes (HR=0.24, 95% CI=0.10-0.59). MST of patients with the rs2854501 CC genotype was 24.4 months, compared with 45.8 and 29.4 months for CT and TT genotypes, respectively (P=0.002) (Figure 2A). Patients with CT/TT genotypes also had a significantly longer MSTs (MST=43.3 for CT/TT genotypes, P=0.001) (Figure 2B). Compared with CC genotypes with XRCC1 rs2854501, risk of bladder cancer recurrence was significantly decreased in the CT (HR=0.10, 95% CI=0.03-0.35) and CT/TT genotypes (HR=0.16, 95% CI=0.06-0.43).
Table 2.
Association between XRCC1 polymorphisms and the RFS of the patients
| Epirubicin | MMC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Recurrence | Total | MST* | P logrank | Adjusted HR (95% CI) | Recurrence | Total | MST* | P logrank | Adjusted HR (95% CI) | |
| rs915927 | ||||||||||
| AA | 35 | 93 | 24.6 | 0.020 | 1.00 (reference) | 31 | 92 | 25.3 | 0.784 | 1.00 (reference) |
| AG | 12 | 32 | 44.5 | 0.21 (0.08-0.53) | 7 | 17 | 35.0 | 0.68 (0.26-1.75) | ||
| GG | 1 | 4 | 15.8 | 1.40 (0.16-11.99) | 1 | 2 | 86.5 | 0.66 (0.08-5.54) | ||
| AA | 35 | 93 | 24.6 | 0.009 | 1.00 (reference) | 31 | 92 | 25.3 | 0.700 | 1.00 (reference) |
| AG+GG | 13 | 36 | 41.3 | 0.24 (0.10-0.59) | 8 | 19 | 40.4 | 0.68 (0.28-1.65) | ||
| rs2854501 | ||||||||||
| CC | 38 | 96 | 24.4 | 0.002 | 1.00 (reference) | 31 | 91 | 25.2 | 0.753 | 1.00 (reference) |
| CT | 8 | 28 | 45.8 | 0.10 (0.03-0.35) | 7 | 18 | 35.0 | 0.66 (0.26-1.67) | ||
| TT | 2 | 5 | 29.4 | 0.65 (0.14-3.10) | 1 | 2 | 86.5 | 0.66 (0.08-5.47) | ||
| CC | 38 | 96 | 24.4 | 0.001 | 1.00 (reference) | 31 | 91 | 25.2 | 0.586 | 1.00 (reference) |
| CT+TT | 10 | 33 | 43.3 | 0.16 (0.06-0.43) | 8 | 20 | 40.2 | 0.66 (0.27-1.60) | ||
Mean survival time.
Figure 1.
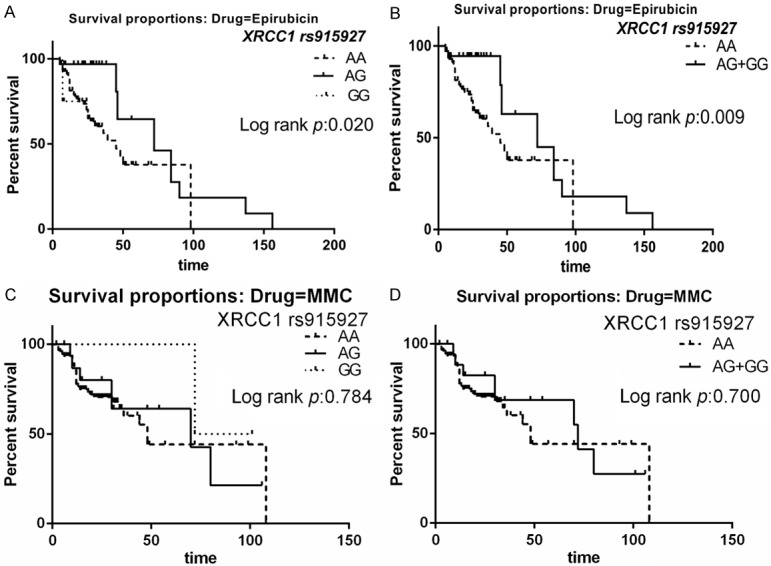
Relapse-free survival (RFS) of the patients with XRCC1 rs915927 polymorphisms. A. Survival curves for AG versus AA in patients treated with EPI. B. Survival curves for AG+GG versus AA in patients treated with EPI. C. Survival curves for AG versus AA in patients treated with MMC. D. Survival curves for AG+GG versus AA in patients treated with MMC.
Figure 2.
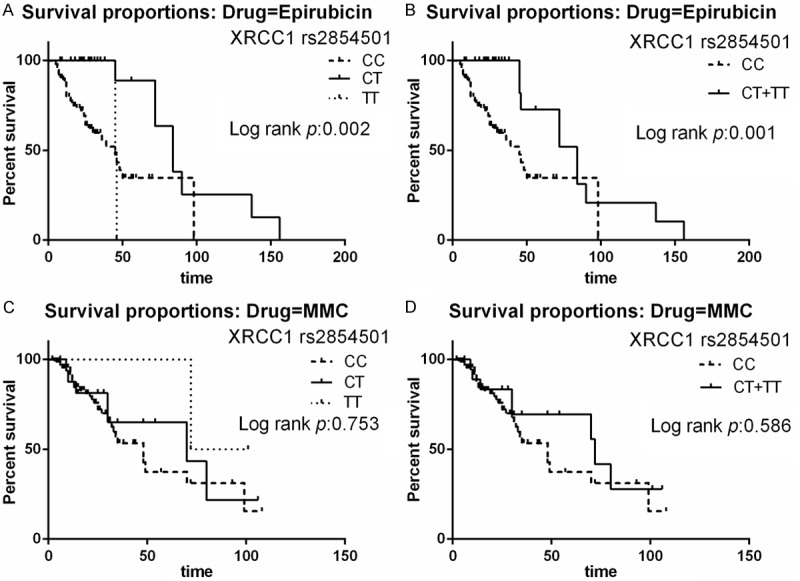
Relapse-free survival (RFS) of the patients with XRCC1 rs2854501 polymorphisms. A. Survival curves for CT versus CC in patients treated with EPI. B. Survival curves for CT+TT versus CC in patients treated with EPI. C. Survival curves for CT versus CC in patients treated with MMC. D. Survival curves for CT+TT versus CC in patients treated with MMC.
RFS of patients receiving MMC treatment
Mean survival time was and 27.9 mos in the MMC group and was not significantly different from the EPI group. We also did not find any significant differences in RFS among participants treated with MMC.
Discussion
In the present study, association between XRCC1 polymorphisms and the risk of bladder recurrence was investigated. We observed that patients receiving EPI with a higher number of genetic variants in XRCC1 (rs915927, rs2854501) had significantly reduced risk of bladder cancer recurrence. The time to recurrence was significantly shorter in G allele carriers than in individuals with the homozygous AA genotype (rs915927). Patients with the AG genotype exhibited a lower risk of recurrence than those with the AA genotype. Similarly, this phenomenon also applied to individuals with the homozygous CC genotype (rs2854501). Patients with the CT genotype also had less recurrence than those with the CC genotype. In participants treated with MMC, our results had no statistical significance.
In current clinical management, several chemotherapeutics, including as EPI and MMC, have been administered intravesically to treat superficial bladder cancer [15]. Since cancer cells proliferate more rapidly than normal cells, the vast majority of antineoplastic drugs target the cell cycle, and the most common approach is to exploit the ability of drugs to damage DNA. Such damage can lead to cell death and better response to therapy. DNA repair pathways can regulate the efficacy of DNA damage-based cancer therapy. DNA damage may result in somatic mutations and stimulate DNA repair processes. It has also been suggested that polymorphisms in DNA repair genes may increase cell repair function capacity and attenuate carcinogenesis [16]. For instance, polymorphisms of the XRCC1 gene have been associated with improved survival in a group of 78 muscle-invasive bladder cancer patients receiving chemotherapy [17].
In bladder cancer patients with comparable biological characteristics and disease stage, fluctuating efficiency of similar instillation schemes suggests that genetic factors may be involved in different mechanisms of anticancer resistance. XRCC1 encodes a protein involved in the BER pathway, which is important for DNA cross links repair induced by epirubicin [18], while MMC stops the replication of cancer cells by form DNA adducts that cause DNA damages [19]. Polymorphisms of XRCC1 may influence the prognosis of chemotherapy in cancers that have been studied for years. Sun et al. found that polymorphisms of XRCC1 were associated with response to platinum-based chemotherapy in non-small-cell lung carcinoma [20]. Increased overall survival as well as disease-free survival were associated with the Gln399Gln genotype of the XRCC1 gene in patients with breast cancer treated with adjuvant therapy [21]. In bladder cancer patients, carriers of the variant allele for XRCC1 rs25489 polymorphism were less likely to die following instillation with BCG or MMC and radiotherapy [22]. Experimental efforts have been made to characterize biochemical activities of XRCC1 polymorphisms, and only rs25489 showed a mild defect in DNA binding capacity [23].
Reasons for the lack of evidence for SNPs and disparities in experimental results following instillation chemotherapy have yet to be fully explored. Sacerdote et al. found that four XRCC1 SNP variants (rs915927, rs2854509, rs2854501, and rs3213255) were associated with survival in bladder cancer patients treated with chemotherapy and conferred lower DNA repair capacity in nucleotide excision repair and double-strand break repair pathways [11]. This finding was confirmed by our study, because we showed that RFS in bladder cancer patients treated with EPI was significantly more favorable for patients with rs915927 AG/GG and rs2854501 CT/TT genotypes than homozygous patients with AA or CC genotypes. Biological effects of rs915927 A and rs2854501 C alleles may be explained by low efficiency of DNA repair capacity in response to DNA damage caused by chemotherapy, leading to apoptosis of tumor cells. Carcinogenesis of recurrent bladder cancer is often multi-genetic, therefore different levels of risk as well as genetic contributors conferred by individual factors may apply [24]. Our studies showed that patients carrying rs915927 AG/GG and rs2854501 CT/TT genotypes for XRCC1 polymorphisms had decreased risk compared with other genotypes.
In conclusion, our results suggest that polymorphisms in XRCC1 SNP variants (rs915927 and rs2854501) were associated with improved clinical outcomes following EPI, but not MMC, treatment. While we did not detect significant effects following MMC treatment, larger sample sizes maybe needed to verify our observations. If our findings are confirmed, these genetic variants could provide a basis for individualized treatment of bladder cancer.
Acknowledgements
The work was funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), by the Program for Development of Innovative Research Team in the First Affiliated Hospital of Nanjing Medical University, Provincial Initiative Program for Excellency Disciplines of Jiangsu Province, and by the National Natural Science Foundation of China [Grant number 81171963 and 81201571]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Lum BL, Torti FM. Adjuvant intravesicular pharmacotherapy for superficial bladder cancer. J Natl Cancer Inst. 1991;83:682–694. doi: 10.1093/jnci/83.10.682. [DOI] [PubMed] [Google Scholar]
- 3.Donat SM. Evaluation and follow-up strategies for superficial bladder cancer. Urol Clin North Am. 2003;30:765–776. doi: 10.1016/s0094-0143(03)00060-0. [DOI] [PubMed] [Google Scholar]
- 4.Shelley MD, Mason MD, Kynaston H. Intravesical therapy for superficial bladder cancer: a systematic review of randomised trials and meta-analyses. Cancer Treat Rev. 2010;36:195–205. doi: 10.1016/j.ctrv.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Witjes JA, Hendricksen K. Intravesical pharmacotherapy for non-muscle-invasive bladder cancer: a critical analysis of currently available drugs, treatment schedules, and long-term results. Eur Urol. 2008;53:45–52. doi: 10.1016/j.eururo.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Oosterlinck W, Lobel B, Jakse G, Malmstrom PU, Stockle M, Sternberg C European Association of Urology (EAU) Working Group on Oncological Urology. Guidelines on bladder cancer. Eur Urol. 2002;41:105–112. doi: 10.1016/s0302-2838(01)00026-4. [DOI] [PubMed] [Google Scholar]
- 7.Soloway MS. Introduction and overview of intravesical therapy for superficial bladder cancer. Urology. 1998;31:5–16. [PubMed] [Google Scholar]
- 8.Popert RJ, Goodall J, Coptcoat MJ, Thompson PM, Parmar MK, Masters JR. Superficial bladder cancer: the response of a marker tumour to a single intravesical instillation of epirubicin. Br J Urol. 1994;74:195–199. doi: 10.1111/j.1464-410x.1994.tb16585.x. [DOI] [PubMed] [Google Scholar]
- 9.Eto H, Oka Y, Ueno K, Nakamura I, Yoshimura K, Arakawa S, Kamidono S, Obe S, Ogawa T, Hamami G, et al. Comparison of the prophylactic usefulness of epirubicin and doxorubicin in the treatment of superficial bladder cancer by intravesical instillation: a multicenter randomized trial. Kobe University Urological Oncology Group. Cancer Chemother Pharmacol. 1994;35(Suppl):S46–51. doi: 10.1007/BF00686919. [DOI] [PubMed] [Google Scholar]
- 10.Wu GQ, Liu NN, Xue XL, Cai LT, Zhang C, Qu QR, Yan XJ. Multiplex Real-time PCR for RRM1, XRCC1, TUBB3 and TS mRNA for Prediction of Response of Non-small Cell Lung Cancer to Chemoradiotherapy. Asian Pac J Cancer Prev. 2014;15:4153–4158. doi: 10.7314/apjcp.2014.15.10.4153. [DOI] [PubMed] [Google Scholar]
- 11.Sacerdote C, Guarrera S, Ricceri F, Pardini B, Polidoro S, Allione A, Critelli R, Russo A, Andrew AS, Ye Y, Wu X, Kiemeney LA, Bosio A, Casetta G, Cucchiarale G, Destefanis P, Gontero P, Rolle L, Zitella A, Fontana D, Vineis P, Matullo G. Polymorphisms in the XRCC1 gene modify survival of bladder cancer patients treated with chemotherapy. Int J Cancer. 2013;133:2004–2009. doi: 10.1002/ijc.28186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Au WW, Salama SA, Sierra-Torres CH. Functional characterization of polymorphisms in DNA repair genes using cytogenetic challenge assays. Environ Health Perspect. 2003;111:1843–1850. doi: 10.1289/ehp.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamerdin JE, Montgomery MA, Stilwagen SA, Scheidecker LK, Tebbs RS, Brookman KW, Thompson LH, Carrano AV. Genomic sequence comparison of the human and mouse XRCC1 DNA repair gene regions. Genomics. 1995;25:547–554. doi: 10.1016/0888-7543(95)80056-r. [DOI] [PubMed] [Google Scholar]
- 14.Ricceri F, Matullo G, Vineis P. Is there evidence of involvement of DNA repair polymorphisms in human cancer? Mutat Res. 2012;736:117–121. doi: 10.1016/j.mrfmmm.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Babjuk M, Oosterlinck W, Sylvester R, Kaasinen E, Bohle A, Palou-Redorta J, Rouprêt M European Association of Urology (EAU) EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Eur Urol. 2011;59:997–1008. doi: 10.1016/j.eururo.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 16.Arizono K, Osada Y, Kuroda Y. DNA repair gene hOGG1 codon 326 and XRCC1 codon 399 polymorphisms and bladder cancer risk in a Japanese population. Jpn J Clin Oncol. 2008;38:186–191. doi: 10.1093/jjco/hym176. [DOI] [PubMed] [Google Scholar]
- 17.Sakano S, Wada T, Matsumoto H, Sugiyama S, Inoue R, Eguchi S, Ito H, Ohmi C, Matsuyama H, Naito K. Single nucleotide polymorphisms in DNA repair genes might be prognostic factors in muscle-invasive bladder cancer patients treated with chemoradiotherapy. Br J Cancer. 2006;95:561–570. doi: 10.1038/sj.bjc.6603290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cullinane C, Cutts SM, Panousis C, Phillips DR. Interstrand cross-linking by adriamycin in nuclear and mitochondrial DNA of MCF-7 cells. Nucleic Acids Res. 2000;28:1019–1025. doi: 10.1093/nar/28.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cummings J, Spanswick VJ, Tomasz M, Smyth JF. Enzymology of mitomycin C metabolic activation in tumour tissue: implications for enzyme-directed bioreductive drug development. Biochem Pharmacol. 1998;56:405–414. doi: 10.1016/s0006-2952(98)00073-2. [DOI] [PubMed] [Google Scholar]
- 20.Sun X, Li F, Sun N, Shukui Q, Baoan C, Jifeng F, Lu C, Zuhong L, Hongyan C, YuanDong C, Jiazhong J, Yingfeng Z. Polymorphisms in XRCC1 and XPG and response to platinum-based chemotherapy in advanced non-small cell lung cancer patients. Lung Cancer. 2009;65:230–236. doi: 10.1016/j.lungcan.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 21.Przybylowska-Sygut K, Stanczyk M, Kusinska R, Kordek R, Majsterek I. Association of the Arg194Trp and the Arg399Gln polymorphisms of the XRCC1 gene with risk occurrence and the response to adjuvant therapy among Polish women with breast cancer. Clin Breast Cancer. 2013;13:61–68. doi: 10.1016/j.clbc.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 22.Sanyal S, De Verdier PJ, Steineck G, Larsson P, Onelov E, Hemminki K, Kumar R. Polymorphisms in XPD, XPC and the risk of death in patients with urinary bladder neoplasms. Acta Oncol. 2007;46:31–41. doi: 10.1080/02841860600812693. [DOI] [PubMed] [Google Scholar]
- 23.Wilson DM 3rd, Kim D, Berquist BR, Sigurdson AJ. Variation in base excision repair capacity. Mutat Res. 2011;711:100–112. doi: 10.1016/j.mrfmmm.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watchko JF, Lin Z, Clark RH, Kelleher AS, Walker MW, Spitzer AR Pediatrix Hyperbilirubinemia Study Group. Complex multifactorial nature of significant hyperbilirubinemia in neonates. Pediatrics. 2009;124:e868–877. doi: 10.1542/peds.2009-0460. [DOI] [PubMed] [Google Scholar]


