Abstract
Juvenile idiopathic arthritis (JIA) is a chronic inflammatory arthritis characterized by periods of remission and relapse. Mean platelet volume (MPV) is an indicator of systemic inflammation. In the present study, we aimed to determine the association between mean platelet volume (MPV), neutrophil/lymphocyte ratio (NLR), platelet distribution width (PDW) and clinical measures of diseases activity in children with JIA. The study included 115 patients with JIA (64 with active disease and 51 with inactive disease) and 64 age-gender matched healthy control subjects. Routine laboratory methods were used to measure white blood cell count (WBC), platelet count (PLT), neutrophil count, lymphocyte count, hemoglobin (Hb), MPV, PDW, NLR, C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR) in all subjects of both the patient and control groups. Active disease was associated with significantly increased MPV (8.23 ± 1.16 fl) compared with inactive disease (7.00 ± 1. 08 fl) and control subjects (6.77 ± 1.08 fl) P<0.001, P<0.001, P=NS, respectively). NLR was significantly higher in patients with active (2.11 ± 1.19) and inactive (2.03 ± 1.51) disease relative to the control subjects (1.33 ± 0.66) (P<0.001, P=0.017, respectively). Mean PDW was significantly higher in patients with active disease (17.84 ± 1.06) compared with the control group (17.19 ± 0.93) (P=0.01). Our results suggest that MPV may be a useful marker of disease activity in patients with JIA. Regular treatment may decrease platelet activation in JIA patients. However, NLR was not a predictive marker of disease activity in patients with JIA.
Keywords: Juvenile idiopathic arthritis, children, mean platelet volume, neutrophil to lymphocyte ratio, disease activity
Introduction
Juvenile idiopathic arthritis (JIA) is a chronic inflammatory autoimmune rheumatic disease that occurs in children and adolescents. A number of other diseases must be excluded in the diagnosis of JIA [1]. The etiology of JIA is unclear, however both genetic and autoimmune factors contribute to disease pathogenesis. The annual incidence and the prevalence of JIA among individuals less than 16 years of age are 3.2/100000 and 19.8/100000, respectively [2].
JIA is a group of diseases that can induce severe articular damage occurring in the form of attacks. Repeated attacks may cause severe morbidity. The primary goal of treatment is to prevent attacks that cause permanent articular damage, foster normal growth, and provide comfort for the patient [3,4]. Among JIA patients, high erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels are used as criteria for determining clinical disease activity [5,6]. There are several important disadvantages to the use of CRP and ESR as a marker of JIA, including changes in serum concentrations associated with age and gender and the existence of co-morbid infection [5,7].
MPV is easily determined at low cost using complete blood count devices. Elevated MPV and increased neutrophil/lymphocyte ratio (NLR) are associated with inflammation; these parameters decreased during disease remission [5,6,8,9]. No previous study has examined MPV and NLR in relation to disease status in patients with JIA. The present study aimed to investigate these markers in the relapse and remission phases of JIA relative to healthy control subjects.
Materials and methods
We retrospectively reviewed the digital records of JIA patients (115 JIA patients, 58 male, 57 female, 64 with active disease and 51 with inactive disease) who were admitted to the Pediatric Rheumatology Department of Dicle University Medical School between 2004 and July 2014.
The analyzed patient records included complete clinical data such as symptoms, past medical history, laboratory data including complete blood count (CBC), MPV, PDW, neutrophil count, lymphocyte count, platelet count (PLT), CRP and ESR.
The control group was composed of 64 healthy children who presented to the hospital for routine checkup or preoperative investigation for elective surgery such as hernia repair or circumcision. JIA was diagnosed according to the classification of International League Associations for Rheumatology (ILAR) [10]. JIA patients were divided into two groups according to Wallace criteria [11] as the active patient group (APG) and inactive patient group (IPG). The criteria for inactive disease included: absence of active arthritis, absence of fever, rash, serositis, splenomegaly, or generalized lymphadenopathy attributable to JIA, absence of active uveitis; normal ESR or CRP levels, and a physician’s global assessment of disease activity indicating clinical disease quiescence [11].
White blood cell (WBC) count, platelet (PLT), neutrophil, and lymphocyte counts, levels of hemoglobin (Hb), MPV, PDW, CRP, and ESR and demographic characteristics were retrieved from the records of the JIA patients. Absolute neutrophil-to-absolute lymphocyte ratio (NLR) was calculated as the ratio of neutrophils to lymphocytes. All above hematological variables were measured and recorded in the healthy control subjects. Blood samples were obtained using a vacutainer and collected in tubes containing standard EDTA. All blood samples were evaluated using the same regularly calibrated analyzer (Abbott CELL-DYN 3700, United States). The study protocol was reviewed and approved by the Local Ethics Committee.
Statistical analysis
The data were analyzed using SPSS (Statistical Package for Social Sciences) 18.0 software. The normality of data distribution was determined using the Kolmogorov Smirnov or Shapiro-Wilk tests. Numeric values compatible with the normal distribution were compared using T test or One-way ANOVA test. Data corresponding to an abnormal distribution were compared using the non-parametric Mann-Whitney U test or Kruskal-Wallis test. The numerical data were expressed as mean plus/minus standard deviation. Categorical values were compared using the Chi-square test. Pearson’s or Spearman’s correlation analyses were used for the relationships between numerical variables. A P value less than 0.05 was considered statistically significant.
Results
The study included 115 children (58 boys, 57 girls) with JIA and 64 healthy children (33 boys, 31 girls). The mean age of the JIA group was 11.32 ± 3.57 years and mean age of the control group was 10.76 ± 3.34 years. There were no significant differences in mean age or gender distribution between the study group and the control group (P=0.64 and P=0.94, respectively). The mean follow-up duration was 2.82 ± 1.72 years. According to ILAR classification, 60 (52.2%) children had oligo-articular JIA, 44 (38.2%) had poly-articular JIA, 7 (6.1%) had enthesitis-related JIA and 4 (3.5%) had systemic JIA. HLAB27 was detected in 10 (8.7%) patients, RF was detected in 5 (4.3%) patients and ANA was detected in 5 (4.3%) patients.
The active disease group exhibited significantly higher MPV, PLT, ESR, and CRP relative to the remission group and the control group (Table 1). There were no statistically significant differences in NLR, WBC and PDW values between JIA patients with active disease and those with disease remission (P>0.05) (Table 1). Patients with active disease had significantly lower hemoglobin levels compared with inactive disease. Remission phase JIA patients had significantly elevated platelet count, ESR, CRP, WBC, and NLR relative to the control group (Table 1).
Table 1.
Comparison of patient characteristics active and inactive and the control group
| Active group (n=64) | Inactive group (n=51) | Control group (n=64) | P | |
|---|---|---|---|---|
| Age, year | 11.32 ± 3.57 | 11.15 ± 3.45 | 10.76 ± 3.34 | NS*, NS**, NS*** |
| Sex (Male/Female) | 33/31 | 25/26 | 33/31 | NS*, NS**, NS*** |
| MPV, fL | 8.23 ± 1.16 | 7.00 ± 1.08 | 6.77 ± 1.08 | <0.001*, <0.001**, NS*** |
| PDW, % | 17.84 ± 1.06 | 17.31 ± 1.09 | 17.19 ± 0.93 | NS*, 0.018**, NS*** |
| PLT count x 103/mm3 | 340.1 ± 91.7 | 282.7± 74.2 | 263.9 ± 45.7 | 0.002*, <0.001**, 0.030*** |
| ESR, mm/h | 25.5 ± 13.5 | 14.2 ± 12.8 | 5.6 ± 4.8 | <0.001*, <0.001**, 0.030*** |
| CRP, mg/dl | 10.15 ± 9.03 | 2.54 ± 2.53 | 0.30 ± 0.25 | <0.001*, <0.001**, <0.001*** |
| WBC, /mm3 | 9.43 ± 3.20 | 10.05 ± 2.79 | 8.26 ± 1.93 | NS*, 0.021**, <0.001*** |
| NLR | 2.11 ± 1.19 | 2.03 ± 1.51 | 1.33 ± 0.66 | NS*, <0.001**, 0.017*** |
| Hemoglobin, g/dl | 11.45 ± 1.42 | 12.89 ± 1.25 | 12.91 ± 1.13 | <0.001*, <0.001**, NS*** |
MPV-mean platelet volume; PDW-platelet distribution width; PLT-Platelet count; ESR-erythrocyte sedimentation rate; CRP-C-reactive protein; WBC-white blood cell; NLR-neutrophil-lymphocyte ratio.
Active disease vs. inactive disease;
Active disease vs. control group;
Inactive disease vs. control group, NS-Not significant.
There was a positive correlation between MPV value and CRP (r=0.523, P<0.001) and a positive correlation between MPV and ESR values (r=0.298, P=0.001) (Figure 1).
Figure 1.
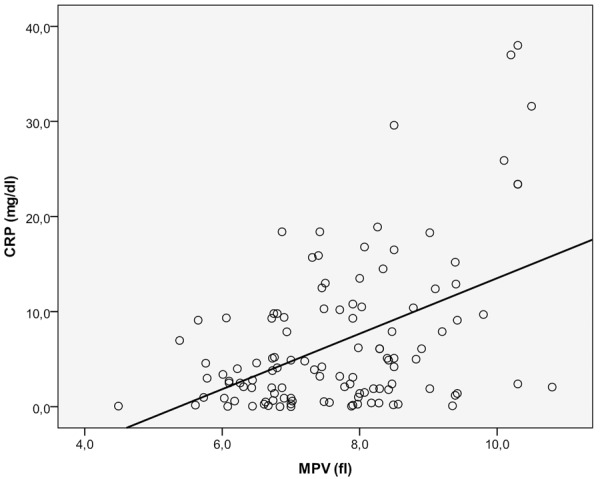
The relationship between MPV and the serum level of CRP. CRP: C reactive protein; MPV: Mean platelet volume.
Discussion
Juvenile idiopathic arthritis (JIA) is a chronic inflammatory arthritis characterized by periods of remission and relapses. Accurate identification of active stage disease is critical for proper management of JIA and the prevention of articular deformities and other morbidities. CRP and ESR are commonly used biomarkers for defining active disease status in JIA and other inflammatory diseases. However, previous studies on inflammatory diseases have suggested that ESR and CRP levels do not reflect clinical disease activity in all cases [12,13]. Moreover, external factors such as patient age and gender, and the existence of co-morbid infection can influence ESR results [14,15]. C-reactive protein suffers from similar limitations as a clinical biomarker [14,15]. JIA is a chronic inflammatory disease resulting in severe effects on the hematopoietic system. Changes in hemoglobin level and platelet count are well established in JIA. Anemia and thrombocytosis are especially distinct during the active phase of inflammatory diseases. In the present study, we found that thrombocyte count was elevated during the active disease phase of JIA compared with the remission phase or healthy control subjects. We also observed significantly lower hemoglobin levels during active disease relative to inactive disease or in healthy subjects. Inflammation is a strong thrombocyte activator [5]. Cytokines such as IL-6, IL-1, TNF-α may play important roles in the pathogenesis of JIA [16,17]. These cytokines promote inflammation and contribute to elevated thrombocyte counts [5].
Mean platelet volume reflects thrombocyte activation and function [18,19]. MPV is a cheap and easily performed test that is often neglected by physicians. MPV may be performed as part of routine blood counts in many laboratories at no additional cost. In the present study, we identified a significant association between MPV and disease activity in JIA. Similarly, MPV is associated with disease activity in patients with rheumatoid arthritis, ulcerative colitis, psoriatic arthritis, ankylosing spondylitis and systemic lupus erythematosus [20-23]. Increased thrombocyte count and elevated enzymatic activity is associated with systemic inflammation in rheumatic disease. MPV reflects global thrombocyte content and enzymatic activity [24]. Moreover Yazici et al., reported a decrease in MPV in ankylosing spondylitis patients after TNF-α antagonist treatment. TNF-α is an inflammatory cytokine that contributes to the pathogenesis of inflammation and thrombocytosis [22].
NLR is calculated by dividing the neutrophil count by lymphocyte number, and can be determined from routine blood differentials at no additional cost. Changes in the relative abundance of leukocyte subgroups occur in parallel with the increase in overall leukocyte count. Lymphocyte count decreases when neutrophil counts are elevated. NLR increases in inflammatory conditions and this increase is considered as an indicator of systemic inflammation [25]. However, there is no association between NLR and disease activity in adult patients with arthritis [25]. In present study, NLR was found to be elevated in both active and inactive JIA patients relative to healthy control subjects. However, there was no difference in NLR between JIA patients with active disease and patients in remission. According to these results, while NLR may be useful for the diagnosis of JIA, it may not be useful in determining disease activity and prognosis.
Increased CRP and ESR have been reported in active inflammation and are often considered useful criteria for demonstrating disease activation in JIA and other inflammatory conditions [11]. In the present study, we observed a positive correlation between MPV, CRP and ESR. This suggests that MPV may have clinical diagnostic utility comparable to CRP and ESR. The retrospective approach used in the present study may be considered the primary limitation.
In conclusion, increased MPV may be useful for the determining disease activation in JIA patients. In addition, NLR may be useful in the initial diagnosis of JIA, although there is no evidence to suggest that NLR is a reliable marker of clinical disease activity. Additional studies are necessary to validate the role of MPV in the diagnosis and management of JIA.
Disclosure of conflict of interest
None.
References
- 1.Olson JC. Juvenile idiopathic arthritis: an update. WMJ. 2003;102:45–50. [PubMed] [Google Scholar]
- 2.Danner S, Sordet C, Terzic J, Donato L, Velten M, Fischbach M, Sibilia J. Epidemiology of juvenile idiopathic arthritis in Alsace, France. J Rheumatol. 2006;33:1377–1381. [PubMed] [Google Scholar]
- 3.Huang JL. New advances in juvenile idiopathic arthritis. Chang Gung Med J. 2012;35:1–14. doi: 10.4103/2319-4170.106171. [DOI] [PubMed] [Google Scholar]
- 4.Kim KH, Kim DS. Juvenile idiopathic arthritis: Diagnosis and differential diagnosis. Korean J Pediatr. 2010;53:931–935. doi: 10.3345/kjp.2010.53.11.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mannaioni PF, Di Bello MG, Masini E. Platelets and inflammation: role of platelet-derived growth factor, adhesion molecules and histamine. Inflamm Res. 1997;46:4–18. doi: 10.1007/PL00000158. [DOI] [PubMed] [Google Scholar]
- 6.Uluca U, Ece A, Sen V, Karabel D, Yel S, Gunes A, Tan I, Sabas M. Usefulness of mean platelet volume and neutrophil-to-lymphocyte ratio for evaluation of children with Familial Mediterranean fever. Med Sci Monit. 2014;20:1578–1582. doi: 10.12659/MSM.892139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, Keystone EC, Genovese MC, Wasko MC, Moreland LW, Weaver AL, Markenson J, Finck BK. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med. 2000;343:1586–1593. doi: 10.1056/NEJM200011303432201. [DOI] [PubMed] [Google Scholar]
- 8.Kisacik B, Tufan A, Kalyoncu U, Karadag O, Akdogan A, Ozturk MA, Kiraz S, Ertenli I, Calguneri M. Mean platelet volume (MPV) as an inflammatory marker in ankylosing spondylitis and rheumatoid arthritis. Joint Bone Spine. 2008;75:291–294. doi: 10.1016/j.jbspin.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 9.Pasceri V, Yeh ET. A tale of two diseases: atherosclerosis and rheumatoid arthritis. Circulation. 1999;100:2124–2126. doi: 10.1161/01.cir.100.21.2124. [DOI] [PubMed] [Google Scholar]
- 10.Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, He X, Maldonado-Cocco J, Orozco-Alcala J, Prieur AM, Suarez-Almazor ME, Woo P International League of Associations for Rheumatology. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390–392. [PubMed] [Google Scholar]
- 11.Wallace CA, Ravelli A, Huang B, Giannini EH. Preliminary validation of clinical remission criteria using the OMERACT filter for select categories of juvenile idiopathic arthritis. J Rheumatol. 2006;33:789–795. [PubMed] [Google Scholar]
- 12.Aletaha D, Nell VP, Stamm T, Uffmann M, Pflugbeil S, Machold K, Smolen JS. Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: validation of a clinical activity score. Arthritis Res Ther. 2005;7:R796–806. doi: 10.1186/ar1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolfe F. Comparative usefulness of C-reactive protein and erythrocyte sedimentation rate in patients with rheumatoid arthritis. J Rheumatol. 1997;24:1477–1485. [PubMed] [Google Scholar]
- 14.Colglazier CL, Sutej PG. Laboratory testing in the rheumatic diseases: a practical review. South Med J. 2005;98:185–191. doi: 10.1097/01.SMJ.0000153572.22346.E9. [DOI] [PubMed] [Google Scholar]
- 15.Kavanaugh A. The role of the laboratory in the evaluation of rheumatic diseases. Clin Cornerstone. 1999;2:11–25. doi: 10.1016/s1098-3597(99)90011-x. [DOI] [PubMed] [Google Scholar]
- 16.Prakken B, Albani S, Martini A. Juvenile idiopathic arthritis. Lancet. 2011;377:2138–2149. doi: 10.1016/S0140-6736(11)60244-4. [DOI] [PubMed] [Google Scholar]
- 17.Yilmaz M, Kendirli SG, Altintas D, Bingol G, Antmen B. Cytokine levels in serum of patients with juvenile rheumatoid arthritis. Clin Rheumatol. 2001;20:30–35. doi: 10.1007/s100670170100. [DOI] [PubMed] [Google Scholar]
- 18.Gasparyan AY, Ayvazyan L, Mikhailidis DP, Kitas GD. Mean platelet volume: a link between thrombosis and inflammation? Curr Pharm Des. 2011;17:47–58. doi: 10.2174/138161211795049804. [DOI] [PubMed] [Google Scholar]
- 19.Leader A, Pereg D, Lishner M. Are platelet volume indices of clinical use? A multidisciplinary review. Ann Med. 2012;44:805–816. doi: 10.3109/07853890.2011.653391. [DOI] [PubMed] [Google Scholar]
- 20.Milovanovic A, Nilsson E, Jaremo P. Relationships between platelets and inflammatory markers in rheumatoid arthritis. Clinica Chimica Acta. 2004;343:237–240. doi: 10.1016/j.cccn.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 21.Canpolat F, Akpinar H, Eskioglu F. Mean platelet volume in psoriasis and psoriatic arthritis. Clinical Rheumatology. 2010;29:325–328. doi: 10.1007/s10067-009-1323-8. [DOI] [PubMed] [Google Scholar]
- 22.Yazici S, Yazici M, Erer B, Erer B, Calik Y, Bulur S, Ozhan H, Ataoglu S. The platelet functions in patients with ankylosing spondylitis: anti-TNF-alpha therapy decreases the mean platelet volume and platelet mass. Platelets. 2010;21:126–131. doi: 10.3109/09537100903470306. [DOI] [PubMed] [Google Scholar]
- 23.Yavuz S, Ece A. Mean platelet volume as an indicator of disease activity in juvenile SLE. Clinical Rheumatology. 2014;33:637–641. doi: 10.1007/s10067-014-2540-3. [DOI] [PubMed] [Google Scholar]
- 24.van der Loo B, Martin JF. A role for changes in platelet production in the cause of acute coronary syndromes. Arterioscler Thromb Vasc Biol. 1999;19:672–679. doi: 10.1161/01.atv.19.3.672. [DOI] [PubMed] [Google Scholar]
- 25.Imtiaz F, Shafique K, Mirza SS, Ayoob Z, Vart P, Rao S. Neutrophil lymphocyte ratio as a measure of systemic inflammation in prevalent chronic diseases in Asian population. Int Arch Med. 2012;5:2. doi: 10.1186/1755-7682-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


