Abstract
The aim of this study was to investigate if anthropometric parameters are associated with both leptin and soluble leptin receptor (sLEPR) levels in newborns and their mothers. This cross-sectional study was performed in 118 mother-newborn pairs. The venous blood sample of mothers was taken before delivery and immediately after delivery an umbilical cord blood sample was collected. Levels of leptin and sLEPR in maternal and umbilical cord sera were assessed by ELISA. Maternal serum concentration of leptin and sLEPR (6.2 and 25.7 ng/ml, respectively) were higher than in umbilical cord blood (2.4 and 14.2 ng/ml, respectively). However, the newborns and their mothers had higher sLEPR levels than leptin levels. In mothers was observed that leptin levels increase with weight gain in pregnancy and decreased sLEPR levels. Cord leptin levels correlated with neonatal birth weight and length, the body circumferences, placental weight and maternal leptin levels. Cord sLEPR levels correlated with maternal sLEPR and leptin levels. Maternal serum concentration of leptin correlated with pre-pregnancy BMI, weight gain, cord sLEPR and leptin levels. Maternal sLEPR concentration correlated with cord sLEPR levels. The leptin and sLEPR levels in mother-newborn pairs are related with anthropometric parameters and an inverse correlation between leptin levels and sLEPR was observed in pairs.
Keywords: Leptin, soluble receptor leptin, neonate, umbilical cord, placenta
Introduction
It has been strongly suggested that the influence of maternal weight determines the relationship of birth weight in the newborn and the subsequent BMI, which it is in relation to the increased supply of energy substrates in the binomial. Maternal overnutrition negatively influences body composition of the fetus and predisposes to the development of obesity-related complications [1]. There is evidence suggesting that cytokines produced by the maternal endometrium and the developing embryo play an important role in this signaling process. Although numerous cytokine-receptor pairs are expressed by the maternal endometrium and the embryo during implantation, their temporal pattern of expression is still poorly understood [2]. Several cytokines and growth factors are known to influence the migration, proliferation and invasion of the trophoblast [3,4]. Leptin has been suggested to be involved in other functions during pregnancy, due to high concentrations produced by the placenta [5].
Leptin is the hormone product of the LEP gene and was originally considered as an adipocyte-derived signaling molecule to participate in modulating satiety and energy homeostasis [6,7]. Actually, the pleiotropic effects of the hormone have been identified; leptin is involved on modulation of several processes such as thermogenesis, homeostasis, angiogenesis, hematopoiesis, osteogenesis, chondrogenesis, neuroendocrine, and immune functions, as well as arterial pressure control [8,9]. Leptin exerts its function through a specific receptor that is expressed in isoforms by alternatively spliced and differ by the relative lengths of their cytoplasmic regions. Within its receptor are included a long form (bLEPR) that predominates in the hypothalamus and a soluble isoform (sLEPR, circulating leptin receptor) generated by the proteolytic cleavage of membrane-bound receptors [10].
It has been proposed that leptin in pregnancy include the regulation of fetal growth and immunomodulation, as well as mobilization of the maternal fat. According to placental perfusion studies, 98.4% of placental leptin is released into the maternal circulation, as well, total and free leptin concentrations are higher in maternal blood during pregnancy and further elevated in pre-eclampsia. Therefore, leptin could be one of the fetal-placental signals altering maternal metabolism to benefit the fetus by mobilizing nutrients [11].
Several studies have demonstrated a strong positive correlation between the serum leptin concentration and amount of body fat [12,13]. On the other hand, the physiological role of leptin in fetal growth has not been well clarified. Reports have demonstrated that leptin levels correlate highly with body mass index in obese children and rise just before the onset of puberty [14]. The aim of this study was to investigate if anthropometric parameters are associated with both leptin and sLEPR levels in newborns and their mothers.
Materials and methods
Participants
We recruited 118 mother-newborn pairs from the Hospital “La Madre y el Niño Guerrerense”, from Chilpancingo City in the state of Guerrero, Mexico. The present study was conducted between January 2013 and March 2014. The study was performed in accordance with the Declaration of Helsinki. All included mothers provided signed informed consent before enrollment. The Ethics Committee of the Hospital “La Madre y el Niño Guerrerense” approved the study protocol. Mothers with pre-eclampsia, hypertension, or diabetes were excluded. Full term neonates were born after uncomplicated pregnancies and were otherwise healthy.
Clinic and anthropometric measurements
The clinical characteristics of the mothers were taken by a trained nurse using standard methods. The weight was measured with a mechanical column, bascule SECA 709 model. To determine height was used vertical stadiometer SECA model 206, and body circumferences were measured using an anthropometric tape. The blood pressure was determined with an automated sphygmomanometer OMRON 712C. We calculated the pre-pregnancy body mass index, according to World Health Organization (WHO). The weight gain was categorized according to the Institute of Medicine of the United States of America (IOM) [15]. The venous blood sample of mothers was taken before delivery. Clinical and anthropometric assessment in the newborn was performed by neonatologist at the same hospital, who determined the weight at birth with a mechanical bascule (SECA 745) and length with mechanical stadiometer for babies (SECA 207). With the anthropometric tape (SECA) were measured the head, abdominal and thoracic circumferences. According to the Mexican Association of Pediatrics we categorize birth weight in underweight (< 2.5 kg), normal-weight (2.5-3.5 kg) and overweight (> 3.5 kg). The newborns and their placentas were weighed immediately after birth.
Laboratory measurements
The venous blood sample of mothers was taken before delivery and immediately after delivery an umbilical cord blood sample was collected. The blood sample was centrifuged at 1200 rpm for 10 min and the serum was stored at -70°C until analysis. Leptin and sLEPR serum concentrations were measured by a sandwich ELISA kit (Human Leptin ELISA and Human Leptin Receptor ELISA, BioVendor Laboratory Medicine Inc, Czech Republic), for these assays the detection limit was 0.2 ng/mL and 0.04 ng/mL, respectively.
Statistical analysis
Data analysis was performed using the statistical package STATA v.9.2. The normality of continuous variables was assessed using the Shapiro-Wilk’s test. Means and standard deviations for symmetric quantitative variables were obtained and the comparison between groups using the t-Student test or one-factor ANOVA. For non-symmetric quantitative variables was obtained medians and percentiles 25 and 75 and comparison between groups was performed using the Mann Whitney or Kruskal Wallis test, were obtained absolute and relative frequencies for qualitative variables and compared between study groups using the chi-square test statistic (X2) were determined. To evaluate correlations between plasma leptin concentration and other parameters, Spearman test was used. A value of P < 0.05 was considered significant statistically.
Results
Clinical and anthropometric characteristics of the mothers are summarized in Table 1. Median maternal age of 25 years old (22-34 yr), median pre-pregnancy BMI of 24 kg/m2 which is representative of normal-weight before pregnancy and median prenatal BMI of 28 kg/m2 characteristic of overweight. However, when we categorize the weight gain of women according to the criteria of the Institute of Medicine of the United States of America (IOM) found that most women (50.4%) had low weight gain during pregnancy and 10.9% were overweight. The women had the blood pressure within the normal range.
Table 1.
Clinical and anthropometric characteristics of the mothers
| Variables | Values |
|---|---|
| Age (years)* | 25 (22-34) |
| Height (cm)† | 1.53 ± 0.06 |
| Pre-pregnancy weight (kg)* | 57 (52-79) |
| Pre-pregnancy BMI (kg/m2)* | 24 (22.3-32.5) |
| Perinatal weight (kg)* | 67 (61-90) |
| Perinatal BMI (kg/m2)* | 28.0 (26.2-37.4) |
| Weight gain (kg)* | 9 (6.2-16.5) |
| Weight gain category n (%) | |
| Underweight | 60 (50.4) |
| Normal-weight | 46 (38.7) |
| Overweight | 13 (10.9) |
| Placental weight (g)* | 625 (540-875) |
| Hip circumference (cm)† | 104.5 ± 10.3 |
| Belly circumference (cm)* | 111 (106-129) |
| Arm circumference (cm)* | 27 (25.2-33) |
| SBP (mmHg)† | 108.53 ± 11.34 |
| DBP (mmHg)† | 70.57 ± 9.61 |
The data shown are median and percentiles (25-75) for nonparametric variables;
mean ± standard deviation for parametric variables;
and frequency as n (%). BMI: Body mass index, SBP: systolic blood pressure, DBP: diastolic blood pressure.
The anthropometric and clinical characteristics of the newborns are shown in Table 2. The results show that female and male neonates had similar somatometric characteristics. However, when we categorize the birthweight of neonates according to Mexican association of Pediatrics found that male neonates had high prevalence of overweight (17.5%) than female neonates (11.4%), although it was not significant.
Table 2.
Anthropometric and clinical characteristics of the newborns
| Variable | Total (n = 118) | Gender | P | |
|---|---|---|---|---|
|
| ||||
| Female (n = 61) | Male (n = 57) | |||
| Gestational age (weeks)† | 38.9 ± 1.3 | 38.9 ± 1.2 | 38.9 ± 1.3 | 0.83 |
| Length (cm)* | 51 (49-53) | 51 (50-53) | 50.5 (49-53) | 0.19 |
| Birthweight (kg)* | 3.16 (2.9-3.3) | 3.18 (2.9-3.3) | 3.13 (2.9-3.3) | 0.90 |
| Birthweight category n (%) | 0.58 | |||
| Underweight < 2.5 kg | 3 (2.5) | 2 (3.3) | 1 (1.8) | |
| Normal-weight 2.5-3.5 kg | 98 (83.1) | 52 (85.3) | 46 (80.7) | |
| Overweight > 3.5 kg | 17 (14.4) | 7 (11.4) | 10 (17.5) | |
| Head circumference (cm)* | 34 (33-36.5) | 34 (33-36.5) | 34 (33-36.5) | 0.50 |
| Thoracic circumference (cm)* | 33 (32-34) | 33 (32-34) | 33.5 (33-35) | 0.04 |
| Abdominal circumference (cm)* | 30.5 (30-35) | 30 (30-36) | 31 (30-34.5) | 0.57 |
| Temperature (°C)* | 36.5 (36.2-37.5) | 36.5 (36.1-37.5) | 36.7 (36.5-37.6) | 0.11 |
The data shown are median and percentiles (25-75) for nonparametric variables;
mean ± standard deviation for parametric variables.
p values were determined by Mann-Whitney and Student’s t test, respectively.
Serum concentration of leptin and sLEPR in newborn-mother pairs are shown in Figure 1A. Maternal serum concentration of leptin and sLEPR (6.2 and 25.7 ng/ml, respectively) were higher than in umbilical cord blood (2.4 and 14.2 ng/ml, respectively). However, the newborns and their mothers had higher sLEPR than leptin levels. Figure 1B shows cord blood leptin and sLEPR levels of the neonates by gender. Leptin was detected in all samples. Leptin levels were higher in females (3.1 ng/ml) than males (1.9 ng/ml) in the total sample, but there was no significant difference in sLEPR levels by gender. Serum leptin and sLEPR levels according to weight gain in the mothers are shown in Figure 1C. In mothers was observed that leptin levels increase with weight gain in pregnancy and decreased sLEPR levels.
Figure 1.
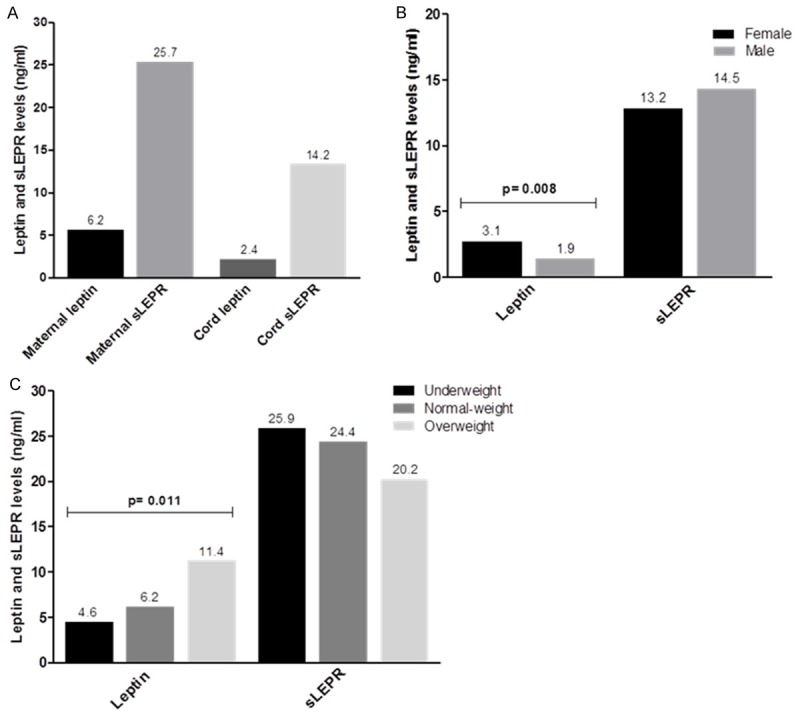
Leptin and sLEPR levels in maternal serum and cord blood. (A) Leptin and sLEPR levels in neonates and their mothers. (B) Cord blood leptin and sLEPR levels of the neonates by gender. (C) Serum leptin and sLEPR levels according to weight gain in the mothers. Data are presented as median and p values were determined by Mann Whitney (B) or Kruskal Wallis (C) test.
There was no significant correlation between the anthropometric characteristics of the mothers and cord blood leptin and sLEPR levels of the newborns. Cord blood leptin levels correlated with neonatal birthweight and length (r = 0.30, P = 0.001), body circumferences, placental weight (r = 0.20, P = 0.034) and maternal leptin levels (r = 0.21, P = 0.026). There was no correlation between cord blood sLEPR levels and anthropometric parameters of the neonates, but if with maternal sLEPR and leptin levels (r = 0.29, P = 0.002 and r = 0.42, P < 0.001, respectively). Maternal serum concentration of leptin correlated with pre-pregnancy BMI (r = 0.32, P < 0.001), weight gain in pregnancy (r = 0.29, P < 0.001) and cord sLEPR and leptin levels (r = 0.42, P < 0.001 and r = 0.21, P = 0.026). Maternal sLEPR correlated negatively with pre-pregnancy BMI (r = -0.18, P = 0.048) and prenatal BMI (r = -0.26, P = 0.004), but it was correlated positively with cord sLEPR (r = 0.29, P = 0.002) (Table 3).
Table 3.
Correlation of leptin and sLEPR in maternal serum and cord blood with selected variables
| Variable | Maternal leptin | Cord leptin | Maternal sLEPR | Cord sLEPR | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| r* | P | r* | P | r* | P | r* | P | |
| Mothers | ||||||||
| Pre-pregnancy BMI | 0.32 | < 0.001 | 0.01 | 0.882 | -0.18 | 0.048 | 0.001 | 0.986 |
| Prenatal BMI | 0.34 | < 0.001 | 0.07 | 0.483 | -0.26 | 0.004 | 0.01 | 0.846 |
| Weight gain | 0.29 | < 0.001 | 0.12 | 0.197 | -0.15 | 0.108 | 0.07 | 0.389 |
| Gestational age | -0.14 | 0.119 | 0.11 | 0.231 | -0.03 | 0.716 | -0.13 | 0.165 |
| Placental weight | 0.17 | 0.059 | 0.20 | 0.034 | -0.03 | 0.780 | 0.03 | 0.785 |
| Belly circumference | 0.28 | 0.002 | -0.04 | 0.621 | -0.11 | 0.218 | 0.09 | 0.314 |
| Leptin levels | --- | --- | 0.21 | 0.026 | -0.09 | 0.311 | 0.42 | < 0.001 |
| sLEPR levels | -0.09 | 0.332 | 0.11 | 0.248 | --- | --- | 0.29 | 0.002 |
| Newborns | ||||||||
| Birthweight | 0.04 | 0.675 | 0.30 | 0.001 | 0.05 | 0.604 | 0.12 | 0.203 |
| Length | -0.02 | 0.827 | 0.30 | 0.001 | 0.04 | 0.706 | 0.06 | 0.484 |
| Head circ. | 0.21 | 0.024 | 0.15 | 0.114 | 0.13 | 0.162 | 0.23 | 0.019 |
| Thoracic circ. | 0.11 | 0.235 | 0.34 | < 0.001 | 0.07 | 0.434 | 0.13 | 0.160 |
| Abdominal circ. | 0.07 | 0.451 | 0.27 | 0.003 | 0.12 | 0.182 | 0.10 | 0.268 |
| Leptin levels | 0.21 | 0.026 | --- | ---- | 0.11 | 0.236 | 0.14 | 0.121 |
| sLEPR levels | 0.42 | < 0.001 | 0.14 | 0.130 | 0.29 | 0.002 | --- | --- |
BMI: body mass index, Head circ: head circumference, Thoracic circ: thoracic circumference, Abdominal circ: abdominal circumference, sLEPR: soluble leptin receptor.
Spearman correlation coefficient.
Discussion
Our study showed that the main determinants of maternal leptin levels are body adiposity and weight gain during pregnancy, but an inverse relation between maternal sLEPR and body adiposity was observed. In neonates, leptin levels correlated with birthweight and anthropometric measures and sLEPR levels with maternal leptin and sLEPR levels.
We found that maternal serum concentration of leptin and sLEPR were higher than in umbilical cord blood. Several studies have provided evidence that high leptin and sLEPR levels are present in maternal serum that in cord blood serum at birth [16-18].
Similarly, it has been reported that leptin levels reflect the mount of adipose tissue and during pregnancy, fetal leptin levels increase in parallel with its development [14]. In adults, levels of circulating leptin are directly proportional to total fat mass [19] and are negatively associated with insulin sensitivity [20]. In another study, circulating leptin level was found to be proportional to adipose tissue mass, and body fat percentage was possibly the best adiposity-related predictor of serum leptin concentrations in human, which may be due to leptin resistance [21]. In the studies on the association between leptin and indirect measures of adiposity, the most frequently used measures were body mass index (BMI) and waist circumference [22].
Furthermore, the increase in the concentration of leptin can be attributed to the mobilization of nutrient sources required for fetal growth such as maternal adipose tissue [23]. We confirmed that BMI and weight gain in pregnancy are the main determinants of leptin levels in pregnant women, but not the placental weight. This correlation suggests body adiposity as the main determinant factor for leptin levels in the mothers.
In this study, the leptin levels in umbilical cord blood are lower than those seen in the mothers. This may be due to the increased prevalence in neonates of normal-weight (83.1%) and underweight (2.5%), therefore to less body fat decreases the leptin levels. Furthermore, cord leptin levels correlated with neonatal length and birthweight, as well placental weight, and these results are consistent with another study that found that the fetus and placenta contribute to the total amount of leptin [24]. While it is known that the placenta secretes leptin, the wide majority of this (at least 95%) is secreted into the maternal circulation, with very little going into the fetal circulation, meaning that cord blood leptin reflects fetal fat mass, with less than 1% estimated to be from the placenta/maternal circulation [25].
There is converging evidence that a strong correlation between leptin levels and neonatal birth weight exists [26,27], therefore lower leptin levels in the cord blood of our neonates suggest that is mostly sourced from fetal adipose tissue. We found that leptin levels in cord blood were highest in female compared to the male neonates, but not cord sLEPR levels. Even though, there was no significant difference in anthropometric measures between female and male newborns, therefore cannot attribute the difference in leptin levels to increase in weight or body circumferences in females. Underlying causes might be the differential amount of fat tissue by gender, the role of the variable sex steroid milieus of the newborn and the heavier placental weight associated with female gender [28].
In another study, it has been reported that serum leptin levels are higher in female neonates than males, but whether the cause of this difference is a suppression of leptin synthesis by androgens remains unclear [29]. Mellati and colleagues suggests that the estrogens may to increase leptin levels in female neonates [30]. Estrogens have been reported to regulate leptin expression by acting on a portion of the estrogen response element in the leptin promoter [31]. While the estrogen and corticosteroids are associated with the enhancement of leptin synthesis, androgens are linked to leptin inhibition [32]. In healthy men, testosterone concentrations were negatively correlated with leptin in serum [33].
We found that the sLEPR concentrations in cord blood and maternal serum, were significantly highest compared to the leptin levels. Similar to the previous reports of sLEPR levels behave in an opposite fashion to leptin levels at birth [18], as in later stages of extrauterine life [34]. The function of sLEPR is not entirely clear but believed to delay the clearance of leptin from the circulation and, thus, increase leptin’s availability [35]. In addition, there is evidence suggesting that sLEPR not only alters the clearance of leptin but also potentiates leptin action [36]. Therefore, our results suggest that, as a consequence of the inverse relationship between maternal levels of leptin and sLEPR, the increase in sLEPR levels may plays a role in the regulation of the leptin levels and its biological activity. Similarly, high sLEPR in cord blood may prevent the leptin from degradation and clearance. Moreover, we found that cord sLEPR levels correlated with maternal serum concentration of leptin and sLEPR, thus hormonal or genetic factors of their mothers can influence sLEPR levels in newborns.
Our study has some limitations. We relied on a single measure of leptin and sLEPR levels at delivery and we did not measure other hormones that are implicated in the regulation of leptin levels and birthweight in the newborns. Taken together, our results indicate that the leptin and sLEPR levels in mother-newborn pairs are related with anthropometric parameters and an inverse correlation between leptin levels and sLEPR was observed in pairs. Biological mechanisms underlying these observations need to be further elucidated.
Acknowledgements
This study was supported by Grants from the Consejo Nacional de Ciencia y Tecnología INFR-2014-02/229958 and Programa de Fortalecimiento Académico del Posgrado de Alta Calidad 2013 and 2014. LAMO received a fellowship of CONACYT (No. 231241).
Disclosure of conflict of interest
None.
References
- 1.Thomas C, Hypponen E, Power C. Prenatal exposure and glucose metabolism in adulthood. Diabetes Care. 2007;30:918–924. doi: 10.2337/dc06-1881. [DOI] [PubMed] [Google Scholar]
- 2.Maymó JL, Pérez Pérez A, Sánchez-Margalet V, Dueñas JL, Calvo JC, Varone CL. Up-regulation of placental leptin by human chorionic gonadotropin. Endocrinology. 2009;50:304–313. doi: 10.1210/en.2008-0522. [DOI] [PubMed] [Google Scholar]
- 3.Fitzgerald JS, Busch S, Wengenmayer T, Foerster K, de la Motte T, Poehlmann TG, Markert UR. Signal transduction in trophoblast invasion. Chem Immunol Allergy. 2005;88:181–199. doi: 10.1159/000087834. [DOI] [PubMed] [Google Scholar]
- 4.Fitzgerald JS, Poehlmann TG, Schleussner E, Markert UR. Trophoblast invasion: the role of intracellular cytokine signalling via signal transducer and activator of transcription 3 (STAT3) Hum Reprod Update. 2008;14:335–344. doi: 10.1093/humupd/dmn010. [DOI] [PubMed] [Google Scholar]
- 5.Magariños MP, Sánchez-Margalet V, Kotler M, Calvo JC, Varone CL. Leptin promotes cell proliferation and survival of trophoblastic cells. Biol Reprod. 2007;76:203–210. doi: 10.1095/biolreprod.106.051391. [DOI] [PubMed] [Google Scholar]
- 6.Kyriakakou M, Malamitsi-Puchner A, Militsi H, Boutsikou T, Margeli A, Hassiakos D, Kanaka-Gantenbein C, Papassotiriou I, Mastorakos G. Leptin and adiponectin concentrations in intrauterine growth restricted and appropriate for gestational age fetuses, neonates, and their mothers. Eur J Endocrinol. 2008;158:343–348. doi: 10.1530/EJE-07-0692. [DOI] [PubMed] [Google Scholar]
- 7.Zare F, Moradizirkohi A, Maghbooli Zh, Hosseinnezhad A, Rahmani M, Larijani B. Relationship between Serum Umbilical Cord and Maternal Leptin and Adiponectin Concentrations with Fetal Growth Parameters. Iranian J Publ Health. 2007:75–79. [Google Scholar]
- 8.Fantuzzi G, Faggioni R. See comment in PubMed Commons below Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol. 2000;68:437–446. [PubMed] [Google Scholar]
- 9.Sagawa N, Yura S, Itoh H, Mise H, Kakui K, Korita D, Takemura M, Nuamah MA, Ogawa Y, Masuzaki H, Nakao K, Fujii S. Role of Leptin in Pregnancy-A review. Placenta. 2002;23:S80–86. doi: 10.1053/plac.2002.0814. [DOI] [PubMed] [Google Scholar]
- 10.Zastrow O, Seidel B, Kiess W, Thiery J, Keller E, Böttner A, Kratzsch J. The soluble leptin receptor is crucial for leptin action: evidence from clinical and experimental data. Int J Obes Relat Metab Disord. 2003;27:1472–1478. doi: 10.1038/sj.ijo.0802432. [DOI] [PubMed] [Google Scholar]
- 11.Laivuori H, Gallaher MJ, Collura L, Crombleholme WR, Markovic N, Rajakumar A, Hubel CA, Roberts JM, Powers RW. Relationships between maternal plasma leptin, placental leptin mRNA and protein in normal pregnancy, pre-eclampsia and intrauterine growth restriction without pre-eclampsia. Mol Hum Reprod. 2006;12:551–556. doi: 10.1093/molehr/gal064. [DOI] [PubMed] [Google Scholar]
- 12.Castellano Filho DS, do Amaral Correa JO, Dos Santos Ramos P, de Oliveira Montessi M, Aarestrup BJ, Aarestrup FM. Body weight gain and serum leptin levels of non-overweight and overweight/obese pregnant women. Med Sci Monit. 2013;19:1043–1049. doi: 10.12659/MSM.884027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, Kern PA, Friedman JM. Leptin levels in human and rodent: Measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1:1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 14.Harigaya A, Nagashima K, Nako Y, Morikawa A. Relationship between concentration of serum leptin and fetal growth. J Clin Endocrinol Metab. 1997;82:3281–3284. doi: 10.1210/jcem.82.10.4321. [DOI] [PubMed] [Google Scholar]
- 15.Weight Gain During Pregnancy: Reexamining the Guidelines. The National Academies Press; 2009. May 28, Institute of Medicine. [PubMed] [Google Scholar]
- 16.Rafeey M, Ouladsahebmadarek E, Rashtchizadeh N, Sheikh Monazah F, Gorbanihaghjo A, Hosseini MB, Nejati N. Correlation between maternal and cord blood leptin and fetal growth. African Journal of Biotechnology. 2007;6:2023–2027. [Google Scholar]
- 17.Schubring C, Kiess W, Englaro P, Rascher W, Dötsch J, Hanitsch S, Attanasio A, Blum WF. Levels of leptin in maternal serum, amniotic fluid, and arterial and venous cord blood: relation to neonatal and placental weight. J Clin Endocrinol Metab. 1997;82:1480–1483. doi: 10.1210/jcem.82.5.3935. [DOI] [PubMed] [Google Scholar]
- 18.Kratzsch J, Schubring C, Stitzel B, Böttner A, Berthold A, Thiery J, Kiess W. Inverse changes in the serum levels of the soluble leptin receptor and leptin in neonates: relations to anthropometric data. J Clin Endocrinol Metab. 2005;90:2212–2217. doi: 10.1210/jc.2004-1454. [DOI] [PubMed] [Google Scholar]
- 19.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, Caro JF. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 20.Wauters M, Considine RV, Yudkin JS, Peiffer F, De Leeuw I, Van Gaal LF. Leptin levels in Type 2 diabetes: associations with measures of insulin resistance and insulin secretion. Horm Metab Res. 2003;35:92–96. doi: 10.1055/s-2003-39054. [DOI] [PubMed] [Google Scholar]
- 21.Mahabir S, Baer D, Johnson LL, Roth M, Campbell W, Clevidence B, Taylor PR. Body Mass Index, percent body fat, and regional body fat distribution in relation to leptin concentrations in healthy, non-smoking postmenopausal women in a feeding study. Nutr J. 2007;6:3. doi: 10.1186/1475-2891-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mente A, Razak F, Blankenberg S, Vuksan V, Davis AD, Miller R, Teo K, Gerstein H, Sharma AM, Yusuf S, Anand SS Study of the Health Assessment And Risk Evaluation; Study of the Health Assessment And Risk Evaluation in Aboriginal Peoples Investigators. Ethnic Variation in adiponectin and leptin levels and their association with adiposity and insulin resistance. Diabetes Care. 2010;33:1629–1634. doi: 10.2337/dc09-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hauguel-de Mouzon S, Lepercq J, Catalano P. The known and unknown of leptin in pregnancy. Am J Obstet Gynecol. 2006;194:1537–1545. doi: 10.1016/j.ajog.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 24.Lappas M, Yee K, Permezel M, Rice GE. Release and regulation of leptin, resistin and adiponectin from human placenta, fetal membranes, and maternal adipose tissue and skeletal muscle from normal and gestational diabetes mellitus-complicated pregnancies. J Endocrinol. 2005;186:457–465. doi: 10.1677/joe.1.06227. [DOI] [PubMed] [Google Scholar]
- 25.Catalano PM, Presley L, Minium J, Hanguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care. 2009;32:1076–1080. doi: 10.2337/dc08-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang SW, Kim SY. The relationship of the levels of leptin, insulin-like growth factor-I and insulin in cord blood with birth size, ponderal index, and gender difference. J Pediatr Endocrinol Metab. 2000;13:289–296. doi: 10.1515/jpem.2000.13.3.289. [DOI] [PubMed] [Google Scholar]
- 27.Petridou E, Mantzoros CS, Belechri M, Skalkidou A, Dessypris N, Papathoma E, Salvanos H, Lee JH, Kedikoglou S, Chrousos G, Trichopoulos D. Neonatal leptin levels are strongly associated with female gender, birth length, IGF-I levels and formula feeding. Clin Endocrinol (Oxf) 2005;62:366–371. doi: 10.1111/j.1365-2265.2005.02225.x. [DOI] [PubMed] [Google Scholar]
- 28.Watanobe H, Habu S. Manipulation of neonatal gonadal steroid milieu and leptin secretion in later life in male and female rats. Regul Pept. 2003;110:219–224. doi: 10.1016/s0167-0115(02)00215-x. [DOI] [PubMed] [Google Scholar]
- 29.Schubring C, Siebler T, Kratzch J, Englaro P, Blum WF, Triep K, Kiess W. Leptin serum concentrations in healthy neonates within the first week of life: relation to insulin and growth hormone levels, skinfold thickness, body mass index and weight. Clin Endocrinol (Oxf) 1999;51:199–204. doi: 10.1046/j.1365-2265.1999.00761.x. [DOI] [PubMed] [Google Scholar]
- 30.Mellati AA, Mazloomzadeh S, Anjomshoaa A, Alipour M, Karimi F, Mazloomi S, Naghi Kazemi SA. Multiple correlations between cord blood leptin concentration and indices of neonatal growth. Arch Med Res. 2010;41:26–32. doi: 10.1016/j.arcmed.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Machinal F, Dieudonne MN, Leneveu MC, Pecquery R, Giudicelli Y. In vivo and in vitro ob gene expression and leptin secretion in rat adipocytes: evidence for a regional specific regulation by sex steroid hormones. Endocrinology. 1999;140:1567–1574. doi: 10.1210/endo.140.4.6617. [DOI] [PubMed] [Google Scholar]
- 32.Reidy SP, Weber JM. Leptin: an essential regulator of lipid metabolism. Comp Biochem Physiol A Mol Integr Physiol. 2000;125:285–298. doi: 10.1016/s1095-6433(00)00159-8. [DOI] [PubMed] [Google Scholar]
- 33.Thomas T, Burguera B, Melton LJ III, Atkinson EJ, O’Fallon WM, Riggs BL, Khosla S. Relationship of serum leptin levels with body composition and sex steroid and insulin levels in men and women. Metabolism. 2000;49:1278–1284. doi: 10.1053/meta.2000.9519. [DOI] [PubMed] [Google Scholar]
- 34.Kratzsch J, Lammert A, Bottner A, Seidel B, Mueller G, Thiery J, Hebebrand J, Kiess W. Circulating soluble leptin receptor and free leptin index during childhood, puberty, and adolescence. J Clin Endocrinol Metab. 2002;87:4587–4594. doi: 10.1210/jc.2002-020001. [DOI] [PubMed] [Google Scholar]
- 35.Huang L, Wang Z, Li C. Modulation of circulating leptin levels by its soluble receptor. J Biol Chem. 2001;276:6343–6349. doi: 10.1074/jbc.M009795200. [DOI] [PubMed] [Google Scholar]
- 36.Cohen SE, Kokkotou E, Biddinger SB, Kondo T, Gebhardt R, Kratzsch J, Mantzoros CS, Kahn CR. High circulating leptin receptors with normal leptin sensitivity in liver-specific insulin receptor knock-out (LIRKO) mice. J Biol Chem. 2007;282:23672–23678. doi: 10.1074/jbc.M704053200. [DOI] [PubMed] [Google Scholar]


