Abstract
There is now growing evidence supporting the association between renal insufficiency and accumulation of plasma homocysteine (Hcy). However, the role of Hcy in the development of diabetic nephropathy (DN) in type 2 diabetic patients is not clearly elucidated. To this end, we performed a prospective observational study in 208 patients and 49 controls. We show that baseline level of Hcy is significantly enhanced in patients with DN and is associated with the severity of the disease. Focusing on patients at early DN stage (n = 157), after four-year follow-up, we find that increase in plasma Hcy level correlates with greater renal failure characterized by faster decline in estimated glomerular filtration rate (eGFR). Using a multivariate linear regression model, we show that plasma Hcy remains significantly associated with eGFR decline after controlling for other progression promoters. Our results support that plasma Hcy is an independent risk factor as well as an early predictor for DN progression in type 2 diabetic patients.
Keywords: Homocysteine, diabetic nephropathy, glomerular filtration rate
Introduction
DN is a serious and progressive complication of diabetes mellitus that affects over 30% of all type 2 diabetic patients [1,2]. It is a major risk factor that accounts for the reduced life expectancy of type 2 diabetic patients [2]. The first manifestation of DN is typically the presence of microalbuminuria, which is characterized by a urine albumin-to-creatinine ratio (u-ACR) greater than 30 mg/g Cr. Without specific interventions, 20-40% of type 2 diabetic patients with microalbuminuria will progress to more advanced stages of DN with severe renal dysfunction, and eventually to renal failure. Indeed, recent studies have shown that DN has become the leading single cause of end-stage renal disease (ESRD) [2,3].
DN develops due to a complex interaction between genetic and environmental pathophysiological factors and is linked to a progressive decline in kidney function. Accordingly, early diagnosis of DN is critical to initiate inventions and to prevent the long term damaging effects of kidney dysfunction. Although abnormal albuminuria is typically considered as an important prognostic marker for the early detection of DN, however, it has several limitations. It has been suggested that microalbuminuria could only be observed after severe renal dysfunctions, such as impaired glomerular filtration barrier, have already occurred so that it is not a good early marker [4-7]. Moreover, a large number of diabetic patients could still develop DN even if their urinary albumin levels are normal, which challenges the sensitivity of albuminuria as a specific marker for DN. Accordingly, more sensitive and specific biomarkers are desired to detect DN at early stage [7-13].
Hcy is a sulfur-containing amino acid generated by methionine demethylation and is not present in food. It was reported that serum Hcy level is high in patients with ESRD [14,15]. A number of studies also showed that enhanced plasma Hcy level is associated with increasing urinary albumin excretion in diabetic patients [16-18]. There is also evidence supporting that Hcy abundance is closely related to renal status in the elderly. These results all suggest that Hcy is a marker of impaired renal function in diabetic patients. However, it remains unclear whether Hcy accumulation is playing a causative role that precedes early renal injury, or is only a secondary effect caused by impaired renal function in diabetic patients.
We propose that this question could be elucidated by examining the significance of baseline Hcy level in predicting the progression of DN in Type 2 diabetes at early stage of the disease. To this end, we have performed a prospective observational study in which type 2 diabetic patients with normo- or microalbuminuria were followed up for ~four years with routine measurement of creatinine and urinal albumin excretion. This allows us to investigate the potential link between Hcy level, albuminuria and glomerular filtration rate as well as the role of Hcy as an independent determinant for DN progression.
Materials and methods
Subjects
A total of 208 Chinese patients with Type 2 diabetes diabetic patients who had been referred to department of nephrology between 2009 and 2014 in XinXiang Central Hospital were enrolled in this study. Diabetes status was biochemically confirmed based on the WHO diagnostic criteria. Patients were stratified by urine Albumin/urine Creatinine (u-ACR) into three groups: normoalbuminuria group (<30 mg/g Cr), microalbuminuria group (30-299 mg/g Cr), and macroalbuminuria group (>300 mg/g Cr). Patients with cardiovascular disease, acute inflammatory disease or other conditions related to nephropathy in addition to retinopathy, neuropathy and macrovascular diseases were excluded. Age and gender -matched nondiabetic controls were randomly selected who fulfilled the following inclusion criteria: normal glucose tolerance (fasting plasma glucose <6.0 mmol/l and HbA1c (A1C) <6%), normal blood pressure (SBP <140 mmHg and DBP <90 mmHg), u-ACR <30 mg/g Cr, Egfr >60 mL/min/1.73 m2, serum creatinine <1.2 mg/dL, with no known history of diabetes, renal disease or cardiovascular disease. The study was approved by the scientific and ethic committee of Xinxiang Central Hospital. Informed consent was obtained from all patients and experiments were performed in accordance with relevant guidelines.
Measurements
Fasting plasma and random spot urine were collected from subjects at their clinic visits when anthropometric measurements were performed. Medical histories were obtained from direct interview with the patients. Fasting plasma Hcy concentration was measured using a automated fluorescence polarization immunoassay (FPIA) from Abbott Diagnostics. Labora-torial variables such as fasting plasma glucose, glycated hemoglobin (HbA1C), plasma cholesterol and triacylglycerol and urinary/serum creatinine and were measured using conventional laboratorial techniques.
The following Modification of Diet in Renal Disease (MDRD) formula was used to calculate eGFR in Chinese people [19]: MDRD = 186 × (serum creatinine [mg/dL]) - 1.154 × (age in years) - 0.203 × 0.742 (if female). All values obtained from the follow-up of a same individual were fit into a linear regression to calculate the annual changes in eGFR.
Statistical analysis
Data analysis was performed using SPSS version 15.0 (SPSS, Chicago, IL, USA) for Windows. The significance of difference between different groups were calculated by ANOVA, followed by Bonferroni’s test for normally distributed values and by the Kruskal-Wallis test for nonparametric values. Uni- or multivariate linear regression analyses were used to determine whether Hcy is an independent predictor of decline in eGFR in diabetic patients. In multivariate models the effect of Hcy was adjusted for progression promoters that were significantly associated with u-ACR abundance (model 2), or all clinic parameters (model 3). A P value <0.05 was considered significant.
Results
Clinical characteristics
The baseline clinical characteristics of the 49 control and 208 type 2 diabetic patients are shown in Table 1. Subjects in the normoalbuminuria, microalbuminuriaand macroalbuminuria groups were well matched with those in the control group in terms of age, gender and body mass index (BMI), except that BMI of patients with microalbuminuria is slightly lower. As expected, patients with worse albuminuria status are more likely to have longer duration of diabetes, higher levels of hemoglobin A1c (HbA1c), higher systolic blood pressure (SBP) and diastolic blood pressure (DBP). Little difference was observed for total cholesterol (TCHOL), triglyceride or low-density lipoprotein (LDL). We also observed a small reduction of High-density lipoprotein (HDL) levels in normoalbuminuria and microablbuminuria groups than control. Consistent with reduced eGFR levels in microalbuminuria and macroalbuminuria groups, enhanced urine albumin levels are in these patients, indicating impaired kidney function. As expected, more hypoglycemic agents and antihypertensive agents were administrated in patients with normoalbuminuria and macroablbuminuria.
Table 1.
Baseline Clinical Characteristics of control and type 2 diabetic patients stratified by albuminuria status
| Control | Normo | Microal | Macroal | p-value | |
|---|---|---|---|---|---|
| Number | 49 | 72 | 85 | 51 | |
| Age (years) | 62.16±8.84 | 60.47±11.44 | 61.79±11.84 | 62.07±11.08 | 0.223 |
| Gender (male/female) | 28/21 | 30/42 | 41/44 | 28/23 | 0.316 |
| BMI (kg/m2) | 23.33±3.85 | 23.68±3.92 | 23.18±3.97 | 22.57±3.78§ | 0.482 |
| Duration of diabetes (years) | - | 6.33±3.31 | 9.61±4.81§ | 10.96±2.93§ | <0.001 |
| SBP (mmHg) | 117.65±9.25 | 121.69±15.72 | 124.83±11.14† | 129.37±12.60† | 0.073 |
| DBP (mmHg) | 74.46±9.34 | 78.25±10.29 | 77.59±8.04 | 80.56±9.37† | 0.021 |
| HbA1c (%) | 4.94±0.97 | 7.13±1.51‡ | 8.22±1.76‡ | 7.95±1.76‡ | <0.001 |
| TCHOL | 202.01±20.64 | 188.55±45.76 | 192.64±33.17 | 203.58±51.47 | 0.117 |
| Triglyceride (mg/dL)* | 126.6 (91.6-153.9) | 141.3 (93.4-179.5) | 136.7 (110.7-163.4) | 142.0 (104.1-176.8)† | 0.268 |
| LDL (mg/dL) | 116.00±26.01 | 111.67±39.04 | 110.61±28.98 | 113.67±35.56 | 0.591 |
| HDL (mg/dL) | 60.66±20.38 | 48.21±16.51† | 51.66±15.34† | 52.20±18.38 | 0.001 |
| eGFR (ml/min/1.73 m2) | 96.05±10.10 | 91.35±15.1 | 88.48±16.71†,‡ | 74.28±16.21†,‡,║,¶ | <0.001 |
| u-ACR (ng/mg)* | 13.3 (7.2-16.3) | 12.9 (8.9-17.1) | 94.5 (55.8-132.3)‡,║ | 829.1 (596.4-1076.8)‡,║,# | <0.001 |
| Homocysteine (μmol/L)* | 8.98±1.74 | 8.50±1.81 | 10.23±1.97‡,║ | 14.45±2.37‡,║,# | <0.001 |
| Hypoglycemic agents, (%) | - | 21 (29.2) | 47 (55.3)║ | 27 (53.0)║ | <0.001 |
| Antihypertensive agents, (%) | - | 16 (22.2) | 56 (55.9)║ | 32 (62.7)║ | <0.001 |
Note: Data are reported as mean ± SD for normally distributed values or median (interquartile range) for non-normally distributed values. P-value indicates the significance of differences between group means as determined by ANOVA.
Logarithm-transformed values were used;
P<0.05 vs. control;
P<0.001 vs. control;
P<0.05 vs. normo;
P<0.001 vs. normo;
P<0.05 vs. micro;
P<0.001 vs. micro.
Abbreviation: BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; TCHOL, total cholesterol; LDL, low-density lipoprotein; HDL, high-density lipoprotein; u-ACR, Urine Albumin/urine Creatinine.
Plasma total Hcy level is significantly enhanced in type 2 diabetic patients with worse albuminuria status
As shown in Figure 1 and Table 1, there is no difference in plasma total Hcy levels between control (8.98±1.74 μmol/L) and diabetic patients with normoalbuminuria (8.50±1.81 μmol/L). However, Hcy level is significantly enhanced in patients with microalbuminuria (10.23±1.97 μmol/L) than those with normoalbuminuria (P<0.001, Bonferroni’s test). Even higher levels of Hcy are observed in patients with macroalbuminuria (14.45±2.37 μmol/L) than microalbuminuria (P<0.001, Bonferroni’s test). These results are in consistent with several prior studies, supporting that changes in Hcy is tightly linked to dysfunction of kidney in diabetic patients.
Figure 1.
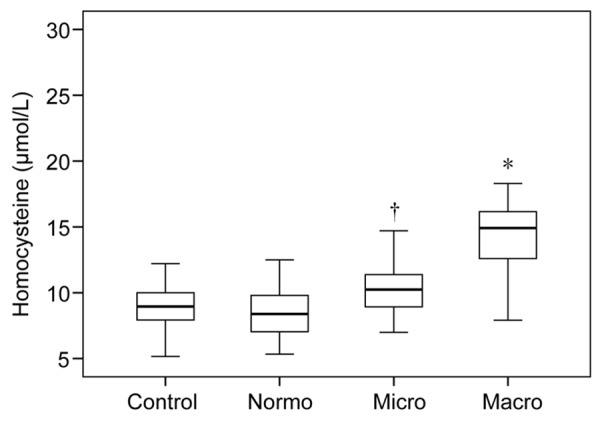
Plasma homocysteine levels in control and type 2 diabetic patients. Box plot graph indicates the quartiles of the values measured in each group. Normo: normoalbuminuria; Micro: microalbuminuria; Macro: macroalbuminuria. *P<0.001 vs. Micro. †P<0.001 vs. Normo (Bonferroni’s test).
Baseline level of Hcy is an independent predictor for the progression of DN at early stage
A high number of diabetic patients are found to have DN shortly after the diagnosis of their diabetes, because diabetes is actually present for many years and damages to the kidney might occur long before the diagnosis is made. Accordingly, early diagnosis of DN is critical and identification of determinants that precede renal failure early in the course of the disease is highly required.
We thus ask whether Hcy may function as an early marker for the development of DN at early stage. To this end, 157 patients with normo- or microalbuminuria were followed up for 46 (40-52) months with routine measurement of creatinine and urinal albumin excretion. This allows us to obtain the annual decline in eGFR which indicates progression of DN in these diabetic patients. During follow-up, we observed a median annual decline in eGFR of 3.52±1.46 ml/min/1.73 m2). As shown in Figure 2, baseline level of Hcy is positively correlated with annual decline in eGFR.
Figure 2.
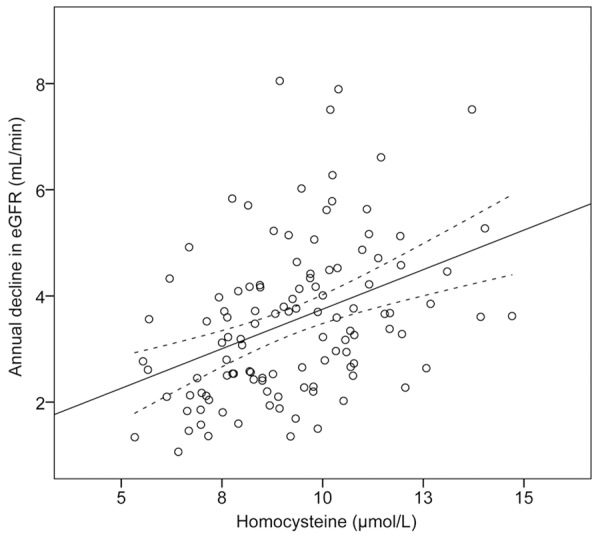
Correlation between baseline homocysteine abundance and annual decline in eGFR. Data from 157 diabetic patients included in the four years’ follow-up were graphed in the scatterplot. The linear regression line and the confidence intervals are also shown.
We then performed a multivariable linear regression analysis to test whether baseline level of Hcy is significantly associated with annual decline in eGFR (Table 2). Using annual decline in eGFR as a dependent variable, in a uni-variate linear regression model (model 1), we found that baseline level of Hcy is significantly correlated with annual decline in eGFR (β = 0.393, P<0.001). We then use a multi-variate linear regression model to control for the influence of potential progression promoters that are significantly linked to albuminuria status (model 2), including u-ACR and eGFR. Hcy remains significantly correlated with annual decline in eGFR (β = 0.262, P = 0.013). After adjusting for all clinical variables (model 3), the correlation between Hcy and annual decline in eGFR is still significant (β = 0.254, P = 0.015). Of the other variables, u-ACR level is significantly linked to annual decline in eGFR (β = 0.289, P = 0.011) and the effect is largely independent of Hcy. These results indicate that high Hcy level might play a role in the pathogenesis of DN and function as an independent predictor for the progression of DN at early stage.
Table 2.
Multiple linear regression analyses of the association between homocysteine abundance and annual decline in eGFR. as a dependent variable in type 2 diabetic patients
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Homocysteine (μmol/L)* | 0.393 (<0.001) | 0.262 (0.013) | 0.254 (0.015) |
| Duration of diabetes (years) | 0.022 (0.816) | 0.04 (0.686) | |
| HbA1c (%) | 0.005 (0.954) | 0.003 (0.975) | |
| HDL (mg/dL) | -0.004 (0.968) | -0.016 (0.883) | |
| eGFR (ml/min/1.73 m2) | 0.138 (0.121) | 0.131 (0.173) | |
| u-ACR (ng/mg)* | 0.295 (0.01) | 0.289 (0.011) | |
| Hypoglycemic agents, (%) | 0.146 (0.13) | 0.142 (0.168) | |
| Antihypertensive agents, (%) | -0.125 (0.244) | -0.156 (0.172) | |
| Age (years) | -0.01 (0.921) | ||
| Gender (male/female) | 0.072 (0.459) | ||
| BMI (kg/m2) | 0.099 (0.331) | ||
| SBP (mmHg) | 0.098 (0.368) | ||
| DBP (mmHg) | -0.168 (0.161) | ||
| Triglyceride (mg/dL)* | 0.039 (0.699) | ||
| LDL (mg/dL) | 0.055 (0.569) |
Note: Annual decline in eGFR was used as a dependent variable in the regression. Model 1: unadjusted; Model 2: adjusted for variables significantly associated with albuminuria status in Table 1; Model 3: adjusted for all variables tested. Association between different variables and the annual decline in eGFR are shown as Standard β (p-value).
Log transformed value was used in the model.
Discussion
In this prospective observational study, we investigated the association of total plasma Hcy with albuminuria and its clinical applicability in predicting decline in kidney function in type 2 diabetic patients. In line with previous studies [18], we showed that Hcy level was significantly elevated in patients with micro- and macro albuminuria. Elevated level of plasma Hcy might result from disturbed Hcy clearance is in the failing kidney. Accordingly, we hypothesize that changes in plasma Hcy level might be a sensitive marker for alterations observed in the diabetic kidney and predictive of progression of DN at early stage [10,20]. To test this hypothesis, we performed a four-year follow-up of diabetic patients with no sign or at early stage of the disease characterized by normo- or microalbuminuria. Our data show that plasma Hcy level is a strong independent predictor for annual eGFR decline over time. We thus suggest that elevated Hcy is an early event linked to the development of DN in type 2 diabetic patients.
There are several possible mechanisms that may lead to plasma Hcy accumulation during DN development in diabetic patients. Several in vitro and in vivo studies suggested that impaired kidney function is associated with defects in renal Hcy clearance, probably by affecting the transsulfuration pathway that catalyzes Hcy into cysteine and α-ketobutyrate [21]. It has also been shown that extrarenal Hcy metabolisms, including the sulfur amino acid metabolism and Hcy remethylation, are impaired during DN development, leading to accumulation of plasma Hcy. In line with our observation that baseline Hcy is an independent predictor for the annual decline of eGFR, there is also evidence suggesting that enhanced Hcy is causally linked to kidney damage in type 2 diabetic patients [21]. Enhanced Hcy level could induce oxidative stress, which may, result in matrix accumulation and hypertension, and in part, in part, be responsible for endothelial dysfunction and impaired elastic properties of the vascular wall [22].
Gene expression is regulated at multiple levels [5,23-28], genetic and/or molecular regulators controlling the expression of enzymes critical for homocysteine production or metabolism might thus affect the risk of DN in these diabetic patients [29,30]. For example, it is well-known that a single SNP of methylenetetrahydrofolate reductase (MTHFR) gene that change alanine to valine (p.A222V) significantly affect baseline homocysteine level. Functional analyses combined with an association study also revealed that SNPs in the promoter region of coactivator- associated arginine methyltransferase 1 (CARM1) gene are associated with expression level of the gene and homocysteine production [31]. Recently, the peroxisome proliferator-activated receptor (PPARγ) is identified as a key regulator of Hcy abundance via modulating Hcy turnover. PPARγ agonist could promote Hcy clearance and reduces blood pressure [32]. These results establish Hcy and its regulators as potential drug targets for DN prevention and therapy.
While at present, it is premature to conclude that high Hcy level is a causative risk factor for DN, there is substantial indirect evidence supporting its damaging effects to the kidney. Further studies are needed to elucidate the signaling pathways and regulatory networks linking Hcy accumulation to DN progression [13,33-35]. Nevertheless, in this study, we observed that at least in a subset of patients, significant plasma Hcy accumulation occurs before the onset of microalbuminuria and is tightly linked to the progression of DN, supporting the model that Hcy is causally linked to renal failure in these patients. We thus suggest that plasma Hcy is a useful marker for the progression of DN in type 2 diabetic patients at early stage.
Disclosure of conflict of interest
None.
References
- 1.Nielsen SE, Reinhard H, Zdunek D, Hess G, Gutiérrez OM, Wolf M, Parving HH, Jacobsen PK, Rossing P. Tubular markers are associated with decline in kidney function in proteinuric type 2 diabetic patients. Diabetes Res Clin Pract. 2012;97:71–76. doi: 10.1016/j.diabres.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Choudhury D, Tuncel M, Levi M. Diabetic nephropathy-- a multifaceted target of new therapies. Discov Med. 2010;10:406–415. [PubMed] [Google Scholar]
- 3.Cohen-Bucay A, Viswanathan G. Urinary markers of glomerular injury in diabetic nephropathy. Int J Nephrol. 2012;2012:146987. doi: 10.1155/2012/146987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaidya VS, Waikar SS, Ferguson MA, Collings FB, Sunderland K, Gioules C, Bradwin G, Matsouaka R, Betensky RA, Curhan GC, Bonventre JV. Urinary biomarkers for sensitive and specific detection of acute kidney injury in humans. Clin Transl Sci. 2008;1:200–208. doi: 10.1111/j.1752-8062.2008.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rossing P, Rossing K, Gaede P, Pedersen O, Parving HH. Monitoring kidney function in type 2 diabetic patients with incipient and overt diabetic nephropathy. Diabetes Care. 2006;29:1024–1030. doi: 10.2337/diacare.2951024. [DOI] [PubMed] [Google Scholar]
- 6.Matteucci E, Rossi L, Mariani S, Fagnani F, Quilici S, Cinapri V, Giampietro O. Blood levels of total homocysteine in patients with type 1 diabetes (with no complications, diabetic nephropathy and/or retinopathy) and in their non-diabetic relatives. Nutr Metab Cardiovasc Dis. 2002;12:184–189. [PubMed] [Google Scholar]
- 7.Brazionis L, Rowley K Sr, Itsiopoulos C, Harper CA, O’Dea K. Homocysteine and diabetic retinopathy. Diabetes Care. 2008;31:50–56. doi: 10.2337/dc07-0632. [DOI] [PubMed] [Google Scholar]
- 8.Huang EJ, Kuo WW, Chen YJ, Chen TH, Chang MH, Lu MC, Tzang BS, Hsu HH, Huang CY, Lee SD. Homocysteine and other biochemical parameters in Type 2 diabetes mellitus with different diabetic duration or diabetic retinopathy. Clin Chim Acta. 2006;366:293–298. doi: 10.1016/j.cca.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 9.Fotiou P, Raptis A, Apergis G, Dimitriadis G, Vergados I, Theodossiadis P. Vitamin status as a determinant of serum homocysteine concentration in type 2 diabetic retinopathy. J Diabetes Res. 2014;2014:807209. doi: 10.1155/2014/807209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bulum T, Blaslov K, Duvnjak L. Plasma Homocysteine is Associated with Retinopathy in Type 1 Diabetic Patients in the Absence of Nephropathy. Semin Ophthalmol. 2014 doi: 10.3109/08820538.2014.912338. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Aydemir O, Turkcuoglu P, Guler M, Celiker U, Ustündağ B, Yilmaz T, Metin K. Plasma and vitreous homocysteine concentrations in patients with proliferative diabetic retinopathy. Retina. 2008;28:741–743. doi: 10.1097/IAE.0b013e31816079fb. [DOI] [PubMed] [Google Scholar]
- 12.Chan CT, Deng W, Li F, DeMott MS, Babu IR, Begley TJ, Dedon PC. Highly predictive reprogramming of tRNA modifications is linked to selective expression of codon-biased genes. Chem Res Toxicol. 2015;28:978–88. doi: 10.1021/acs.chemrestox.5b00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez MF, Krastins B, Sarracino DA, Byram G, Vogelsang MS, Prakash A, Peterman S, Ahmad S, Vadali G, Deng W, Inglessis I, Wickham T, Feeney K, Dec GW, Palacios I, Buonanno FS, Lo EH, Ning M. Proteomic signatures of serum albumin-bound proteins from stroke patients with and without endovascular closure of PFO are significantly different and suggest a novel mechanism for cholesterol efflux. Clin Proteomics. 2015;12:2. doi: 10.1186/1559-0275-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sabanayagam C, Shankar A. Association between plasma homocysteine and microalbuminuria in persons without hypertension, diabetes mellitus, and cardiovascular disease. Clin Exp Nephrol. 2011;15:92–99. doi: 10.1007/s10157-010-0361-5. [DOI] [PubMed] [Google Scholar]
- 15.Sen U, Tyagi SC. Homocysteine and Hypertension in Diabetes: Does PPARgamma Have a Regulatory Role? PPAR Res. 2010;2010:806538. doi: 10.1155/2010/806538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hellgren M, Melander A, Ostgren CJ, Rastam L, Lindblad U. Inverse association between plasma homocysteine, sulphonylurea exposure and physical activity: a community-based sample of type 2 diabetes patients in the Skaraborg hypertension and diabetes project. Diabetes Obes Metab. 2005;7:421–429. doi: 10.1111/j.1463-1326.2004.00431.x. [DOI] [PubMed] [Google Scholar]
- 17.Glowinska B, Urban M, Peczynska J, Florys B, Szydlowska E. Elevated concentrations of homocysteine in children and adolescents with arterial hypertension accompanying Type 1 diabetes. Med Sci Monit. 2001;7:1242–1249. [PubMed] [Google Scholar]
- 18.Hovind P, Tarnow L, Rossing P, Teerlink T, Stehouwer CD, Emeis JJ, Parving HH. Progression of diabetic nephropathy: role of plasma homocysteine and plasminogen activator inhibitor-1. Am J Kidney Dis. 2001;38:1376–1380. doi: 10.1053/ajkd.2001.29261. [DOI] [PubMed] [Google Scholar]
- 19.Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, Xu JS, Huang SM, Wang LN, Huang W, Wang M, Xu GB, Wang HY. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17:2937–2944. doi: 10.1681/ASN.2006040368. [DOI] [PubMed] [Google Scholar]
- 20.Lim CP, Loo AV, Khaw KW, Sthaneshwar P, Khang TF, Hassan M, Subrayan V. Plasma, aqueous and vitreous homocysteine levels in proliferative diabetic retinopathy. Br J Ophthalmol. 2012;96:704–707. doi: 10.1136/bjophthalmol-2011-301044. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Shi M, Zhang H, Yan L, Xie M, Zhuang L, Zhu Y, Chen J. Relation of homocysteine to early nephropathy in patients with Type 2 diabetes. Clin Nephrol. 2012;77:305–310. doi: 10.5414/cn107296. [DOI] [PubMed] [Google Scholar]
- 22.Xu C, Wu Y, Liu G, Liu X, Wang F, Yu J. Relationship between homocysteine level and diabetic retinopathy: a systematic review and meta-analysis. Diagn Pathol. 2014;9:167. doi: 10.1186/s13000-014-0167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin S, Deng W, Hu L, Kong X. The impact of nucleosome positioning on the organization of replication origins in eukaryotes. Biochem Biophys Res Commun. 2009;385:363–368. doi: 10.1016/j.bbrc.2009.05.072. [DOI] [PubMed] [Google Scholar]
- 24.Yin S, Deng W, Zheng H, Zhang Z, Hu L, Kong X. Evidence that the nonsense-mediated mRNA decay pathway participates in X chromosome dosage compensation in mammals. Biochem Biophys Res Commun. 2009;383:378–382. doi: 10.1016/j.bbrc.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 25.Yin S, Wang P, Deng W, Zheng H, Hu L, Hurst LD, Kong X. Dosage compensation on the active X chromosome minimizes transcriptional noise of X-linked genes in mammals. Genome Biol. 2009;10:R74. doi: 10.1186/gb-2009-10-7-r74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yin S, Yang J, Lin B, Deng W, Zhang Y, Yi X, Shi Y, Tao Y, Cai J, Wu CI, Zhao G, Hurst LD, Zhang J, Hu L, Kong X. Exome sequencing identifies frequent mutation of MLL2 in non-small cell lung carcinoma from Chinese patients. Sci Rep. 2014;4:6036. doi: 10.1038/srep06036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lei H, Zhai B, Yin S, Gygi S, Reed R. Evidence that a consensus element found in naturally intronless mRNAs promotes mRNA export. Nucleic acids research. 2013;41:2517–2525. doi: 10.1093/nar/gks1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang P, Yin S, Zhang Z, Xin D, Hu L, Kong X, Hurst LD. Evidence for common short natural trans sense-antisense pairing between transcripts from protein coding genes. Genome biology. 2008;9:R169. doi: 10.1186/gb-2008-9-12-r169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim SS, Song SH, Kim IJ, Yang JY, Lee JG, Kwak IS, Kim YK. Clinical implication of urinary tubular markers in the early stage of nephropathy with type 2 diabetic patients. Diabetes Res Clin Pract. 2012;97:251–257. doi: 10.1016/j.diabres.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 30.Barratt J, Topham P. Urine proteomics: the present and future of measuring urinary protein components in disease. CMAJ. 2007;177:361–368. doi: 10.1503/cmaj.061590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu X, Hao Y, Wang L, Li H, Lu X, Cao J, Hu Y, Mo X, Peng X, Gu D. Functional analysis of single-nucleotide polymorphisms in the regulation of coactivator-associated arginine methyltransferase 1 expression and plasma homocysteine levels. Circ Cardiovasc Genet. 2014;7:642–649. doi: 10.1161/CIRCGENETICS.113.000408. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez WE, Sen U, Tyagi N, Kumar M, Carneal G, Aggrawal D, Newsome J, Tyagi SC. PPAR gamma agonist normalizes glomerular filtration rate, tissue levels of homocysteine, and attenuates endothelial-myocyte uncoupling in alloxan induced diabetic mice. Int J Biol Sci. 2008;4:236–244. doi: 10.7150/ijbs.4.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan CT, Pang YL, Deng W, Babu IR, Dyavaiah M, Begley TJ, Dedon PC. Reprogramming of tRNA modifications controls the oxidative stress response by codon-biased translation of proteins. Nat Commun. 2012;3:937. doi: 10.1038/ncomms1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng WJ, Nie S, Dai J, Wu JR, Zeng R. Proteome, phosphoproteome, and hydroxyproteome of liver mitochondria in diabetic rats at early pathogenic stages. Mol Cell Proteomics. 2010;9:100–116. doi: 10.1074/mcp.M900020-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ben Ameur R, Molina L, Bolvin C, Kifagi C, Jarraya F, Ayadi H, Molina F, Granier C. Proteomic approaches for discovering biomarkers of diabetic nephropathy. Nephrol Dial Transplant. 2010;25:2866–2875. doi: 10.1093/ndt/gfq258. [DOI] [PubMed] [Google Scholar]


