Abstract
Non-small cell lung cancer (NSCLC), which account for the most of lung carcinoma, is sometimes difficult to differentiate from benign lung diseases presented with nodular shadow in imaging scan. There is a need to find another non-invasive way to diagnosis early-stage NSCLC. To examine the potential diagnostic value of SCC, CFYRA 21-1 and CEA for the differentiation of early-stage NCSCL from benign lung diseases, we analyzed serum levels of tumor markers in 278 patients, including 248 patients with NSCLC and 30 patients with benign lung diseases. These benign lung diseases were presented with evidence of a high likelihood of having lung cancer. After surgical operation, diagnosis of lung cancer and benign lung disease were confirmed by histological examination. Preoperative tumor marker levels were quantified. Mann-Whitney U test was used to compare median levels of SCC, CFYRA 21-1 and CEA between the benign group and lung cancer group. Analysis of variance results were used for differences between different clinical stages of NSCLC. ROC was used to evaluate the diagnostic value of tumor markers. The median levels of Cyfra21-1, SCC and CEA were much higher in NSCLC than those in benign lung diseases. And we found that the mean levels of tumor marker were higher in advanced stage of NSCLC. The combination of tumor markers resulted in a higher sensitivity (91.3%) and a lower specificity (86.7%). In conclusion, the combination of positive SCC, positive CEA and positive Cyfra21-1 appear to be helpful in distinguishing early-stage NSCLC from benign lung disease which presented with suspicious pulmonary masses.
Keywords: Cyfra21-1, SCCA, CEA, NSCLC, lung carcinoma, benign lung disease
Introduction
Lung carcinoma is one of the most leading death prevailing cancers worldwide [1,2]. NSCLC account for 85% in lung cancer, including type of squamous cell carcinoma (SCC), adenocarcinoma (AC), large cell carcinoma (LCC) and others. Most patients, by the time of diagnosis, were within advanced stages and therefore lost the best opportunity to be cured.
Nowadays, low dose of CT is used in patients who are highly suspicious of having lung carcinoma, and possess high sensitive to help to find and identify early-stage lung carcinoma [3-5]. However, the specificity of CT in lung carcinoma diagnosis is poor [6,7]. There is a need to find another way to diagnosis early-stage lung carcinoma, especially NSCLC.
Tumor markers are widely used in lung carcinoma management such as diagnosis, the evaluation of treatment effectiveness, and monitoring the recurrence after therapy. Carcinoembryonic antigen (CEA), cytokeratin 19 fragments (CYFRA 21-1) and squamous cell carcinoma antigen (SCC) are commonly recommended in NSCLC management [8-11]. But, because of their low sensitivity, they have not been generally recommended as a tool for the early screening of lung carcinoma. However, the sensitivity of tumor markers is dependent on the prevalence rates of lung cancer. In patient populations with high prevalence rates of lung cancer, the sensitivity of the tumor marker should demonstrate a high score. Additionally, there is little report about cut-off levels of tumor markers based on patients presented with evidence of a high likelihood of having lung carcinoma.
In our study, we examined the potential diagnostic value of SCC, Cyfra21-1 and CEA for the differentiation of early-stage NCSCL from various benign lung diseases. These diseases showed nodular shadow or lesion and were difficult to distinguish from lung carcinoma based on imaging scan.
Materials and methods
Ethics statement
The study was approved by the Human Research Ethics Committee of the Cancer Institute and Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, and written informed consent was provided by all patients.
Patients
Between January 2012 and June 2013, 278 newly diagnosed and previously untreated early-stage NSCLC patients and 30 benign lung diseases patients were enrolled. These patients were all diagnosed by histological examination after surgical operation in the Department of Thoracic Surgery, Cancer Institute and Hospital, Chinese Academy of Medical Sciences (CAMS). Diagnosis of malignant disease was confirmed pathologically and classified according to World Health Organization (WHO) 2013 classification and UICC guidelines of TNM classification, respectively. Benign diseases were also diagnosed based on each diagnostic criterion. Benign diseases with nodular shadow or lesion which were difficult to distinguish from lung cancer were included. Before surgical operation, all patients were highly suspected with lung cancer based on CT scan.
Detection of tumor markers
All the serum samples (pre-treatment) were detected upon collecting from patients in 2 hours. Serum CYFRA 21-1 and CEA levels were detected with a Cyfra21-1 and CEA test kit (Roche Diagnostics Corp, China) using a cobas e601 analyzer. Serum SCC levels was detected with a SCC test kit (Abbott Diagnostics Corp, China) using a ARCHITECT i2000 analyzer.
Statistical analysis
Study analysis included information regarding tumor marker levels as a continuous and dichotomous variable (we used cut-off levels calculated from ROC curve, < 2.54 ng/mL or ≥ 2.54 ng/mL for CYFRA 21-1, < 2.13 ng/mL or ≥ 2.13 ng/mL for CEA, < 0.95 ng/mL or ≥ 0.95 ng/mL for SCC). As the tumor marker levels were not normally distributed, the results of the tumor marker level were reported as median. The association between pairs of variables was assessed with spearman correlation coefficients. Mann-Whitney U test was used to compare median levels of SCC, CFYRA 21-1 and CEA between the benign group and NSCLC group. Analysis of variance results were used for differences between different clinical stages of NSCLC. P values < 0.05 were considered statistically significant. We used ROC curve to calculate cut-off levels to evaluate the diagnostic value of tumor markers. Statistical analysis was carried out using SPSS (Statistical Package for the Social Sciences) 21.0 software.
Results
Patient characteristics
The characteristics of 278 NSCLC patients and 30 patients with benign disease were listed in Table 1. Median age in NSCLC patients was 63 (range: 42-82) years old. Median age in benign lung disease patients was 50 (range: 32-64) years old. There are 204 patients (73.4%) having smoking history and 74 patients (26.6%) never smoking in NSCLC group. And there are 19 patients (63.3%) having smoking history and 11 patients (36.7%) never smoking in benign lung disease group. Of NSCLC patients, 96 patients (34.5%) were stage I, 156 patients (56.1%) were stage II, 26 patients (9.4%) were stage III. Because only the operable patients were enrolled, there were no stage IV patients in this study. Two hundred and six patients (74.1%) had adenocarcinoma, 66 patients (23.7%) had squamous cell carcinoma, 6 patients (2.2%) had ASC (adenosquamous carcinoma of the lung). Benign lung diseases included benign arcoidosis (n = 12), Pulmonary tuberculosis (n = 4), organizing pneumonia (n = 6), lymphadenitis (n = 6) and Hamartoma (n = 2).
Table 1.
Characteristics of subjects
| NSCLC (n = 278) | Benign lung disease (n = 30) | |
|---|---|---|
| Gender | ||
| Male | 152 (54.7%) | 8 (26.7%) |
| Female | 126 (45.3%) | 22 (73.3%) |
| Age, Years | ||
| Median | 63 | 50 |
| Range | 42-82 | 32-64 |
| Smoking history | ||
| Yes | 204 (73.4%) | 19 (63.3%) |
| Never | 74 (26.6%) | 11 (36.7%) |
| Stage | ||
| I | 96 (34.5%) | |
| II | 156 (56.1%) | |
| III | 26 (9.4%) | |
| Histology | ||
| Adenocarcinoma | 206 (74.1%) | |
| Squamous cell carcinoma | 66 (23.7%) | |
| Adenosquamous carcinoma | 6 (2.2%) | |
| Characteristics of benign lung disease | ||
| Benign sarcoidosis | 12 (40%) | |
| Pulmonary tuberculosis | 4 (13.3%) | |
| Organizing pneumonia | 6 (20%) | |
| Lymphadenitis | 6 (20%) | |
| Hamartoma | 2 (6.7%) |
Median tumor marker levels were higher in patients with NSCLC compared with those with benign lung disease
The median levels of Cyfra21-1, SCC and CEA in benign and lung cancer patients were shown in Table 2 (Median, range of CEA in NSCLC: 2.54, 0.20-67.55 ng/mL, Median, range of CEA 21-1 in benign lung diseases: 1.13, 0.48-2.97 ng/mL, Median, range of Cyfra21-1 in NSCLC: 3.01, 0.73-68.99 ng/mL, Median, range of Cyfra21-1 in benign lung disease: 1.96, 0.96-2.52 ng/ml, Median, range of SCC in NSCLC: 0.90, 0.2-12.90 ng/mL, Median, range of SCC in benign lung disease: 0.70, 0.40-1.00 ng/mL). Cyfra21-1, SCC and CEA levels in NSCLC patients were clearly higher than those in patients with benign lung disease (Mann-Whitney U test, P < 0.01).
Table 2.
Median tumor marker levels in patients
| Benign lung disease | NSCLC | Statistic test | |
|---|---|---|---|
| CEA ( ng/ml, Median, range) | 1.13, 0.48-2.97 | 2.54, 0.20-67.55 | P = 0.000 |
| Cyfra21-1 (ng/ml, Median, range) | 1.96, 0.96-2.52 | 3.01, 0.73-68.99 | P = 0.000 |
| SCC (ng/ml, Median, range) | 0.70, 0.40-1.00 | 0.90, 0.2-12.90 | P = 0.000 |
Mann-Whitney U test was used to compare median level of Cyfra21-1, SCC and CEA between the benign group and NSCLC group. P values < 0.05 were considered statistically significant.
Mean tumor marker levels were higher in advanced stage of NSCLC
The average levels of Cyfra21-1, SCC and CEA in different histology of NSCLC were shown in Table 3 (average score ± SD of CEA in stage I: 2.47 ± 1.85 ng/mL, average score ± SD of CEA in stage II: 4.78 ± 9.04 ng/mL, average score ± SD of CEA in stage III: 11.05 ± 14.06 ng/mL; average score ± SD of Cyfra21-1 in stage I: 2.73 ± 1.34 ng/mL, average score ± SD of Cyfra21-1 in stage II: 3.67 ± 2.06 ng/mL, average score ± SD of Cyfra21-1 in stage III C: 14.35 ± 17.44 ng/mL; average score ± SD of SCC in stage I: 0.95 ± 0.40 ng/mL, average score ± SD of SCC in stage II: 1.21 ± 1.04 ng/mL, average score ± SD of SCC in stage III: 2.69 ± 3.56 ng/mL).
Table 3.
Mean tumor marker levels were higher in advanced stage of NSCLC
| Stage | ANOVA | |||
|---|---|---|---|---|
|
|
||||
| I | II | III | ||
| CEA (ng/ml, Mean ± SD) | 2.47 ± 1.85 | 4.78 ± 9.04 | 11.05 ± 14.06 | P = 0.004 |
| Cyfra21-1 (ng/ml, Mean ± SD) | 2.73 ± 1.34 | 3.67 ± 2.06 | 14.35 ± 17.44 | P = 0.000 |
| SCC (ng/ml, Mean ± SD) | 0.95 ± 0.40 | 1.21 ± 1.04 | 2.69 ± 3.56 | P = 0.000 |
Analysis of variance results for differences between groups; P values < 0.05 were considered statistically significant.
Diagnostic value of tumor markers for differentiation of early-stage NSCLC from benign lung disease
Figure 1 showed the receiver operating curve (ROC) curves for tumor markers. Based on the ROC curve, cut-off values have been set for all the individual tumor markers (Table 4). Table 5 showed the sensitivities, specificities of Cyfra21-1, SCC and CEA in the diagnosis of lung cancer. Sensitivity and specificity of Cyfra21-1 for detecting malignant nodules in patients with NSCLC was 61.9% and 93.3% using cut-off level (2.54 ng/ml), respectively. Sensitivity and specificity of SCC for detecting malignant nodules in patients with NSCLC was 46.7% and 93.3% using cut-off level (0.95 ng/ml), respectively. The sensitivity and specificity of CEA for detecting malignant nodules in patients with NSCLC was 63.3% and 93.3% using cut-off level (2.13 ng/ml), respectively. Then, we analyzed the sensitivity and specificity with the combination of positive Cyfra21-1, positive SCC and positive CEA at the cut-off level, this resulted in a lower specificity (86.7%) but in a higher sensitivity (91.3%) compared with Cyfra21-1, SCC or CEA alone.
Figure 1.
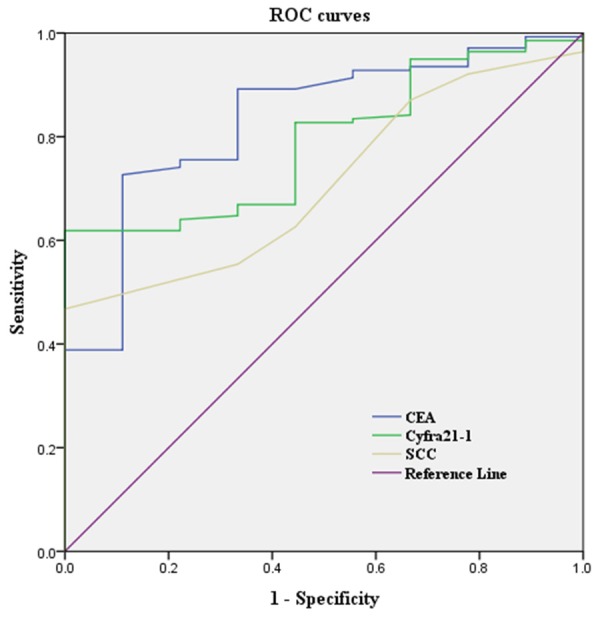
Area under the receiver operating characteristic curve values used for predicting NSCLC.
Table 4.
Results of the ROC analysis
| AUC | Cut-off (ng/ml) | P-value | |
|---|---|---|---|
| CEA | 0.834 | 2.13 | < 0.01 |
| Cyfra21-1 | 0.797 | 2.54 | < 0.01 |
| SCC | 0.711 | 0.95 | < 0.05 |
Table 5.
Combination of positive CYFRA 21-1, positive SCC and positive CEA
| Tumor marker (ng/mL) | Sensitivity (%) | Specificity (%) |
|---|---|---|
| Cyfra21-1 ≥ 2.54 | 61.9 | 93.3 |
| CEA ≥ 2.13 | 63.3 | 93.3 |
| SCCA ≥ 0.95 | 46.7 | 93.3 |
| Cyfra21-1 ≥ 2.54, CEA ≥ 2.13 or SCC ≥ 0.95a | 91.3 | 86.7 |
In the Cyfra21-1≥ 2.54, CEA ≥ 2.13 or SCC ≥ 0.95 group, positive patients were considered to be those having at least one marker above the cut-off level, while negative patients were considered to be those having all markers below the cut-off level.
Discussion
We evaluated the diagnosis value of Cyfra21-1, CEA and SCC in differentiation of early-stage NSCLC from benign lung disease. We found that tumor marker levels were higher in NSCLC patients than those patients with benign lung disease (Table 2). To evaluate the diagnostic value of tumor markers, we used ROC curves to calculate cut-off levels and AUC. We observed a moderate specificity (86.7%) and high sensitivity (91.3%) when combined cut-off levels of tumor markers were used.
The prevalence rate for lung carcinoma may affect the diagnostic value. Cancer Institute and Hospital, Chinese Academy of Medical Sciences is a specialized oncology hospital, so the percentage of lung carcinoma patients is high among the lung disease patients, and the prevalence rate of lung carcinoma is higher. Therefore, contrast the former reports [12,13], we observed a higher specificity when set the tumor markers at the cut-off level (Table 5). Although the sensitivity of each tumor markers at the cut-off levels for NSCLC diagnosis were not so high, the combination of positive SCC, positive CEA and Cyfra21-1 was greater than SCC, CEA or Cyfra21-1 alone (Table 5).
Many studies had recommended that tumor markers may be helpful in diagnosing lung carcinoma. In these studies, healthy peoples, patients with obstructive respiratory diseases or inflammatory pulmonary diseases were used as controls [14-17]. However, these benign diseased can be distinguished easily from lung carcinoma. In contrast, our study focused on the differentiation of lung carcinoma from benign lung disease suspected to have lung carcinoma using imaging techniques. Therefore, our study may more useful for the diagnostic differentiation of lung carcinoma, especially NSCLC, from benign lung diseases which is presented with evidence of a high likelihood of having lung carcinoma.
Currently, CT has been used as a detection tool. Although its sensitivity is high, the specificity of CT in lung cancer diagnosis is poor. The sensitivity of dynamic CT scan for identifying a malignant solitary pulmonary nodule (SPN) is 98-100% and the specificity is 54-93% [18]. In our study, tumor markers exhibit lower sensitivity than dynamic CT imaging, but the specificity is higher than that of CT scan. Therefore, imaging scan combined with tumor markers might be helpful in distinguishing NSCLC from benign lung disease.
In conclusion, the combination of positive SCC, positive CEA and positive Cyfra21-1 appear to be helpful in distinguishing early-stage NSCLC from benign lung disease which presented with suspicious pulmonary masses. However, the cutoff level of a specific tumor marker may be variable in different kinds of people due to age, sex, nationality etc. Therefore, cutoff levels obtained from a single study may not fit all patients. Based on this consideration, more studies, together with other detections, are needed in the future to confirm this.
Disclosure of conflict of interest
None.
References
- 1.Addario BJ. Lung cancer is a global epidemic and requires a global effort. Ann Transl Med. 2015;3:26. doi: 10.3978/j.issn.2305-5839.2015.01.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malvezzi M, Bertuccio P, Rosso T, Rota M, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2015: does lung cancer have the highest death rate in EU women. Ann Oncol. 2015;26:779–86. doi: 10.1093/annonc/mdv001. [DOI] [PubMed] [Google Scholar]
- 3.Crucitti P, Gallo IF, Santoro G, Mangiameli G. Lung cancer screening with low dose CT. Experience at Campus Bio-Medico of Rome on 1500 patients. Minerva Chir. 2015 [Epub ahead of print] [PubMed] [Google Scholar]
- 4.de Groot PM, Carter BW, Godoy MC, Munden RF. Lung cancer screening-why do it? Tobacco, the history of screening, and future challenges. Semin Roentgenol. 2015;50:72–81. doi: 10.1053/j.ro.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Schulz C. [Lung cancer screening and management of small pulmonary nodules] . Dtsch Med Wochenschr. 2015;140:317–322. doi: 10.1055/s-0041-100760. [DOI] [PubMed] [Google Scholar]
- 6.Lee H, Um SW. Probability of lung cancer based on the size threshold and volume-doubling time for lung nodules detected in low-dose CT screening. Ann Transl Med. 2015;3:21. doi: 10.3978/j.issn.2305-5839.2015.01.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mortani BJ Jr. Lung Cancer Screening Overdiagnosis: Reports of Overdiagnosis in Screening for Lung Cancer Are Grossly Exaggerated. Acad Radiol. 2015;22:976–982. doi: 10.1016/j.acra.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Sugio K, Sugaya M, Hanagiri T, Yasumoto K. [Tumor marker in primary lung cancer] . J UOEH. 2004;26:473–479. doi: 10.7888/juoeh.26.473. [DOI] [PubMed] [Google Scholar]
- 9.Schneider J. Tumor markers in detection of lung cancer. Adv Clin Chem. 2006;42:1–41. doi: 10.1016/s0065-2423(06)42001-1. [DOI] [PubMed] [Google Scholar]
- 10.Moro D, Villemain D, Vuillez JP, Delord CA, Brambilla C. CEA, CYFRA21-1 and SCC in non-small cell lung cancer. Lung Cancer. 1995;13:169–176. doi: 10.1016/0169-5002(95)00485-8. [DOI] [PubMed] [Google Scholar]
- 11.Lai RS, Hsu HK, Lu JY, Ger LP, Lai NS. CYFRA 21-1 enzyme-linked immunosorbent assay. Evaluation as a tumor marker in non-small cell lung cancer. Chest. 1996;109:995–1000. doi: 10.1378/chest.109.4.995. [DOI] [PubMed] [Google Scholar]
- 12.Huang MS, Jong SB, Lin MS, Chong IW, Tsai MS, Lin HC, Hwang JJ. Cytokeratin fragment 19 (CYFRA 21-1) as a tumor marker in non-small cell lung cancer. Kaohsiung J Med Sci. 1996;12:62–68. [PubMed] [Google Scholar]
- 13.Pastor A, Menendez R, Cremades MJ, Pastor V, Llopis R, Aznar J. Diagnostic value of SCC, CEA and CYFRA 21.1 in lung cancer: a Bayesian analysis. Eur Respir J. 1997;10:603–609. [PubMed] [Google Scholar]
- 14.Szturmowicz M, Sakowicz A, Rudzinski P, Zych J, Wiatr E, Zaleska J, Rowinska-Zakrzewska E. The clinical value of Cyfra 21-1 estimation for lung cancer patients. Int J Biol Markers. 1996;11:172–177. doi: 10.1177/172460089601100306. [DOI] [PubMed] [Google Scholar]
- 15.Kanazawa H, Yoshikawa T, Yamada M, Shoji S, Fujii T, Kudoh S, Hirata K, Yoshikawa J. CYFRA 21-1, a cytokeratin subunit 19 fragment, in bronchoalveolar lavage fluid from patients with interstitial lung disease. Clin Sci (Lond) 1998;94:531–535. doi: 10.1042/cs0940531. [DOI] [PubMed] [Google Scholar]
- 16.Upham J, Campbell B. Utility of squamous cell carcinoma antigen (SCC Ag) as a tumour marker in pulmonary malignancy. Respir Med. 1992;86:201–203. doi: 10.1016/s0954-6111(06)80055-7. [DOI] [PubMed] [Google Scholar]
- 17.Grunnet M, Sorensen JB. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer. 2012;76:138–143. doi: 10.1016/j.lungcan.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 18.Gould MK, Fletcher J, Iannettoni MD, Lynch WR, Midthun DE, Naidich DP, Ost DE. Evaluation of patients with pulmonary nodules: when is it lung cancer? ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132:108S–130S. doi: 10.1378/chest.07-1353. [DOI] [PubMed] [Google Scholar]


