Abstract
Purpose: To investigate the relationship between blood glucose levels, age, body mass index (BMI), and benign prostatic hyperplasia (BPH) in patients with newly diagnosed type 2 diabetes. Methods: A total of 141 BPH patients with newly diagnosed type 2 diabetes participated in this study. Their glucose level, international prostate symptom score (IPSS), prostate volume (PV), and maximum urinary flow rate (Qmax) were determined and analyzed. Results: Compared to patients in 60-69 years of age, those in 70-79 years of age had higher IPSS and PV values (11.10±2.68 vs. 16.09±2.64, respectively; P<0.01; 38.67±4.65 vs. 44.76±2.84, respectively; P<0.01) as did patients ≥80 y (11.10±2.68 vs. 19.87±3.35, respectively; P<0.01; 38.67±4.65 vs. 51.38±3.74, respectively; P<0.01). The Qmax was lower in the ≥80 y group compared to the 60-69 y group (7.91±2.13 vs. 13.50±1.75, respectively; P<0.01). IPSS, PV, and insulin resistance index (HOMA-IR) were higher in patients with a BMI ≥28 kg/m2 group as compared to those with a BMI <24 kg/m2 group. IPSS and PV values were higher in patients with HbA1c levels ≥6.5% than in those with HbA1c<6.5% (16.30±3.31 vs. 9.87±1.07, respectively; P<0.01; 45.69±3.97 vs. 36.64±3.30, respectively; P<0.01), and the Qmax was lower (10.61±1.98 vs. 14.40±0.82, respectively; P<0.01). Conclusions: Aging, obesity, high glucose level, and insulin resistance increase the risk of BPH progression in elderly patients with newly diagnosed type 2 diabetes. Managing body weight and lowering the level of glycosylated hemoglobin may slow the progression of BPH in people with type 2 diabetes.
Keywords: Type 2 diabetes mellitus, benign prostatic hyperplasia, blood glucose level, obesity
Introduction
Benign prostatic hyperplasia (BPH) is a common benign disease that causes voiding dysfunction in elderly male patients and, to various extents, impairs their quality of life. Original studies of BPH pathogenesis identified androgen and aging as risk factors [1], and more recent studies have identified additional risk factors, such as genetics, diet, immune status, metabolism, and inflammation [2,3]. Studies of BPH patients with metabolic disorders, such as diabetes and obesity, have found a positive correlation between fasting glucose level and the progression of BPH [4]. A positive correlation has also been shown between hyperlipidemia and the progression of BPH [5]. Although there have been multiple studies on the progression of BPH in patients with systemic metabolic disorders [6], the impact of glucose and lipid metabolism disorders on BPH is still not clear. Here, we chose elderly patients with newly diagnosed type 2 diabetes and studied their BPH in correlation to body mass index (BMI) and glucose levels, aiming to explore the relationships between aging, obesity, hyperglycemia, and BPH.
Methods
Study subjects
A total of 141 BPH patients who were newly diagnosed with type 2 diabetes in our hospital were enrolled in this study from January 2013 to December 2013. Their ages ranged from 60 to 85 years. Patients with prostate cancer or a medical history of prostate surgery were excluded. Informed consents were received from all participants, and the study was approved by local ethics committees.
Data collection
All patients’ height and weight were recorded for calculation of BMI. Fasting blood glucose (FBG), 2-hour postprandial blood glucose (PBG), glycosylated hemoglobin (HbAlc), and fasting insulin (FINS) were determined in the hospital diagnostic laboratory. Insulin resistance index (HOMA-IR) was determined using the following formula: HOMA-IR = FBG×FINS/22.5.
IPSS was obtained by standardized interview after patients were diagnosed with type 2 diabetes. PV was determined by means of transrectal ultrasound. Maximum urinary flow rate was measured by uroflowmetry. PSA/fr-ee PSA (FPSA) was also determined. Patients with elevated PSA levels underwent prostate biopsy in order to rule out prostate cancer.
Diagnostic criteria
The diagnosis of type 2 diabetes was made according to the criteria of the American Diabetes Association (ADA). HbA1c of 6.5% was used as the threshold of diagnosing type 2 diabetes [7]. The diagnosis criteria of BPH were as follows: (1) prostate specific antigen (PSA) <4 μg/L, no abnormal nodules detected by digital rectal examination, and no abnormal echo via abdominal ultrasound; (2) PSA >4 μg/L, and prostate cancer was excluded by prostate biopsy; and (3) prostate volume (PV) >30 mL, estimated by transrectal ultrasound, with or without IPSS >7.
Obesity was defined using the criteria made by the Working Group on Obesity in China (WGOC) [8]. Specifically, a patient with a BMI of 18.5-23.9 kg/m2 was considered to have normal body weight, those with a BMI from 24.0-27.9 kg/m2 were considered to be overweight, and patients with a BMI ≥28.0 kg/m2 were considered to be obese.
Statistical methods
All continuous variables were presented as mean ± standard deviation. SPSS for Windows 13.0 software (Chicago, IL, USA) was used for data analysis. Two-sample t-test was used to analyze the differences between two groups. Pearson correlation coefficient was used to detect the correlation of continuous variables. Multiple linear regression analysis was performed to study the risk factors of IPSS. Statistical significance was defined as two-sided P-values <0.05.
Results
Comparison of IPSS, PV, and Qmax by age, BMI and HbA1c
The average age of the 141 BPH patients was 69.7±6.6 years. In older patients, IPSS and PV were higher while Qmax was lower. As shown in Table 1, IPSS and PV were significantly higher in patients aged 70-79 years (IPSS: 16.09±2.64; PV: 44.76±2.84 cm3) and 80-85 years (IPSS: 19.87±3.35; PV: 51.38±3.74 cm3) compared with those aged 60-69 years (IPSS: 11.10±2.68; PV: 38.67±4.65 cm3), respectively. Qmax was significantly lower in patients aged 70-79 years (11.02±1.30 mL/s) and 80-85 years (7.91±2.13 mL/s) than in those aged 60-69 years (13.50±1.75 mL/s), respectively.
Table 1.
BPH related measurements of study subjects by age, BMI and HbA1c
| Variable | N | IPSS | P | PV (cm3) | P | Qmax (mL/s) | P |
|---|---|---|---|---|---|---|---|
| Age (years) | |||||||
| 60-69 | 77 | 11.10±2.68 | 38.67±4.65 | 13.50±1.75 | |||
| 70-79 | 50 | 16.09±2.64 | <0.01 | 44.76±2.84 | <0.01 | 11.02±1.30 | <0.01 |
| 80-85 | 14 | 19.87±3.35 | <0.01 | 51.38±3.74 | <0.01 | 7.91±2.13 | <0.01 |
| BMI (kg/m2) | |||||||
| <24 | 44 | 9.49±0.86 | 35.59±2.94 | 14.70±0.64 | |||
| 24-27.9 | 59 | 13.17±1.68 | <0.01 | 42.36±1.59 | <0.01 | 12.31±0.81 | <0.01 |
| ≥28 | 38 | 19.57±1.43 | <0.01 | 49.23±3.18 | <0.01 | 8.81±1.48 | <0.01 |
| HbA1c (%) | |||||||
| <6.5 | 56 | 9.87±1.07 | 36.64±3.30 | 14.40±0.82 | |||
| ≥6.5 | 85 | 16.30±3.31 | <0.01 | 45.69±3.97 | <0.01 | 10.61±1.98 | <0.01 |
In addition, IPSS and PV elevated with increasing BMI. IPSS and PV were significantly higher in the BMI ≥28 kg/m2 group (IPSS: 19.57±1.43; PV: 49.23±3.18 cm3) and BMI of 24-27.9 kg/m2 group (IPSS: 13.17±1.68; PV: 42.36±1.59 cm3) compared with the BMI <24 kg/m2 group (IPSS: 9.49±0.86; PV: 35.59±2.94 cm3), respectively. However, Qmax decreased while BMI increased. Qmax was significantly lower in patients with BMI of 24-27.9 kg/m2 (12.31±0.81 mL/s) and BMI ≥28 kg/m2 (8.81±1.48 mL/s) than in those with BMI <24 kg/m2 (14.7±0.64 mL/s), respectively.
Furthermore, IPSS and PV were higher and Qmax was lower in patients with higher HbA1c. IPSS and PV were significantly higher in patients with HbAlc ≥6.5% compared with those with HbAlc <6.5% (IPSS: 16.30±3.31 vs. 9.87±1.07; PV: 45.69±3.97 cm3 vs. 36.64±3.30 cm3). Qmax was significantly lower in patients with HbAlc ≥6.5% than in those with HbAlc <6.5% (10.61±1.98 mL/s vs. 14.40±0.82 mL/s).
We also explored the association between obesity, Hyperglycemia and BPH progression with all risk factors as continuous variables. As shown in Table 2, age, BMI, HbA1c, FBG, PBG and HOMA-IR were all significantly positively correlated with IPSS and PV and significantly negatively correlated with Qmax.
Table 2.
Correlation between age, BMI, HbAlc, FBG, PBG, HOMA-IR and IPSS, PV, Qmax
| IPSS | PV (cm3) | Qmax | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| v | r | P | r | P | r | P |
| Age (year) | 0.737 | 0.000 | 0.713 | 0.000 | -0.731 | 0.000 |
| BMI (kg/m2) | 0.863 | 0.000 | 0.872 | 0.000 | -0.868 | 0.000 |
| HbAlc (%) | 0.706 | 0.000 | 0.706 | 0.000 | -0.711 | 0.000 |
| FBG (mmol/L) | 0.654 | 0.000 | 0.628 | 0.000 | -0.644 | 0.000 |
| PBG (mmol/L) | 0.629 | 0.000 | 0.613 | 0.000 | -0.629 | 0.000 |
| HOMA-IR | 0.853 | 0.000 | 0.849 | 0.000 | -0.853 | 0.000 |
Association between age, BMI, HbA1c and IPSS
Multivariate linear regression analysis was also used to determine the association between age, BMI, HbA1c and IPSS. Since HbAlc, FBG and PBG, all as indicators of Hyperglycemia, were highly correlated with each of the others, only HbAlc was used in the linear regression analysis. As shown in Table 3 and Figure 1, IPSS was significantly positively associated age, BMI and HbAlc, respectively. One-year increase in age was significantly associated with an elevation of 0.141 in IPSS, after adjusting for BMI and HbAlc. One-unit increase of BMI corresponded significantly with an elevation of 0.714 in IPSS, after adjusting for age and HbAlc. One-percent increase of HbAlc was significantly associated with an elevation of 0.352 in IPSS, controlling for age and BMI. Furthermore, BMI was more associated with IPSS compared with age and HbAlc.
Table 3.
Multivariate linear regression analysis of risk factors associated with BPH progression
| Independent variable | Parameter Estimate | Standardized Parameter | P |
|---|---|---|---|
| Age (years) | 0.141 | 0.226 | <0.001 |
| BMI (kg/m2) | 0.714 | 0.642 | <0.001 |
| HbA1c (%) | 0.352 | 0.151 | 0.0008 |
Figure 1.
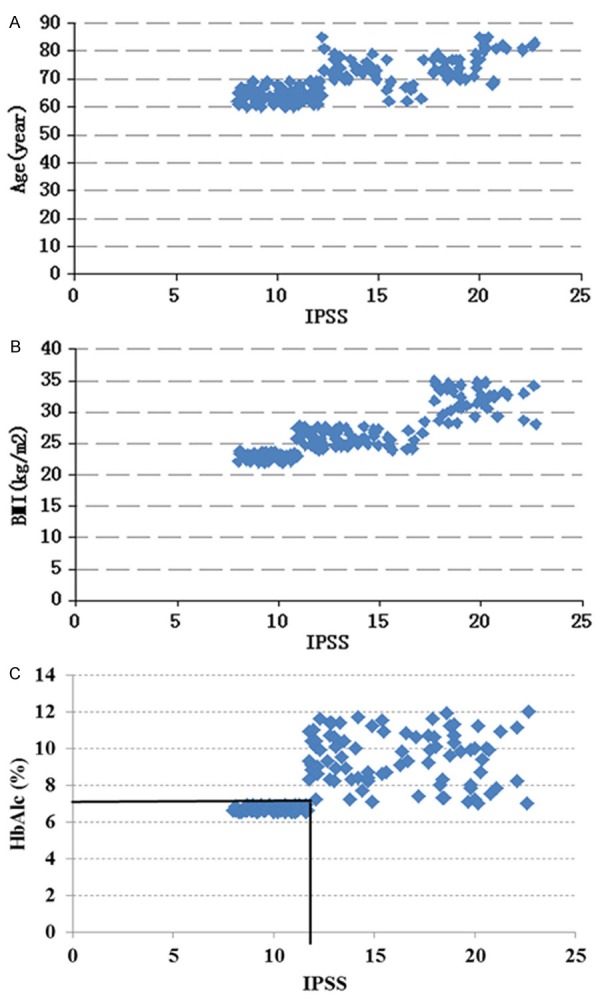
A. Scatter diagram between IPSS and Age; B. Scatter diagram between IPSS and BMI; C. Scatter diagram between IPSS and HbA1c.
Discussion
Diabetes and obesity are characterized by high plasma glucose and abnormal lipid levels, and are considered to be systematic metabolic diseases. In addition to damaging body tissues, the impaired glucose metabolism and dyslipidemia also damage multiple organs and cause systemic diseases, such as coronary heart disease and stroke. In patients with BPH, or lower urinary tract symptoms (LUTS), diabetes and obesity are commonly seen as co-morbidities. Actually, this co-existence has been known for more than 40 years [9], and recent studies suggest that BPH may not be a single disease, but rather manifestations due to systemic alterations. For instance, Jerde and Bushman [10] indicated that alteration of sex steroid hormone metabolism caused by both obesity and diabetes could lead to ‘pro-inflammatory’ conditions in whole body, resulting in the release of chemokines that may contribute to prostate enlargement. In addition, in 158 men with LUTS, larger prostates were associated not only with higher incidences of diabetes, obesity, and hypertension, but also higher serum insulin levels and lower levels of low-density lipoprotein (LDL) [11], which may imply a link between the metabolic syndrome and prostatic enlargement.
In this study, we have demonstrated that IPSS and PV increase with increasing blood glucose levels and BMI in elderly patients with newly diagnosed type 2 diabetes. This trend has never been previously reported. In addition, we have shown that Qmax decreases with increasing blood glucose levels and BMI. We think it is the first step to revealing the effect of obesity and hyperglycemia in the etiology of BPH in elderly patients with newly diagnosed type 2 diabetes. Further studies are needed to understand the underlying mechanisms.
The results of this study showed significant differences in IPSS and PV between the different BMI groups. The highest levels of IPSS and PV were observed in the obese group. This result is consistent with previous studies. For example, Lee et al. [12] reported that BMI and waist circumference were positively correlated with prostate volume (i.e., prostate volume increased by 0.41 mL with each additional 1 kg/m2 increase in BMI). Parsons et al. [13] also reported that the incidence of BPH was 3.5-fold higher in severely obese patients was than in patients with normal body weight. The high incidence of BPH among obese patients might be related not only with the imbalance between androgen and estrogen, but also with the presence of insulin resistance and hyperinsulinemia [14]. Vikram et al. [14] reported that in obese patients, increased levels of free fatty acids in plasma inhibit pyruvate dehydrogenase and phosphofructokinase, causing an accumulation of glucose-6-phosphate, which in turn inhibits hexokinase II. These chain reactions eventually result in poor glucose absorption into cells, i.e., insulin resistance. To compensate, more insulin is secreted by pancreatic β cells, resulting in secondary hyperinsulinemia. More insulin-like growth factor-1 (IGF-1) is observed in hyperinsulinemia, and the IGF-1 may promote enlargement of the prostate. The present study demonstrated that insulin resistance index was much higher in patients with a BMI >28 kg/m2, which supports the link between insulin resistance and BPH. More attention should, therefore, be paid to the presence of BPH in elderly obese patients with type 2 diabetes.
According to previous studies, blood glucose levels are positively correlated with prostate volume; the higher the fasting blood glucose level, the larger the prostate volume [15]. Diabetes may, therefore, be a risk factor for BPH [16]; however, it is still not known whether blood sugar management could inhibit the development of BPH. This study showed that IPSS and PV were higher in patients with HbAlc values ≥6.5% than in those with HbAlc <6.5%, while Qmax was lower in the HbAlc ≥6.5% group. This implies that the level of glycemic control in diabetes might affect the progress of BPH, i.e., the lower the blood sugar level, the better the improvement in IPSS, PV, and Qmax in patients with BPH. Therefore, for elderly patients with diabetes, good blood glucose management may facilitate the prevention and deceleration of BPH development. For elderly patients with BPH and diabetes, HbAlc should be reduced to 7% or less, as this may inhibit the development of BPH as well as other diabetic complications.
Most previous studies concluded that BPH is significantly associated with age [17]. In this study, IPSS and PV increased with age, and Qmax decreased with age in elderly patients who were newly diagnosed with type 2 diabetes. Our findings are, therefore, consistent with previous studies.
Conclusion
In summary, age, obesity, and high blood glucose levels all contribute to the development of BPH. Better control of body weight and blood sugar management may be beneficial in preventing and decelerating BPH in elderly patients with diabetes.
Acknowledgements
This project was supported by grants and contracts from the Beijing Science & Technology Committee, China (Grant No. 7122073) to G.B.M and a grant from the Clinic-Basic Fund of Capital Medical University (Grant No. 14JL41) to ZC and the Capital Clinical Research Foundation of Beijing Municipal Commission of Science and Technology (Z131107002213024) to Yuan Xu. The authors report no declarations of interest.
Disclosure of conflict of interest
None.
References
- 1.Vovk EI, Vertkin AL, Zairat’iants OV, Mishutchenko OP. [Benign prostate hyperplasia as an age-related problem] . Arkh Patol. 2008;70:55–59. [PubMed] [Google Scholar]
- 2.Vignozzi L, Rastrelli G, Corona G, Gacci M, Forti G, Maggi M. Benign prostatic hyperplasia: a new metabolic disease? J Endocrinol Invest. 2014;37:313–322. doi: 10.1007/s40618-014-0051-3. [DOI] [PubMed] [Google Scholar]
- 3.Bostanci Y, Kazzazi A, Momtahen S, Laze J, Djavan B. Correlation between benign prostatic hyperplasia and inflammation. Curr Opin Urol. 2013;23:5–10. doi: 10.1097/MOU.0b013e32835abd4a. [DOI] [PubMed] [Google Scholar]
- 4.De Nunzio C, Aronson W, Freedland SJ, Giovannucci E, Parsons JK. The correlation between metabolic syndrome and prostatic diseases. Eur Urol. 2012;61:560–570. doi: 10.1016/j.eururo.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Jiang M, Strand DW, Franco OE, Clark PE, Hayward SW. PPARgamma: a molecular link between systemic metabolic disease and benign prostate hyperplasia. Differentiation. 2011;82:220–236. doi: 10.1016/j.diff.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nandeesha H. Benign prostatic hyperplasia: dietary and metabolic risk factors. Int Urol Nephrol. 2008;40:649–656. doi: 10.1007/s11255-008-9333-z. [DOI] [PubMed] [Google Scholar]
- 7.American Diabetes A. Standards of medical care in diabetes--2013. Diabetes Care. 2013;36(Suppl 1):S11–66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou BF Cooperative Meta-Analysis Group of the Working Group on Obesity in China. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults--study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15:83–96. [PubMed] [Google Scholar]
- 9.Bourke JB, Griffin JP. Hypertension, diabetes mellitus, and blood groups in benign prostatic hypertrophy. Br J Urol. 1966;38:18–23. doi: 10.1111/j.1464-410x.1966.tb09675.x. [DOI] [PubMed] [Google Scholar]
- 10.Jerde TJ, Bushman W. IL-1 induces IGF-dependent epithelial proliferation in prostate development and reactive hyperplasia. Sci Signal. 2009;2:ra49. doi: 10.1126/scisignal.2000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammarsten J, Hogstedt B, Holthuis N, Mellstrom D. Components of the metabolic syndrome-risk factors for the development of benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 1998;1:157–162. doi: 10.1038/sj.pcan.4500221. [DOI] [PubMed] [Google Scholar]
- 12.Lee S, Min HG, Choi SH, Kim YJ, Oh SW, Kim YJ, Park Y, Kim SS. Central obesity as a risk factor for prostatic hyperplasia. Obesity (Silver Spring) 2006;14:172–179. doi: 10.1038/oby.2006.21. [DOI] [PubMed] [Google Scholar]
- 13.Parsons JK, Carter HB, Partin AW, Windham BG, Metter EJ, Ferrucci L, Landis P, Platz EA. Metabolic factors associated with benign prostatic hyperplasia. J Clin Endocrinol Metab. 2006;91:2562–2568. doi: 10.1210/jc.2005-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vikram A, Jena G, Ramarao P. Insulin-resistance and benign prostatic hyperplasia: the connection. Eur J Pharmacol. 2010;641:75–81. doi: 10.1016/j.ejphar.2010.05.042. [DOI] [PubMed] [Google Scholar]
- 15.Kim WT, Yun SJ, Choi YD, Kim GY, Moon SK, Choi YH, Kim IY, Kim WJ. Prostate size correlates with fasting blood glucose in non-diabetic benign prostatic hyperplasia patients with normal testosterone levels. J Korean Med Sci. 2011;26:1214–1218. doi: 10.3346/jkms.2011.26.9.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parsons JK. Benign Prostatic Hyperplasia and Male Lower Urinary Tract Symptoms: Epidemiology and Risk Factors. Curr Bladder Dysfunct Rep. 2010;5:212–218. doi: 10.1007/s11884-010-0067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu C, Kapoor A. Dutasteride for the treatment of benign prostatic hyperplasia. Expert Opin Pharmacother. 2013;14:1399–1408. doi: 10.1517/14656566.2013.797965. [DOI] [PubMed] [Google Scholar]


