Abstract
Spontaneous esophageal perforation, also known as Boerhaave’s syndrome, is a rare but potentially life-threatening condition, especially in elderly patients with more complications, speedy development and higher mortality. Successful handling of the disease depends on a timely diagnosis and the appropriate choice of treatment. Unfortunately, late diagnosis is common because of the non-specific clinical presentation. We here present a 72-year-old patient of spontaneous esophageal perforation who complained of chest pain, but sharply deteriorated with septic shock. With a vomiting history and gastrointestinal-genic bacterium identified in the chest fluid, the patient was highly suspected for esophageal perforation, though the oral methylene blue test was negative for three times. The diagnosis was finally established by esophagoscopy on the 10th day. The perforation was successfully healed by active conservative management and the patient was discharged home on the 43rd day eating normal diet.
Keywords: Spontaneous esophageal perforation, Boerhaave’s syndrome, chest pain, septic shock, conservative management
Introduction
Spontaneous esophageal perforation also known as Boerhaave’s syndrome, which means transmural rupture of the esophagus in healthy people, is a rare but potentially life-threatening condition. It is supposed to be the combined result of a sudden rise in intraluminal esophageal pressure produced during vomiting and the negative intrathoracic pressure. The diagnosis of Boerhaave’s syndrome is often missed or delayed especially in elderly patients, because a wide range of symptoms are atypically presented on admission, easily leading to misdiagnosis of other more common diseases, e.g. acute myocardial infarction, angina pectoris, acute pancreatitis or peptic ulcer perforation. Once an appropriate treatment is absent, a series of complications will take place, such as pneumothorax, empyema, acute respiratory distress syndrome and septic shock. One research showed half of the patients with spontaneous esophageal perforation who died were aged over 80 years [1].
Case report
A 72-year-old man attended the emergency department of our hospital with a chest pain. 15 hours before admission, he felt a sudden onset left side chest pain, radiating to the back, followed by shortness of breath and fever, body temperature up to 38.8°C. On physical examination his blood pressure was 101/79 mmHg, pulse rate was 106 bpm, body temperature was 37.6°C and oxygen saturation was 87%. The breath sounds were equal bilaterally in the upper lung fields, but diminished in the left lower lobe. There were no cardiovascular or abdominal signs.
The results of laboratory studies showed a white blood cell (WBC) count of 18.7×109/L, neutrophils % of 63.5% and C-reactive protein (CRP) <8 mg/L. Arterial blood gases showed respiratory failure of type I. Electrocardiogram showed blocked premature atrial beats. Thoracic computed tomography (CT) revealed bilateral inflammation and pleural effusion, particularly in the left side (Figure 1).
Figure 1.
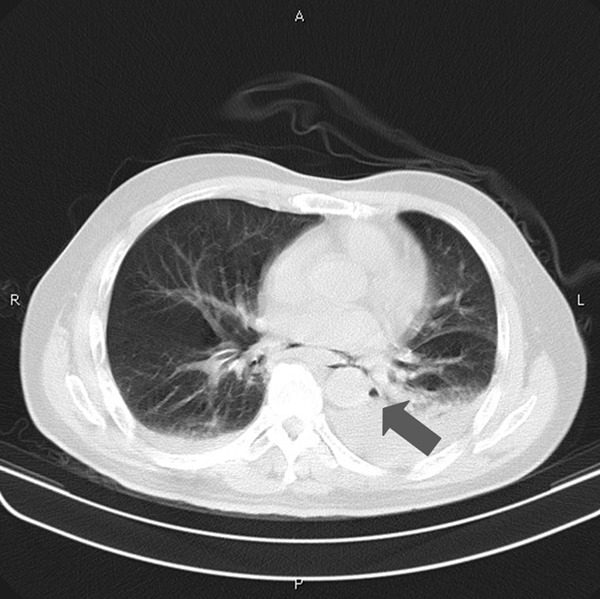
Chest CT scan on admission indicated copious left pleural effusion. Arrow shows subtle pneumomediastinum.
After admission, non-invasive ventilation, antibiotics were supported. But the patient’s condition deteriorated sharply five hours later with hypotension and tachycardia. The oxygen saturation dropped to 80%. Septic shock was highly suspected. Besides promptly anti-shock treatment and intubated mechanical ventilation, pleural puncture was done, drawing out 120 ml dark brown pus. Considered of thoracic empyema, the patient received a chest tube insertion and a strong antibiotic therapy. 1.3 L pus was drained from the chest tube for the first 24 hours.
For the next two days, the patient managed to his hemodynamical stability with the inotropic support and ventilation. On the 4th day, the patient was afebrile and weaned off mechanical ventilation. Microbiological investigation of the chest fluid identified Bacillus cereus and Streptococcus intermedius with no evidence of malignant cells or tuberculosis. Subsequent CT revealed reduced but encapsulated pleural effusion (Figure 2). Urokinase was then injected from the chest tube daily to prevent the encapsulation.
Figure 2.
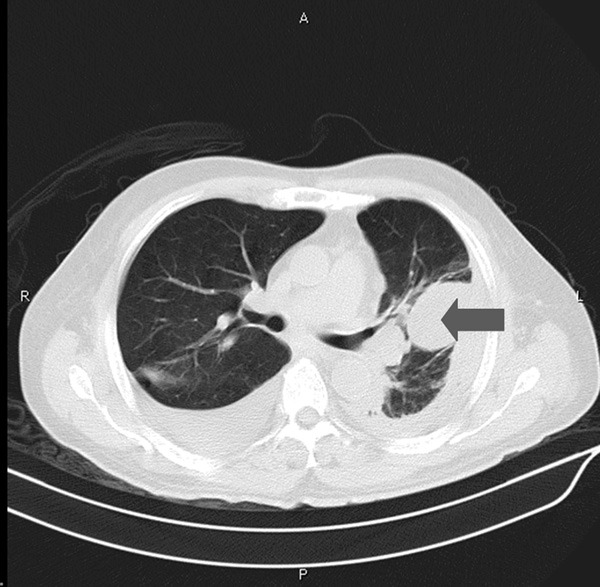
Chest CT taken on the 4th day. No pneumomediastinum was identified. Arrow showed encapsulated pleural effusion.
With a thoroughly history taken on the 4th day, the patient admitted to having forceful vomiting after eating a lot of noodles before admission, and the chest pain begun just at that time. This aroused the suspicion of esophageal perforation. The patient was orally given methylene blue solution for three times from the 5th day, but it did not appear in the chest drainage. It was not until the food residue (cabbage leaves) was noticed in the chest drainage on the 9th evening did we confirm there was an esophagopleural fistula. The 10th day esophagoscopy revealed a rupture of about 1.5-2 cm in the lower esophagus near the cardia (Figure 3). Spontaneous esophageal perforation was thus diagnosed.
Figure 3.
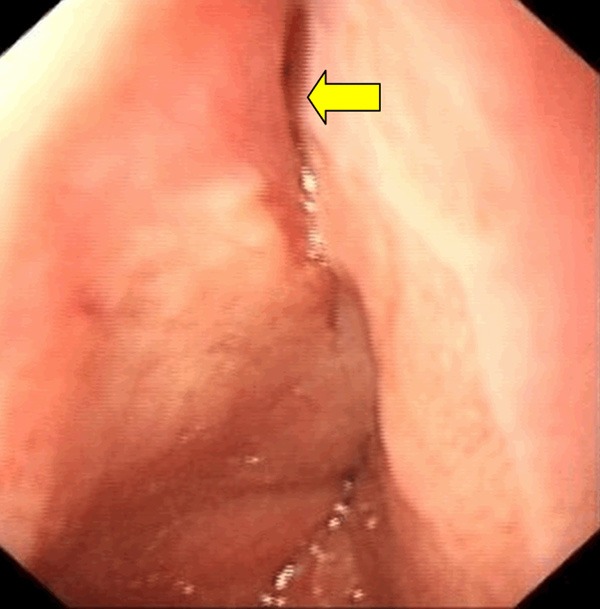
Esophagoscopy image taken on the 10th day. Arrow showed the site of the perforation is near the cardia.
As the patient refused further surgical management and endoscopic stent insertion, we adopt conservative management. By enteral nutrition via indwelling jejunal tube, gastrointestinal decompression, continuous thoracic drainage, pleural lavage, antibiotics and other supportive treatment, the patient’s inflammatory markers returned to normal on the 15th day. The hypaque contrast esophagography demonstrated no esophageal leakage on the 35th day. The patient was then started on oral feeding. He was discharged home on the 43rd day eating normal diet.
Conclusion
Boerhaave’s syndrome represents a diagnostic dilemma in elderly patients. It is reported that the Mackler triad (history of forceful vomiting, subxiphoid chest pain, and subcutaneous emphysema) is encountered in half of the cases. In our case, subcutaneous emphysema didn’t appear, yet the history of vomiting was still very indicative. More than 90% of the cases are caused by forceful vomiting after excessive eating or drinking. Elderly patients usually pay less attention to the vomiting than the chest pain, so history should be taken thoroughly on admission.
Computed tomography is the most accurate diagnostic tool in the detection of Boerhaave’s syndrome. It may show mediastinal and subcutaneous emphysema, pleural effusion and pneumothorax. It is also helpful in localizing the site of perforation and for surgical drainage. When taken early, however, the findings can be normal because it usually takes several hours to make mediastinal emphysema and pleural effusion to become prominent after the perforation. Reviewing the case, first CT showed copious pleural effusion in the left side. Besides, there had been subtle pneumomediastinum imaging (Figure 1), which was neglected by both clinicians and radiologists. Subsequent CT after four days, however, showed no signs of pneumomediastinum presumably due to the insertion of the chest tube (Figure 2).
For the patients who have a chest tube inserted the methylene blue dye test can be done. When methylene blue is taken orally, it gives a bluish discolouration to the chest tube effluent. In our patient, the methylene blue dye test was false negative for three times. Presumably the time was late, the rupture was small and close to the cardia, it closed temporary after a diet, so the liquid dye was unable to enter the pleural cavity. Water-soluble contrast esophagography can define the anatomical site and extent of the rupture. But there is also a false-negative rate of 10% to 36% due to tissue edema and other factors [2].
Food residues appear in the thoracic drainage is the powerful evidence. The pleural fluid with low pH and very high amylase are typical of esophageal perforation. Bacterial culture of the effusion is also an indicative factor. We found Bacillus cereus and Streptococcus intermedius in the effusion. These bacterial mostly appear in gastrointestinal tract and oral cavity and are unusual as the causative microbial agents in the pleural cavity. Arin Saha et al reported a case of a 54-year-old woman with a Boerhaave’s syndrome that presented as Enterococcal bacterial pericardial effusion [3]. Cytological examination of pleural fluid showing the presence of benign squamous epithelial cells, ingested food material and Candida, can provide early, fast and accurate diagnosis of the disease [4]. It is important to note that when an unusual microbe is found in the effusion, careful steps need to be taken to exclude esophageal perforation.
The treatment of Spontaneous esophageal perforation is not consistency. Some scholars believe that primary repair is necessary, no matter how long the time between perforation and treatment [5,6]. Some investigators recommend conservative management, especially in patients whose manifestation is non-acute and non-specific, with a well-contained perforation as proven on imaging [7,8]. In the present reports, the overall survival of those who had non-operative treatment was no different from that of the operative group [1,9,10].
As the patient refused further surgical management and endoscopic stent insertion, we adopted conservative management actively, including antibiotics, percutaneous drainage of empyema, maintain nutrition and endoscopic approach. Percutaneous drainage is an important treatment for empyema. Pleural catheter should be placed as soon as possible to recruit the lung tissue. Intrapleural infusion of fibrinolytic agent can dissolve fibrin, liquefy purulent material, reduce or eliminate the formation of pleural adhesions and increase the drainage. Reviewing our case, the insertion of the thoracostomy tube was the vital step to resuscitate the patient from the deterioration of the septic shock. After the pus was drained, pleural injection of urokinase and antibiotics therapy based on bacterial susceptibility also effectively eliminate the infection.
This case highlights a number of important issues: Spontaneous esophageal perforation is uncommon, but the mortality rate is high, especially in elderly patients with more complications, speedy development and higher mortality. For acute onset, after forceful vomiting, patients with chest pain, upper abdominal pain, dyspnea, subcutaneous emphysema, pneumomediastinum or fever should be suspected for esophageal perforation. Pleural fluid analysis is useful in supporting the clinical diagnosis, while some tests as oral methylene blue, esophageal imaging have the possibility of false-negative results. Conservative management can also be appropriate in elderly patients.
Disclosure of conflict of interest
None.
References
- 1.Wahed S, Dent B, Jones R, Griffin SM. Spectrum of oesophageal perforations and their influence on management. Br J Surg. 2014;101:e156–e162. doi: 10.1002/bjs.9338. [DOI] [PubMed] [Google Scholar]
- 2.Tsalis K, Blouhos K, Kapetanos D, Kontakiotis T, Lazaridis C. Conservative management for an esophageal perforation in a patient presented with delayed diagnosis:a case report review of the literature. Cases J. 2009;2:6784–6786. doi: 10.4076/1757-1626-2-6784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saha A, Jarvis M, Thorpe JA, O’Regan DJ. Atypical presentation of Boerhaave’s syndrome as Enterococcal bacterial pericardial effusion. Interact Cardiovasc Thorac Surg. 2007;6:130–132. doi: 10.1510/icvts.2006.139667. [DOI] [PubMed] [Google Scholar]
- 4.Khalbuss WE, Hooda S, Auger M. Cytomorphology of Boerhaave’s syndrome: A critical value in cytology. Cytojournal. 2013;10:8. doi: 10.4103/1742-6413.111811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho K, Jheon S, Ryu KM, Lee EB. Primary esophageal repair in Boerhaave’s syndrome. Dis Esophagus. 2008;21:660–663. doi: 10.1111/j.1442-2050.2008.00833.x. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Zhang R, Zhou Y, Li X, Cheng Q, Wang Y, Liu K, Wang X. Our experience on management of Boerhaave’s syndrome with late presentation. Dis Esophagus. 2009;22:62–67. doi: 10.1111/j.1442-2050.2008.00858.x. [DOI] [PubMed] [Google Scholar]
- 7.Abbas G, Schuchert MJ, Pettiford BL, Pennathur A, Landreneau J, Landreneau J, Luketich JD, Landreneau RJ. Contemporaneous management of esophageal perforation. Surgery. 2009;146:749–756. doi: 10.1016/j.surg.2009.06.058. [DOI] [PubMed] [Google Scholar]
- 8.De Schipper JP, Pull ter Gunne AF, Oostvogel HJ, van Laarhoven CJ. Spontaneous rupture of the oesophagus: Boerhaave’s syndrome in 2008. Dig Surg. 2009;26:1–6. doi: 10.1159/000191283. [DOI] [PubMed] [Google Scholar]
- 9.Abbas G, Schuchert MJ, Pettiford BL, Pennathur A, Landreneau J, Landreneau J, Luketich JD, Landreneau RJ. Contemporaneous management of esophageal perforation. Surgery. 2009;146:749–755. doi: 10.1016/j.surg.2009.06.058. [DOI] [PubMed] [Google Scholar]
- 10.Keeling WB, Miller DL, Lam GT, Kilgo P, Miller JI, Mansour KA, Force SD. Low mortality after treatment for esophageal perforation: a single-center experience. Ann Thorac Surg. 2010;90:1669–1673. doi: 10.1016/j.athoracsur.2010.06.129. [DOI] [PubMed] [Google Scholar]


