Abstract
Background: Secondary hyperparathyroidism (sHPT) is a common acquired disorder in patients with chronic renal failure. Despite the development of new therapeutic agents, a majority of patients will require parathyroidectomy. The aim of this study was to evaluate total parathyroidectomy with auto-transplantation of trace amounts of parathyroid tissue as a surgical option in uremia sHPT treatment. Methods: Clinical data of 50 sHPT patients who underwent total parathyroidectomy with auto-transplantation between January 2011 and December 2013 were reviewed retrospectively. Symptoms such as bone pain and fractures, concentrations of intact parathyroid hormone (iPTH), levels of ionized calcium and serum phosphorus, and activity of alkaline phosphatase were recorded before and after parathyroidectomy. Results: After operation, signs of pruritus, bone pain and muscle weakness was disappeared, iPTH level and serum phosphate concentration were declined markedly. No serious postoperative complications were observed. Follow-up observation was around 28 months. One female patient (2%) died 3 months after surgery due to heart failure, and another patient (2%) had persistent disease. All other patients recovered during the follow-up period. Conclusions: Total parathyroidectomy with auto-transplantation of trace amounts of parathyroid tissue was considered to be a feasible, safe and effective surgical option for the treatment of sHPT.
Keywords: Secondary hyperparathyroidism, chronic renal failure, total parathyroidectomy, auto-transplantation
Introduction
Secondary hyperparathyroidism (sHPT) is a common acquired disorder develops in chronic renal failure treated with long-term dialysis (> 2 years). sHPT may lead to serious complications including metabolic bone diseases, severe atherosclerosis and undesirable cardiovascular events [1]. The stimuli for the development of sHPT relevant to renal failure include hypocalcemia, hyperphosphatemia, and low 1,25-dihydroxy-Vitamin D3 (1,25(OH)2D3). Hypocalcemia increases the parathyroid hormone gene expression per cell, the secretion of mature parathyroid hormone (PTH) and the number of parathyroid cells [2-4]. This excess PTH can lead to renal osteodystrophy, calciphylaxis, ectopic calcifications, abnormal fat and sugar metabolism, refractory pruritis and anemia [5,6].
If condition is available, kidney replacement is the only causal treatment to correct the abnormalities in mineral metabolism [7]. However, as there is limited amount of available kidneys for transplantation, medical therapies and parathyroidectomy became alternative ways for the treatment of sHPT. Regarding to medical therapies using the new approved cinacalcet (60 mg/day) or paricalcitol (15 μg/week), the annual costs were 5828.40 € and 4485.20 €, respectively, while the cost for parathyroidectomy surgery was 3755.38 €/case in German [5]. Medical treatment with cinacalcet for more than 9 months or paricalcitol for more than 12 months will exceed the costs of surgical therapy. Additionally, medical treatment using cinacalcet or paricalcitol was not always works. Thus, parathyroidectomy is required in most cases and would be more acceptable by a larger portion of patients.
There are various surgical approaches for parathyroidectomy, including total parathyroidectomy, subtotal parathyroidectomy and total parathyroidectomy with auto-transplantation [8,9]. However, no consensus on the optimal operative management for sHPT has been reached [10-12]. Total parathyroidectomy with auto-transplantation and subtotal parathyroidectomy are currently considered as standard surgical procedures for the treatment of sHPT [13].
The aim of this study is to evaluate total parathyroidectomy with auto-transplantation of trace amounts of parathyroid tissue as a surgical option in uremia sHPT treatment. In this study, clinical data of 50 sHPT patients who underwent total parathyroidectomy with autotransplantion of trace amounts of parathyroid tissue between January 2011 and December 2013 were reviewed retrospectively. Demographic data was recorded before the operation. Symptoms such as bone pain and fractures, concentrations of intact parathyroid hormone (iPTH), levels of ionized calcium and serum phosphorus, and activity of alkaline phosphatase were measured before and after the surgery. From our study, it was suggested that total parathyroidectomy with trace amounts of parathyroid tissue autotransplantation is considered to be a feasible, safe and effective surgical option for the patients with sHPT.
Materials and methods
Patients
This study was approved by the Jinan Military General Hospital Research Ethics Board. The diagnosis of severe sHPT was established in all patients on the basis of clinical, biochemical, radiological and histological evidence. The criteria for uremia secondary hyperparathyroidism in this study included [14]: age ≥ 18 years, dialysis treatment period ≥ 12 months, intact parathyroid hormone (iPTH) > 500 pg/mL, enlarged parathyroid glands visualized via imaging techniques, findings indicating osteitis fibrosa cystica or high bone turnover, and at least one factor refractory to medical treatment (hypocalcaemia, serum calcium-phosphate production over 70 mg/dL, osteodystrophy or calciphylaxis, bone pain or pathological fracture). Patients with a history of neck explorations for thyroid/parathyroid disorders or malignant disease of the thyroid glands were excluded from this study.
Total of 50 sHPT patients (male 26 and female 24) met the criteria of sHPT between January 2011 and December 2013 were included in this study. Their mean age was 44.1±13.7 years (range 21-71) and their dialysis history was 8.0±4.8 years (range 3-23 years). All patients underwent total parathyroidectomy with trace amount of autotransplantion.
Preoperative examination
Upon admitted, patients’ medical history was asked, and special attention was placed on signs of bone pain and joint deformity. Routine examinations, including blood routine test, hepatorenal function examination, serum iPTH level analysis, X-ray examination of cranium, hands and pelvis, and parathyroid glands scanning, were conducted on all patients before surgery. If necessary, ultrasonic cardiogram test was performed. Preoperative localization studies include neck ultrasonography and 99mTc-MIBI SPECT scans. Levels of serum-ionized calcium, phosphorus, alkaline phosphatase and iPTH were measured before, during and daily after parathyroidectomy surgery.
Surgical approach
During the surgery, 100 mL calcium gluconate (10%, Tianjin pharmaceutical co., LTD.) was applied to all patients in a rate of 10 mL/h. The neck and presternal area were explored without sternotomy for the identification of the four parathyroid glands. Intraoperative iPTH was measured to confirm total removal of parathyroid glands. If 3 or less parathyroid glands were identified or intraoperative iPTH was greater than 400 pg/mL, transcervical thymectomy and bilateral exploration of the carotid sheath were carried out. After all parathyroid glands were removed, the most normal-appearing non-nodular areas of the parathyroid gland were divided into pieces of 1 mm3 in size. 30 mg of parathyroid tissue was implanted into the muscular bed of the patients’ sternocleidomastoid muscle and the implanted site was marked with titanic clips for future reference.
Postoperative management
The day after surgery, incentive attention was played on the iPTH level, ALP activity, serum calcium and phosphorous concentration. Besides intravenous calcium, patients also received oral calcitriol (0.5 μg, Shanghai Roche Pharmaceuticals Ltd) twice and calcium carbonate (2.25 μg, Zhuhai special economic zone biochemistry pharmaceutical factory) three times. Routine or no heparin dialysis was performed depending on the patient’s conditions.
During the first four days after surgery, all patients received intravenous calcium 100 mL per day in a speed of 10 mL/h. with or without oral calcium. The serum calcium and phosphorus concentration was monitored every day to adjust the supplemented rate of intravenous calcium in order to maintain the serum calcium concentration higher than 1.8 mM/L. Fructose diphosphate (Anhui double crane pharmaceutical co., LTD) was applied if serum phosphorus concentration was lower than 1.0 mM/L.
After the serum calcium level was stabilized (7~15 days) and hypocalcemic symptoms was disappeared, patients were discharged. A follow-up visits at 1, 3, 6, 12 months, and every six months thereafter are executed (totally around 28 months). Follow-up visits consist of telephone interview, in-clinic visits, direct mail, and email.
Statistical analysis
The continuous data were tested by t-test, and categorical data were tested by the chi-square test or Fisher’s exact test. Differences were considered significant at p-value < 0.05.
Results
The patients’ demographic and clinical data were summarized in Table 1. Their average age was 44.1±13.7 years and range from 21 to 71 years. Their dialysis duration was 8.0±4.8 years and range from 3-23 years. Before operation, the average iPTH concentration was 2375±907 pg/mL which was dramatically elevated. The phosphorus and calcium concentration was 2.45±0.55 and 2.48±0.20 mM/L, respectively. The alkaline phosphatase activity was 687±498 U/L, greatly exceeding the normal level.
Table 1.
Demographics, symptoms, and preoperative or postoperative biochemistry
| Sex (Male/Female) | 26/24 |
| Age (Years) | 44.1±13.7 (21-71) |
| Dialysis (Years) | 8.0±4.8 (3-23) |
| iPTH (pg/mL) | 2375±907 |
| Phosphorus (mM/L) | 2.45±0.55 |
| Alkaline phosphatase (U/L) | 687±498 |
| Serum-ionised calcium (mM/L) | 2.48±0.20 |
| Ca*P (mg2/dl2) | 73.1±17.4 |
Data expressed as mean ± SD and range. Reference values: Serum-ionised calcium: 1.13-1.33 mM/L; Phosphorus: 0.97~1.62 mM/L; Alkaline phosphatase: 35-129 U/L; Ca*P: 30-40 mg2/dl2.
All patients underwent total parathyroidectomy with trace amounts (30 mg) of auto-transplantation. Totally 201 parathyroid glands were indentified and removed from all patients. The majority of the removed parathyroid glands were found in normal anatomical positions (n=186), and other glands were found within the thymus (n=10), carotid sheath (n=4) or paraesophageal region (n=1). 47 patients had 4 glands, and more than 4 glands were removed in two patients. Three glands were discovered in 1 patient. The average length of hospital stay was 5 days (rang from 3 to 10 days). Postoperatively, no vocal cord paralysis or substantial bleeding, hematoma or seroma requiring reoperation was recorded. The average follow-up period was 28±5 (range: 9-62) months. One female patient died 3 months after surgery because of hard failure, and the iPTH concentration of another patient elevated again after a short-term decrease who was confirmed with parathyroid gland shifted to the retrosternal that was not removed during the surgery. All other patients recovered during the follow-up period.
After the surgery, the clinical symptoms, such as bone pain and muscle weakness were disappeared in all formerly symptomatic patients. The iPTH levels before, during and after the surgery were showed in Table 2 and Figure 1. During the surgery, the detected iPTH was dramatically reduced to a level lower than 400 pg/mL (up to 80 fold), indicating the successfully removal of parathyroid glands. After operation, the serum iPTH levels continuously decreased and maintained at a level between 40 to 50 pg/mL.
Table 2.
Levels of iPTH, calcium, phosphorus, alkaline phosphatase and phosphorus × calcium before, during and after surgery
| Parameters | Preoperative | Intraoperative | Postoperative | ||
|---|---|---|---|---|---|
|
| |||||
| 1 d | 3 d | 7 d | |||
| iPTH (pg/mL) | 2375±907 | 313±217* | 46±56* | 41±78* | 46±82* |
| Calcium (mM/L) | 2.48±0.20 | 2.34±0.20 | 1.88±0.35* | 1.79±1.92* | 1.92±0.44* |
| Phosphorus (mM/L) | 2.45±0.55 | 2.22±0.57 | 2.00±1.72* | 1.29±0.39* | 1.09±0.31* |
| AKP (U/L)# | 687±498 | - | 899±779* | 723±567 | 1133±953* |
| Ca*P (mg2/dl2)# | 73.1±13.4 | 62.4±16.9* | 44.9±35.9* | 26.8±10.4* | 23.2±8.4* |
Data was presented as mean ± SD.
AKP represents activity of alkaline phosphatase.
Ca*P, represents product of calcium and phosphorus.
Indicates statistic difference compared to preoperative data with P < 0.05.
Figure 1.
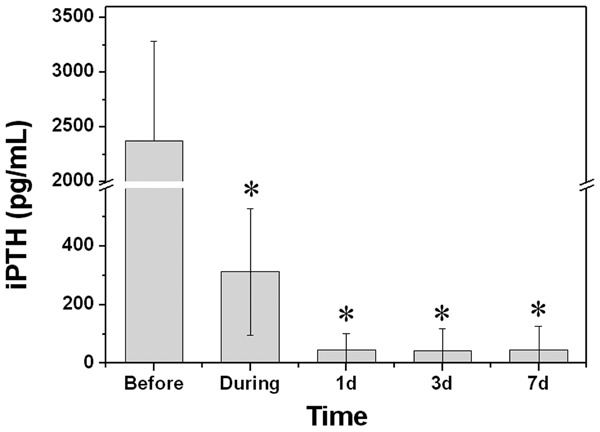
Levels of iPTH before, during, and different time points after the operation. *Indicates significant difference from that before operation (P < 0.01).
The reduction of serum calcium during the surgery was not so obvious comparing to iPTH (Table 2). After operation, the serum calcium concentration continuously decreased and maintained in a level lower than 2 mM/L. Regarding to the serum phosphorus, its concentration kept decreasing in one week to a level of 1.09±0.31 mM/L which fell in the normal range (0.97~1.62 mM/L) (Table 2). The product of calcium and phosphorus dropped from 73.1±13.4 mg2/dl2 before operation to 23.2±8.4 mg2/dl2 one week after surgery (Figure 2). Different from other parameters, the activity of alkaline phosphatase was significantly increased after the surgery to a level higher than 1000 U/L (Table 2).
Figure 2.
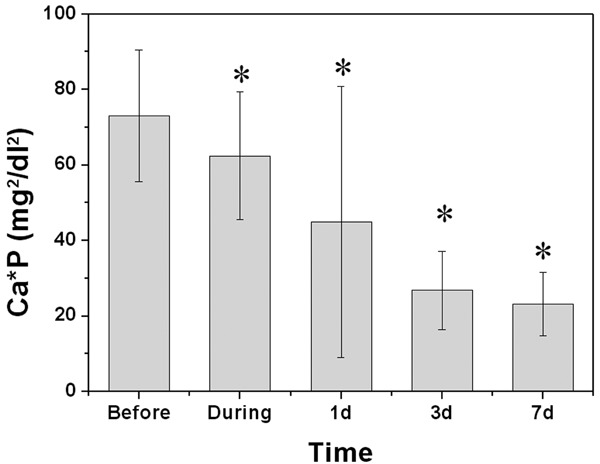
Product of serum-ionized calcium and phosphorus concentration before, during, and different time points after the operation. *Indicates significant difference from that before operation (P < 0.01).
Discussion
sHPT is common in patients with chronic renal failure, affecting most of those who are receiving hemodialysis [15]. In patients with renal failure, the synthesis of 1,25(OH)2D3 secretion and phosphate excretion is greatly decreased, leading to hypocalcemia and hyperphosphatemia. Hypocalcemia and hyperphosphatemia stimulate the secretion of parathyroid hormone and hyperplasia of parathyroid cell. With time, sHPT is characterized by persistently elevated iPTH levels and complicated by important disturbances in mineral metabolism and skeletal resistance to the calcemic actions of parathyroid hormone [16-18]. The alterations of calcium and phosphorus metabolism can further resulted in soft-tissue and vascular calcification, cardiovascular disease, and the risk of death [19,20]. And it is believed that the rates of symptomatic hyperparathyroidism increase with the duration of dialysis treatment [21].
Although kidney replacement is the only causal treatment to correct the abnormalities in mineral metabolism, it is still difficult in providing kidney transplantation for all patients with chronic renal failure [7]. Thus, alternative treatment technique is in great demand for the therapy of sHPT. Vitamin D analogues and calcimimetics have been demonstrated to be effective in sHPT treatment, but this prophylactic therapy is not always works [22]. Despite the development of new agents for sHPT, such as calcimimetics, new phosphate binders and less calcemic vitamin D analogues, many patients require parathyroidectomy due to the high cost of these new medicine and limited effect on long-standing sHPT [5].
Three different surgical procedures for parathyroidectomy are reported in the literature: subtotal parathyroidectomy, total parathyroidectomy with auto-transplantation of some of the excised tissue into defined areas and total parathyroidectomy without transplantation [9,23]. Since the potential complication of a dynamic bone disease or severe ongoing hypocalcemia, total parathyroidectomy was not introduced into clinical practice. Subtotal parathyroidectomy and total parathyroidectomy with auto-transplantation are currently considered as the standard procedures in the treatment of sHPT [9,14,24] and thus total parathyroidectomy was employed in our study.
During the operation, the iPTH concentration can be used to reveal the function of the grafted tissues. Intraoperative iPTH lower than 400 pg/mL has been frequently employed to assure the completely removal of all parathyroid glands [25,26]. In our study, introaperative iPTH assay has been performed in all patients. Auto-transplantation only performed until all parathyroid glands has been removed with detected iPTH lower than 400 pg/mL. At the routine postoperative iPTH level examination, almost all patients presented iPTH levels in the normal range in one week which was consistent with the literature [27].
After surgery, attention must be kept on patients’ level of calcium, phosphorus, and alkaline phosphatase. The lack of osteoclastic activity caused by a decrease in PTH postoperatively may lead to a precipitous fall in calcium levels, a condition called “hungry-bone syndrome” [28]. Since the autographed parathyroid tissue required 2~3 weeks to function in releasing PTH, hungry bone syndrome might happen in patients received operation. It is necessary that all patients received calcium and calcitriol supplement for low calcium levels. In our study, calcium and calcitriol was supplemented to all patients during the surgery and the day after the surgery. Two days post the operation, calcium, calcitriol and/or fructose diphosphate was supplemented depending on the detected calcium or phosphorus concentrations.
It was worth noting that during our 28 months follow-up examination, expect one female patient died from hard failure, no recurrence has been observed. The prevalence recurrence rate of parathyroidectomy with auto-transplantation has been reported to be 3% to 38% in the literature (3% to 38%) [29]. The low recurrence rate in our study might due to the relative short follow-up period (28 months) compare to that of the literature (~3 years).
Conclusion
In this study, 50 sHPT patients who underwent total parathyroidectomy with auto-transplantation from January 2011 to December 2013 were reviewed retrospectively. After operation, signs of pruritus, bone pain and muscle weakness was disappeared, and levels of serum PTH and phosphate were declined markedly. Patients were discharge in an average of 10 days (7-15 days). After a mean follow-up of 28 months, most of sHPT patients (98%) were recovered without recurrence. Thus we believed that total parathyroidectomy with auto-transplantation of trace amounts of parathyroid tissue might be a feasible, safe and effective surgical option for the patients with sHPT.
Disclosure of conflict of interest
None.
References
- 1.Malberti F, Marcelli D, Conte F, Limido A, Spotti D, Locatelli F. Parathyroidectomy in patients on renal replacement therapy: An epidemiologic study. J Am Soc Nephrol. 2001;12:1242–1248. doi: 10.1681/ASN.V1261242. [DOI] [PubMed] [Google Scholar]
- 2.Roth SI, Raisz LG. Effect of calcium concentration on ultrastructure of rat parathyroid in organ culture. Lab Invest. 1964;13:331–45. [PubMed] [Google Scholar]
- 3.Mayer GP, Keaton JA, Hurst JG, Habener JF. Effects of plasma calcium concentration on the relative proportion of hormone and carboxyl fragments in parathyroid venous-blood. Endocrinology. 1979;104:1778–1784. doi: 10.1210/endo-104-6-1778. [DOI] [PubMed] [Google Scholar]
- 4.Naveh-Many T, Friedlaender MM, Mayer H, Silver J. Calcium Regulates Parathyroid Hor- mone Messenger Ribonucleic Acid (mRNA), but not Calcitonin mRNA in Vivo in the Rat. Dominant Role of 1,25-Dihydroxyvitamin D. Endocrinology. 1989;125:275–280. doi: 10.1210/endo-125-1-275. [DOI] [PubMed] [Google Scholar]
- 5.Schneider R, Kolios G, Koch BM, Fernandez ED, Bartsch DK, Schlosser K. An economic comparison of surgical and medical therapy in patients with secondary hyperparathyroidism-the German perspective. Surgery. 2010;148:1091–1099. doi: 10.1016/j.surg.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Iannazzo S, Carsi M, Chiroli S. A cost-utility analysis of cinacalcet in secondary hyperparathyroidism in five European countries. Appl Health Econ Health Policy. 2012;10:127–38. doi: 10.2165/11597980-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.Lewin E, Wang W, Olgaard K. Reversibility of experimental secondary hyperparathyroidism. Kidney Int. 1997;52:1232–1241. doi: 10.1038/ki.1997.448. [DOI] [PubMed] [Google Scholar]
- 8.Conzo G, Perna AF, Savica V, Palazzo A, Della Pietra C, Ingrosso D, Satta E, Capasso G, Santini L, Docimo G. Impact of parathyroidectomy on cardiovascular outcomes and survival in chronic hemodialysis patients with secondary hyperparathyroidism. A retrospective study of 50 cases prior to the calcimimetics era. Bmc Surg. 2013;13(Suppl 2):S4. doi: 10.1186/1471-2482-13-S2-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naranda J, Ekart R, Pedcovnik-Balon B. Total Parathyroidectomy with Forearm Autotransplantation as the Treatment of Choice for Secondary Hyperparathyroidism. J Int Med Res. 2011;39:978–987. doi: 10.1177/147323001103900333. [DOI] [PubMed] [Google Scholar]
- 10.Schneider R, Slater EP, Karakas E, Bartsch DK, Schlosser K. Initial Parathyroid Surgery in 606 Patients with Renal Hyperparathyroidism. World J Surg. 2012;36:318–326. doi: 10.1007/s00268-011-1392-0. [DOI] [PubMed] [Google Scholar]
- 11.Hayes JF, Gross GF, Schuman ES. Surgical management of renal hyperparathyroidism in the dialysis patient. Am J Surg. 1982;143:569–571. doi: 10.1016/0002-9610(82)90164-7. [DOI] [PubMed] [Google Scholar]
- 12.Madorin C, Owen RP, Fraser WD, Pellitteri PK, Radbill B, Rinaldo A, Seethala RR, Shaha AR, Silver CE, Suh MY, Weinstein B, Ferlito A. The surgical management of renal hyperparathyroidism. Eur Arch Otorhinolaryngol. 2012;269:1565–1576. doi: 10.1007/s00405-011-1833-2. [DOI] [PubMed] [Google Scholar]
- 13.Schlosser K, Veit JA, Witte S, Fernandez ED, Victor N, Knaebel HP, Seiler CM, Rothmund M. Comparison of total parathyroidectomy without autotransplantation and without thymectomy versus total parathyroidectomy with autotransplantation and with thymectomy for secondary hyperparathyroidism: TOPAR PILOT-Trial. Trials. 2007;8:22. doi: 10.1186/1745-6215-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakman G, Parsak CK, Balal M, Seydaoglu G, Eray IC, Saritas G, Demircan O. Outcomes of Total Parathyroidectomy with Autotransplantation versus Subtotal Parathyroidectomy with Routine Addition of Thymectomy to both Groups: Single Center Experience of Secondary Hyperparathyroidism. Balkan Med J. 2014;31:77–82. doi: 10.5152/balkanmedj.2014.9544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Owda A, Elhwairis H, Narra S, Towery H, Osama S. Secondary Hyperparathyroidism in Chronic Hemodialysis Patients: Prevalence and Race. Ren Fail. 2003;25:595–602. doi: 10.1081/jdi-120022551. [DOI] [PubMed] [Google Scholar]
- 16.Silver J, Levi R. Regulation of PTH synthesis and secretion relevant to the management of secondary hyperparathyroidism in chronic kidney disease. Kidney Int. 2005;67:S8–S12. doi: 10.1111/j.1523-1755.2005.09501.x. [DOI] [PubMed] [Google Scholar]
- 17.Slatopolsky E, Brown A, Dusso A. Pathogenesis of secondary hyperparathyroidism. Kidney Int. 1999;56:S14–S19. doi: 10.1046/j.1523-1755.1999.07304.x. [DOI] [PubMed] [Google Scholar]
- 18.Yuan CM, Nee R, Narayan R, Abbott KC. Treatment of Secondary Hyperparathyroidism With Parathyroidectomy Instead of Cinacalcet: Time to Pick the Low-Hanging Fruit? Am J Kidney Dis. 2012;60:179–181. doi: 10.1053/j.ajkd.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB. Coronary-Artery Calcification in Young Adults with End-Stage Renal Disease Who Are Undergoing Dialysis. N Engl J Med. 2000;342:1478–1483. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 20.Raggi P, Boulay A, Chasan-Taber S, Amin N, Dillon M, Burke SK, Chertow GM. Cardiac calcification in adult Hemodialysis patients-A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol. 2002;39:695–701. doi: 10.1016/s0735-1097(01)01781-8. [DOI] [PubMed] [Google Scholar]
- 21.Santos RO, Ohe MN, Carvalho AB, Neves MC, Kunii I, Lazaretti-Castro M, Abrahao M, Cervantes O, Vieira JGH. Total parathyroidectomy with presternal intramuscular autotransplantation in renal patients: a prospective study of 66 patients. J Osteoporos. 2012;2012:631243. doi: 10.1155/2012/631243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drueeke TB, Ritz E. Treatment of Secondary Hyperparathyroidism in CKD Patients with Cinacalcet and/or Vitamin D Derivatives. Clin J Am Soc Nephrol. 2009;4:234–241. doi: 10.2215/CJN.04520908. [DOI] [PubMed] [Google Scholar]
- 23.Stanbury SW, Lumb GA, Nicholson WF. Elective subtotal parathyroidectomy for renal hyperparathyroidism. Lancet. 1960;1:793–799. doi: 10.1016/s0140-6736(60)90678-4. [DOI] [PubMed] [Google Scholar]
- 24.He QQ, Zhuang DY, Zheng LM, Fan ZY, Zhou P, Zhu JJ, Duan SN, Li YM, Ge Y, Lv Z, Cao L. Total parathyroidectomy with trace amounts of parathyroid tissue autotransplantation as the treatment of choice for secondary hyperparathyroidism: a single-center experience. BMC Surg. 2014;14:26. doi: 10.1186/1471-2482-14-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan HWH, Chu KH, Fung SKS, Tang HL, Lee W, Cheuk A, Yim KF, Tong MKL, Lee KC. Prospective study on dialysis patients after total parathyroidectomy without autoimplant. Nephrology. 2010;15:441–447. doi: 10.1111/j.1440-1797.2009.01257.x. [DOI] [PubMed] [Google Scholar]
- 26.Kim WY, Lee JB, Kim HY. Efficacy of intraoperative parathyroid hormone monitoring to predict success of parathyroidectomy for secondary hyperparathyroidism. J Korean Surg Soc. 2012;83:1–6. doi: 10.4174/jkss.2012.83.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tominaga Y, Matsuoka S, Uno N, Tsuzuki T, Hiramitsu T, Goto N, Nagasaka T, Watarai Y, Uchida K. Removal of Autografted Parathyroid Tissue for Recurrent Renal Hyperparathyroidism in Hemodialysis Patients. World J Surg. 2010;34:1312–1317. doi: 10.1007/s00268-010-0412-9. [DOI] [PubMed] [Google Scholar]
- 28.Goldfarb M, Gondek SS, Lim SM, Farra JC, Nose V, Lew JI. Postoperative Hungry Bone Syndrome in Patients with Secondary Hyperparathyroidism of Renal Origin. World J Surg. 2012;36:1314–1319. doi: 10.1007/s00268-012-1560-x. [DOI] [PubMed] [Google Scholar]
- 29.Kinnaert P, Salmon I, Decoster-Gervy C, Vienne A, De Pauw L, Hooghe L, Tielemans C. Long-term results of subcutaneous parathyroid grafts in uremic patients. Arch Surg. 2000;135:186–190. doi: 10.1001/archsurg.135.2.186. [DOI] [PubMed] [Google Scholar]


