Abstract
Objective: To explore the effects of ketamine abuse on the concentration of dopamine (DA), a monoamine neurotransmitter, and the mRNA expression of dopamine type 2 (D2) receptors in brain tissue, we used male Wistar rats to model ketamine abuse through chronic intraperitoneal infusion of ketamine across different doses. Methods: The rats were sacrificed 45 minutes and 1, 2, and 3 weeks after initiating the administration of ketamine or normal saline, as well as 3 days following discontinuation. Brain tissue was harvested to examine the concentration of 2,5-dihydroxyphenylacetic acid and homovanillic acid, the primary metabolites of DA, as well as the expression of D2 receptor mRNA. In addition, behavioral changes were observed within 30 minutes of administration, and withdrawal symptoms were also documented. A factorial experimental design was used to investigate variations and correlations in the primary outcome measures across the four doses and five time points. Brain DA concentrations were significantly higher in the ketamine-treated groups compared with the saline-treated group, with 30 mg/kg > 10 mg/kg > 60 mg/kg > saline (P < 0.05). The D2 receptor mRNA expression exhibited an inverse downregulation pattern, with 30 mg/kg < 10 mg/kg < 60 mg/kg < saline (P < 0.05). In the 10 mg/kg and 30 mg/kg ketamine-treated groups, the DA concentration and D2 receptor mRNA level in the brain tissue correlated with the dose of ketamine (r = 0.752, r = -0.806), but no significant correlation was found in the 60 mg/kg group. Result: These findings indicated that chronic dosing with ketamine increased the concentration of DA in rat brain tissue by increasing DA release or interrupting DA degradation. D2 receptor mRNA expression likely decreased because of stimulation with excessive DA. Conclusion: High-dose (60 mg/kg) ketamine had potent paralyzing effects on the central nervous system of rats and weakened the excitatory effects of the limbic system. Brain DA and D2 receptor mRNA may be associated with ketamine abuse.
Keywords: Ketamine, rat brain, dopamine, dopamine type 2 receptor mRNA, abuse
Introduction
Ketamine, also called ‘K-powder’, is a derivative of N-1-pehnycyclohexy-piperidine and an antagonist of N-methyl-D-aspartic acid (NMDA) receptors [1]. Besides being an analgesic and anesthetic, ketamine excites the limbic system, and causes vivid hallucinations and separation status. Ketamine also has the potential to engender psychological dependence, and is recreationally abused. The mechanisms underlying ketamine abuse and chronic brain toxicity are under-studied. Studies have shown that heroin, cocaine, and amphetamine addiction is closely associated with the actions of dopamine (DA) and dopamine receptors (DR) in the nucleus accumbens area of the brain [2,3]. The primary mechanism involves the activation of the cerebral reward system; namely, the mesencephalon-limbic dopamine system [4]. In this system, DA neurons in the brainstem ventral tegmental area (VTA) project to the nucleus accumbens septum (NAs), the main areas receiving projects from the VTA. This VTA-NAs dopamine system is the core site mediating the rewarding effects of drug abuse. In the presence of these drugs, discharge of VTA dopaminergic neurons is potentiated, leading to increased release of dopamine into the NAs, modulation of dopaminergic signaling, and the rewarding effects of drug abuse. Additionally, DR has been implicated in the occurrence and progression of drug addiction, by allowing the drug to affect intracellular signaling pathways and alter gene expression.
Some studies show that ketamine can stimulate particular neurons to release DA [5]. This may be because ketamine blocks NMDA receptors on gamma-aminobutyric acid (GABA) neurons inside the thalamic reticular nucleus in a noncompetitive manner, and this reduces GABA release, dis-inhibits dopaminergic neurons, and leads to the production of excessive DA [6]. These effects may be associated with the abuse of ketamine. Meo et al. reported that ketamine could induce rats to exhibit significant conditioned place preferences, showing a potent potential to engender psychological dependence, which differs from traditional opiates [7]. Rodríguez-Arias et al. examined type 2 DA (D2) receptor knock-out mice, and found that the rewarding effects of morphine were completely inhibited in these animals, but physical withdrawal symptoms were retained. Therefore, it is likely that D2 receptors play a critical role in psychological dependence. Likewise, it has been reported that the number of brain D2 receptors is lower in cocaine abusers [8].
An increasing number of drug abusers indulge in the hallucinations and illusions caused by ketamine. It remains unknown whether psychological dependence is associated with DA and D2 receptors located in VTA-NAs, and whether brain levels of DA and D2 receptors in chronic abusers correlate with the dose and duration of ketamine abuse. In this study, we modeled ketamine abuse in Wistar rats by chronically injecting predefined doses of ketamine intraperitoneally. We aimed to examine variations in DA levels and D2 receptor mRNA expression in the brain. We also documented the withdrawal symptom of ketamine and the variations in these two markers following drug discontinuation. We attempted to investigate the associations between ketamine abuse and brain levels of DA and D2 receptor mRNA in the hopes of elucidating the pharmacological mechanisms underlying ketamine-induced neurotoxicity and ketamine abuse.
Materials and methods
Animals and specimens
We purchased adolescent male Wistar rats (n = 120), initially weighing 160-180 g, from the Laboratory Animal Center of Hebei Medical University (certificate No., 711044). All rats were housed in standard rodent cages (six animals per cage), and allowed free access to rodent chow and tap water. The housing environment was maintained on a 12-hour light/dark cycle, at 17°C, at a humidity of 45-65%. The animals were acclimated to this environment for 1 week before we conducted any experiments.
Rats (n = 120) were randomly assigned to a 0 mg/kg (saline control), 10 mg/kg (low dose), 30 mg/kg (medium dose), or 60 mg/kg (high-dose) group. Ketamine abuse was modeled by intraperitoneal injection at 07:00 am daily, whereas normal saline was given under the same protocol to the control group. Drug-elicited behaviors in the rats were observed following dosing. The rats were sacrificed at 45 minutes and 1, 2, and 3 weeks after initiation of drug or normal saline administration, and 3 days following discontinuation (six animals per time point). The behavioral changes associated with withdrawal symptoms were documented within 3 days of drug discontinuation. The left and right cerebral hemispheres were harvested following sacrifice for the examination of DA levels and D2 receptor mRNA expression, respectively.
Brain DA assay
Left hemisphere tissue, 0.1 g, was precisely weighed, homogenized in 1-mL of 0.15 mol·L-1 perchloric acid for 20 seconds, and centrifuged at 15 000 r·min-1 for 20 minutes. The supernatant was filtered through a 0.22-μm filter membrane, aliquoted, and stored at -80°C.
HPLC was performed using a Waters Symmetry C18 reversed phase chromatographic column, with a 3.9 mm × 150 mm, 5 μm pore (Waters Corporation, Milford, MA, USA) at room temperature. The mobile phase was composed of a 5.4 g NaH2PO4 and 2.4 g citric acid added to a 0.25 mL EDTA aqueous solution (100 μmol/L) and dissolved in 300 mL of ultrapure water. The solution was filtered through a 0.22-μm aqueous solution filter membrane. The filtered solution was supplemented with 0.184 g octanesulfonic acid sodium salt and 50 mL acetonitrile, and reconstituted to 500 mL using filtered and double-distilled water. The flow rate of the mobile phase was set at 0.6 mL/min. The HPLC system (CoulArray Multi-channel Electrochemical Array Detector for HPLC, Dionex Corporation, Sunnyvale, CA, USA) consisted of a 5600A electrochemical analyzer, ESA58 binary pump system, 542 automatic sample loader, and 6210 carbon graphite electrode. The electric potentials were set at -150 mV, +450 mV, +500 mV, and +550 mV. The sample load was 10 μL per test. The peak area was used to determine the brain DA concentration.
Brain D2 Receptor mRNA Assay
Anterior brain tissue (100 mg) was dissected on a chilled glass plate, collected into a 1.5 mL Eppendorf tube (Eppendorf, Hamburg, Germany), and supplemented with 1 mL of Trizol. Brain tissue was homogenized using a cell disrupter. The RNA sample was extracted using a mixture of 4:1 (v/v) chloroform (400 μL) and isopropanol (100 μL). The mixture was inverted and centrifuged at 13 000 r·min-1 for 5 minutes. The upper-layer aqueous phase was moved into a 1.5 mL Eppendorf tube, supplemented with 400 μL isopropanol, and centrifuged at 13 000 r·min-1 for 3 minutes. The upper-layer liquid was discarded. The precipitate was resuspended in 500 μL ethanol (80%) using a vortex, and centrifuged at 13 000 r·min-1 for 3 minutes. The supernatant was removed, and the precipitate was rewashed in 80% ethanol. The tube was inverted for air drying. RNA samples were dissolved in 40 μL diethylpyrocarbonate water and stored at -70°C.
For reverse transcription-PCR, diethylpyrocarbonate water (6 μL), 100 μmol/L of the random primers (1 μL), and 1 μg of the RNA template (4 μL) were centrifuged and mixed. The mixture was placed in a PCR thermal cycler (Applied Biosystems, Inc., Carlsbad, CA, USA) at 70°C for 5 minutes. The mixture was further supplemented with a 5-fold concentration of buffer (4 μL), 20 μg/μL of RNA inhibitor (1 μL), and 10 mmol/L of dNTP (2 μL), and then centrifuged and vortexed. After allowing the reaction to proceed at 25°C for 5 minutes, the reverse transcription enzyme was added at a concentration of 200 μg/μL and a volume of 1 μL, and the reaction was allowed to further proceed at 25°C for 10 minutes, 42°C for 60 minutes, and 72°C for 10 minutes.
For real-time quantitative fluorescent PCR, we used a quantitative fluorescent PCR system that consisted of 100 μmol/L of upstream primer (1 μL), 100 μmol/L of downstream primer (1 μL), 100 μmol/L of the robe (1.5 μL), 10-fold concentration of buffer (50 μL), 2.5 μmol/L of dNTP (30 μL), 5 μg/μL of Taq polymerase (5 μL), and water (420 μL). The mixture was vortexed and aliquoted to 18 μL, and further supplemented with 2 μL of the target gene or housekeeping gene cDNA (2 μL), and then vortexed and centrifuged. The thermal cycling conditions we used for the quantitative fluorescent PCR were pre-denaturing at 94°C for 2 minutes, denaturing at 94°C for 20 seconds, and annealing and extending at 60°C for 45 seconds, for a total of 40 cycles. The Ct-values for the target and housekeeping genes were documented, and the 2-ΔΔCt value was used to semiquantitatively express D2 receptor mRNA expression.
Statistical analysis
All data are expressed as the mean ± SD. SPSS 13.0 (SPSS, Inc., Chicago, IL, USA) was used with a factorial design in the analysis of variance in DA levels and D2 receptor mRNA expression in the brains of the ketamine and saline-exposed rats among the predefined doses and time course. A value of P < 0.05 was considered statistically significant.
Results
Drug-elicited behaviors
Compared with the saline-treated group, the ketamine treatment groups exhibited higher drug-elicited behaviors starting 1 minute following intraperitoneal injection, which lasted for about 20 minutes. These behaviors manifested mainly as falling down after standing erect because of unstable posture and trunk whirling. These behaviors returned to normal after approximately 1 hour. The rats in the 30 mg/kg group exhibited more drug-elicited behaviors than those in the 10 mg/kg group, whereas those in the 60 mg/kg group exhibited less drug-elicited behaviors because of quadriplegia. Three weeks following dosing, the duration of the static term was longer: the rats remained inert for roughly 5 minutes, and subsequently started whirling and falling after standing erect. Heavy music aggravated these behaviors. No significant physical withdrawal symptoms, such as weight loss, irritability, or increased exploratory behavior [9], appeared following drug discontinuation.
Brain DA concentrations
Brain DA concentrations across the groups are shown in Table 1 and Figure 1, and those of DOPAC and homovanillic acid, the primary metabolites of DA, are shown in Tables 2 and 3.
Table 1.
Concentrations of DA in the brains of the rats in the different treatment groups (ng/mL, n = 6)
| Time | 0 mg/kg group | 10 mg/kg group | 30 mg/kg group | 60 mg/kg group |
|---|---|---|---|---|
| 45 min | 118.033±7.975 | 121.383±12.418 | 130.720±9.679 | 121.800±13.138 |
| 1 week | 118.000±14.519 | 124.167±9.745 | 134.667±9.522 | 122.833±10.870 |
| 2 week | 116.167±4.956 | 134.167±14.061 | 145.167±12.576 | 121.500±5.244 |
| 3 week | 119.633±10.381 | 143.167±6.940 | 157.667±12.832 | 125.333±7.501 |
| 3 week + 3 day | 118.833±8.232 | 123.833±11.017 | 130.000±11.576 | 124.667±10.289 |
Figure 1.
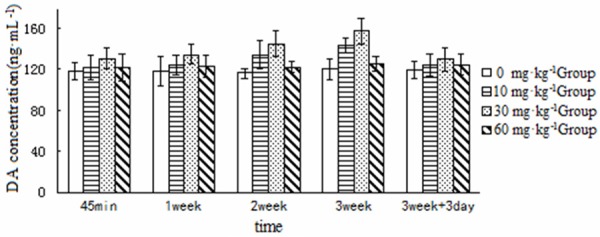
Concentrations of DA in the brains of the rats in the different treatment groups at the different time points examined (ng/mL).
Table 2.
Concentrations of DOPAC in brains of rats in the different treatment groups (ng/mL, n = 6)
| Time | 10 mg/kg group | 30 mg/kg group | 60 mg/kg group | 0 mg/kg group |
|---|---|---|---|---|
| 45 min | 11.360±1.499 | 13.117±1.951 | 12.117±1.798 | 11.053±1.352 |
| 1 week | 10.900±1.783 | 11.967±1.550 | 13.840±1.228 | 13.050±1.958 |
| 2 weeks | 10.982±1.836 | 13.333±2.306 | 12.133±2.187 | 11.074±1.649 |
| 3 weeks | 10.455±1.719 | 10.533±0.857 | 10.242±0.564 | 10.745±2.687 |
| 3 weeks + 3 day | 10.793±1.203 | 12.733±1.731 | 11.933±1.724 | 9.793±1.008 |
Table 3.
Concentrations of homovanillic acid in the brains of the rats in the different treatment groups (ng/mL, n = 6)
| Time | 10 mg/kg group | 30 mg/kg group | 60 mg/kg group | 0 mg/kg group |
|---|---|---|---|---|
| 45 min | 11.398±2.118 | 9.52±0.798 | 12.767±1.934 | 10.468±1.373 |
| 1 week | 9.398±1.090 | 12.700±0.974 | 10.823±1.089 | 10.163±1.013 |
| 2 weeks | 9.057±1.463 | 11.328±1.747 | 9.058±1.466 | 6.582±1.023 |
| 3 weeks | 9.545±1.607 | 10.725±2.017 | 10.022±2.121 | 12.062±2.955 |
| 3 weeks + 3 day | 9.782±1.344 | 10.157±1.345 | 12.022±1.280 | 8.333±0.329 |
Brain D2 receptor mRNA Expression
Brain D2 receptor mRNA expression was analyzed semiquantitatively and is shown in Table 4 and Figure 2.
Table 4.
D2 DRmRNA (2-ΔΔCt value) in the brains of the rats in the different treatment groups (n = 6)
| Time | 0 mg/kg group | 10 mg/kg group | 30 mg/kg group | 60 mg/kg group |
|---|---|---|---|---|
| 45 min | 1.047±0.170 | 0.347±0.060 | 0.142±0.039 | 0.740±0.166 |
| 1 week | 0.913±0.100 | 0.245±0.092 | 0.109±0.027 | 0.494±0.167 |
| 2 week | 1.131±0.211 | 0.142±0.061 | 0.056±0.021 | 0.499±0.118 |
| 3 week | 0.966±0.163 | 0.085±0.027 | 0.031±0.008 | 0.477±0.172 |
| 3 week + 3 day | 1.037±0.130 | 0.184±0.125 | 0.086±0.034 | 0.694±0.175 |
Figure 2.
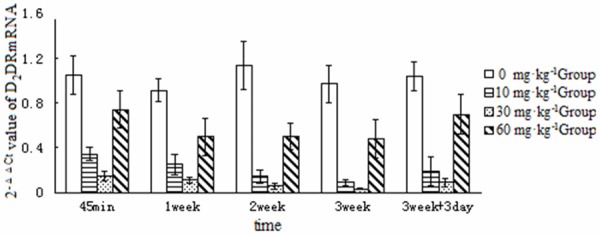
2-ΔΔCt Values for dopamine D2 receptor mRNA expression in the brains of rats in different treatment groups at the different time points examined.
Discussion
Establishment of a rat model of ketamine abuse
In the present study, ketamine was intraperitoneally injected into rats to establish a psychological dependence model. A previous study reported that 10 mg/kg and 80 mg/kg of ketamine administered intraperitoneally are the minimum and maximum doses of ketamine that induce significant drug-elicited movements in rats, respectively [10]. In our preliminary study, however, 80 mg/kg ketamine engendered rapid anesthetic effects in rats, and rendered the rats lethargic for up to 1 hour. Therefore, we used a maximum dose of 60 mg/kg in the present study. 30 mg/kg has been reported to optimally produce drug-elicited movements in rats. Therefore, this dose was used as the median dose.
Correlations between the dose of ketamine administration and brain DA levels and D2 receptor mRNA expression
Tables 2 and 3 show that the levels of the DA metabolites DOPAC and homovanillic acid were comparable among the groups, suggesting that DA has a generally constant metabolism in rat brain tissue. Therefore, DA concentrations can be compared among these groups.
Five time points versus four dose groups were used in the present study to examine potential correlations. Analysis of variance for DA levels and D2 receptor mRNA expression revealed significant differences among the high, medium, and low dose groups at each time point (P < 0.05). DA concentrations were significantly higher in the 10, 30, and 60 mg/kg groups than in the saline-treated group, with 30 mg/kg > 10 mg/kg > 60 mg/kg > control (P < 0.05). D2 receptor mRNA levels in the ketamine-treated groups showed a multi-fold reduction as compared with the control group, with 30 mg/kg < 10 mg/kg < 60 mg/kg < control (P < 0.05). A bivariate Pearson’s correlation analysis of DA levels and D2 receptor mRNA expression versus serum ketamine levels in the ketamine-treated groups (10-30 mg/kg) at each time point shows that serum ketamine levels were correlated with brain DA levels and D2 receptor mRNA expression (r = 0.752 to -0.806) in the rats treated with the low and medium dose. In the higher dose group, DA levels were higher but D2 receptor mRNA expression was lower. Brain DA concentrations were lower in the 60 mg/kg group than in the 30 mg/kg group, whereas D2 receptor mRNA expression was higher in the 60 mg/kg group than in the 30 mg/kg group. These contradictory findings may have resulted from differential anesthesia, a pharmacological effect of ketamine [11]. 60 mg/kg may be a dose that approximates the anesthetic effects seen in clinical practice, which potentiates central paralysis but weakens excitatory effects on the limbic system.
The drug-elicited behaviors of rats following ketamine dosing are consistent with our aforementioned findings. Miyamoto et al. reported that drug-elicited movement in rats is associated with brain DA systems [12]. In our study, following intraperitoneal injection of ketamine, the 30 mg/kg group exhibited the highest level of drug-elicited movements. In contrast, ketamine at 60 mg/kg resulted in quadriplegia and less drug-elicited movement.
Our results show that the ketamine exposure increases brain DA concentrations but decreases D2 receptor mRNA expression. Ketamine may increase the release of DA or interrupt the degradation of DA, which then increases the concentrations of DA in the brain. Alternatively, following chronic dosing, ketamine accumulates in the body, and the concentration of DA increases in the brain. Moreover, excessive brain concentrations of DA may subject DR to continuous and excessive stimulation, and lead to downregulation of these receptors. Some authors have proposed the concept of a reward deficiency syndrome [13]. Reward deficiency syndrome refers to when DA released from the reward pathway cannot effectively activate D2 receptors because of D2 receptor loss or insufficiency, and cannot meet the requirements for satisfaction in normal living. Therefore, individuals with reward deficiency syndrome are more likely to abuse heroin, marijuana, cocaine, and other addictive drugs. The pathological stimulus signals the reward pathway to release DA, which results in euphoria. Drug addicts have reduced D2 receptors in the presence of withdrawal symptom or shortly after drug discontinuation, which lasts up to several months, even though D2 receptor expression increases slightly following drug discontinuation [14].
Effects of ketamine on brain DA levels and D2 receptor mRNA expression
Five time points were used in this study. These were 45 minutes and 1, 2, and 3 weeks after the initiation of dosing, and 3 days after drug discontinuation. The effects of ketamine on rat brain DA levels and D2 receptor mRNA expression were examined across multiple doses using the same dosing procedure. The 10 and 30 mg/kg ketamine treatment groups exhibited higher brain DA concentrations than the saline-treated group at 45 minutes and 1, 2, and 3 weeks after dosing, with a sequentially increasing trend (P < 0.05). D2 receptor mRNA expression was significantly lower in the ketamine treatment groups than in the control group, with a sequentially decreasing trend (P < 0.05). Brain DA concentrations decreased slightly 3 days after drug discontinuation as compared with 2 and 3 weeks of dosing, but were still higher than in the saline-treated group (P < 0.05). Three days after drug discontinuation, D2 receptor mRNA expression slightly normalized as compared with 2 and 3 weeks of dosing, but was still lower than in the control group (P < 0.05).
DA concentrations increased in the brains of the rats in the 60 mg/kg group as compared with the control group, whereas D2 receptor mRNA expression decreased. These two markers were not different at 45 minutes and 1, 2, and 3 weeks of dosing, showing no temporal variation. This may be associated with the weakened excitatory effects of high-dose ketamine on the limbic system.
In this study, DA concentrations were significantly higher and D2 receptor mRNA expression was significantly lower in the ketamine treatment groups than in the control group at 45 minutes and 1, 2, and 3 weeks following the initiation of dosing with ketamine. DA concentrations slightly normalized in the ketamine treatment groups 3 days after drug discontinuation, but were still above those in the control group. D2 receptor mRNA expression increased slightly following drug discontinuation, but still was below that in the control group. These findings support the hypothesis that the mesolimbic dopamine system is involved in drug addiction and the reward deficiency syndrome. This suggests that both DA and D2 receptor mRNA may be associated with ketamine abuse. However, the pharmacological mechanisms of drug abuse are complex, and further studies are needed to elucidate how DA and D2 receptor mediate ketamine abuse.
Acknowledgements
This work was supported by the National Science & Technology Key Projects Program for the Eleventh Five-year Plan Period (Grant No. 2007BAK26B05) from The Ministry of Science and Technology of the People’s Republic of China and by the Shanxi Natural Science Foundation Committee, China (Grant Nos. 2007011105 , 20110321082). Points of view in this document are those of the authors and do not necessarily represent the official position of the Ministry of Science and Technology of the People’s Republic of China and the Shanxi Natural Science Foundation Committee, China.
Disclosure of conflict of interest
None.
References
- 1.Svensson TH. Dysfunction brain dopamine systems induced by psychotomimetic NMDA receptor antagonist and the effects of antipsychotic drugs. Brain Res Brain Res Rev. 2000;31:320–329. doi: 10.1016/s0165-0173(99)00048-x. [DOI] [PubMed] [Google Scholar]
- 2.Sami BH, Erin P, Brigitte C, Christian K, Byron C, Jones BC, Cassel JC. Interactions between EtOH and cocaine, amphetamine, or MDMA in the rat: thermoregulatory and locomotor effects. Psychopharmacology. 2008;197:67–82. doi: 10.1007/s00213-007-1007-5. [DOI] [PubMed] [Google Scholar]
- 3.Jin XG. Research Progress of mental side effects and neurotoxic effects of ketamine. Foreign Medicine: Anesthesiology and Resusciation. 2003;24:35–38. [Google Scholar]
- 4.Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, Kim SY, Adhikari A, Thompson KR, Andalman AS, Gunaydin LA, Witten IB, Deisseroth K. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493:537–41. doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farber NB, Kim SH, Dikranian K, Jiang XP, Heinkel C. Receptor mechanisms and circuitry underlying NMDA antagonist neurtoxicity. Mol Psychiatry. 2002;7:32–43. doi: 10.1038/sj.mp.4000912. [DOI] [PubMed] [Google Scholar]
- 6.Irifune M, Shimizu T, Nomoto M. Ketamine-induced hyperlocomotion associated with alteration of presynaptic components of dopamine neuronsin the nucleus accumbens of mice. Pharmacol Biochem Behav. 1991;40:399–407. doi: 10.1016/0091-3057(91)90571-i. [DOI] [PubMed] [Google Scholar]
- 7.Meo LN, Qang JS, Fang Q, Lu YH, Zhai HF, Lu L. Ketamine Induces Rat Conditioned Place Preference. Chinese Journal of Drug Dependence. 2006;15:277–279. [Google Scholar]
- 8.Rodríguez-Arias M, Broseta I, Aguilar MA, Miñarro J. Lack of specific effects of selective D1 and D2 dopamine antagonists vs risperidone on morphine-induced hyperactivity. Pharmacol Biochem Behav. 2000;66:189–197. doi: 10.1016/s0091-3057(00)00207-0. [DOI] [PubMed] [Google Scholar]
- 9.Gilles J, Rivière W, Brooks Gentry S. Michal O Disposition of MA and its metabolite amphetamine in brain and other tissues in rats after intravenous administration. J Pharmacol Exp Ther. 2000;292:1042–1047. [PubMed] [Google Scholar]
- 10.Parashchanka A, Schelfout S, Coppens M. Role of novel drugs in sedation outside the operating room: dexmedetomidine, ketamine and remifentanil. Curr Opin Anaesthesiol. 2014;27:442–7. doi: 10.1097/ACO.0000000000000086. [DOI] [PubMed] [Google Scholar]
- 11.Cruickshank CC, Dyer KR. A review of the clinical pharmacology of methamphetamine. Addiction. 2009;104:1085–1099. doi: 10.1111/j.1360-0443.2009.02564.x. [DOI] [PubMed] [Google Scholar]
- 12.Miyamoto S, Leipzig JN, Lieberman JA, Duncan GE. Effects of ketamine, MK-801, and amphetamine on regional brain 2-deoxyglucose uptake in freely moving mice. Neuropsychopharmacology. 2000;22:400–412. doi: 10.1016/S0893-133X(99)00127-X. [DOI] [PubMed] [Google Scholar]
- 13.Cruickshank CC, Dyer KR. A review of the clinical pharmacology of methamphetamine. Addiction. 2009;104:1085–1099. doi: 10.1111/j.1360-0443.2009.02564.x. [DOI] [PubMed] [Google Scholar]
- 14.Morgan CJ, Curran HV. Independent scientific committee on drugs. Ketamine use: a review. Addiction. 2012;107:27–38. doi: 10.1111/j.1360-0443.2011.03576.x. [DOI] [PubMed] [Google Scholar]


