Abstract
We have characterized 11 women diagnosed with aortic dissection during the course pregnancy or puerperium by analysis of 1,271 patients enrolled at the Second Xiangya Hospital in central south university of China from 2010 to 2013. The age of these patients ranged from 22 to 39 years old (30.6 ± 5.6 years old). Among which, 5 cases were noted secondary to Marfan syndrome. Three patients were diagnosed at the late stage of pregnancy, 6 cases were in the postpartum stage, and 2 cases occurred in the early stage of pregnancy. Six patients were Stanford A-type, 4 patients were Stanford B-type, and the rest one was diagnosed as aorta aneurysm. The major symptom for 9 patients was manifested by chest/back pain, 1 patient was characterized by chest pain along with dyspnea, while the other one was featured by dyspnea only. Five out of 6 A-type patients were undergone surgical treatment, and 3 patients were survived, while the rest 1 patient refused surgical treatment and died soon in a local hospital. All 4 B-type patients were survived after surgical therapy, and the patient with aorta aneurysm was also survived after surgical treatment. Together, our data suggest that pregnancy associated aortic dissection is more common in late pregnancy or puerperium, and Marfan’s syndrome is likely the highest risk factor to this special group of patients. A quick and accurate diagnosis along with appropriate surgical treatments would be necessary to save the lives for both mother and baby.
Keywords: Pregnancy, puerperium, aortic dissection, Marfan’s syndrome
Introduction
Acute aortic dissection during the course of pregnancy or puerperium is a rare but urgent and thorny clinical event, which endangers the safety of both pregnant women and fetuses. A population-based study including 34,1381 women (15 to 45 years old) from Vienna of Austria with 10-year follow up identified 15 patients with acute aortic dissection (0.4 case per 100,000 person-years), and prehospital mortality rate was recorded to be 53%, while in-hospital mortality rate was only 6.6% [1]. Unfortunately, there is a significant lack for the information relevant to this particular group of patients in China. We thus in the current report analyzed 1,271 patients with aortic dissection enrolled at the Second Xiangya Hospital from 2010 to 2013. We have identified 11 patients with onset of aortic dissection during pregnancy or puerperium and followed up these patients for 1 year. We now present the clinical manifestations, related risk factors, diagnosis and prognosis for these patients.
Study subjects
General data for 1,271 patients enrolled with aortic dissection at the Second Xiangya Hospital in Central South University of China from 2010 to 2013 were reviewed, and 11 patients were characterized associated with pregnancy or puerperium. All characterized patients were followed up for at least one year, and the studies were approved by the Human Assurance Committee at the Second Xiangya Hospital of Central South University. Informed consent was obtained from all study subjects.
Results
Demographic information and clinical manifestations for the study subjects
The characterized 11 patients with aortic dissection during the course of pregnancy or puerperium were summarized as detailed in Table 1. In general, the youngest patient was only 22 years old, while the oldest one was 39 years old, and the average age for these patients is 30.6 ± 5.6 years. Three cases were diagnosed in the late trimester of pregnancy, and 2 cases were in the early trimester of pregnancy, while 6 cases were noted in the state of puerperium. Three patients showed a history of high blood pressure, and 5 patients were diagnosed with Marfan’s syndrome, while one patient manifested both hypertension and Marfan’s syndrome. The initial symptoms for 10 patients were featured by the chest and/or back pain, and the other case was characterized by dyspnea. Six patients were diagnosed as Stanford type A, and 4 patients were Stanford type B, while one patient was diagnosed as aortic aneurysm according to the guidelines published by the European Society of Cardiology [2]. CT Angiography (CTA) and transthoracic echocardiography (TTE) were employed for diagnostic purpose.
Table 1.
Patient information and characteristics
| Patient | Age | Time of onset | Symptom | Hypertension | Marfans’ syndrome | Subtype (Standford) | Diagnostic approach |
|---|---|---|---|---|---|---|---|
| 1 | 22 | 37 weeks gestation | Chest and back pain | No | No | B | CTA |
| TTE | |||||||
| 2 | 24 | 36 weeks gestation | back pain | No | Yes | B | CTA |
| 3 | 35 | 12 days after labor | Chest pain | No | No | A | CTA |
| 4 | 32 | 7 days after labor | Dyspnea | Yes | Yes | A | CTA |
| TTE | |||||||
| 5 | 39 | 20 days after labor | Chest pain | No | No | A | CTA |
| TTE | |||||||
| 6 | 35 | 33weeks gestation | Back pain | No | Yes | A | CTA |
| TTE | |||||||
| 7 | 26 | 4 days after labor | Chest and back pain | No | No | A | CTA |
| 8 | 29 | 7 days after labor | Chest and back pain | Yes | No | B | CTA |
| 9 | 26 | 10 weeks gestation | Chest and back pain | No | Yes | Aorta aneurysm | CTA |
| TTE | |||||||
| 10 | 33 | 12 weeks gestation | Chest pain | No | Yes | A | CTA |
| 11 | 36 | 9 days after labor | Chest pain and dyspnea | Yes | No | B | CTA |
Approaches for clinical treatment and outcomes
Six type A patients were treated on the basis of Bentall surgery. One of which was in the late trimester of pregnancy, a cesarean section was first carried out for delivery of her baby. Because her chest pain became worse and worse, she was next advised for Bentall surgery 9 days after her cesarean section, and both her baby and herself were survived (patient 6). Similarly, patient 7 manifested onset of type A aortic dissection 4 days after giving birth, she was scheduled for Bentall surgery, and survived well after 1 year of follow up studies. One type A patient was diagnosed in the stage of puerperium, she was treated with Bentall surgery, total arch replacement and descending aorta stent implantation (DASI). Unfortunately, she developed low cardiac output syndrome and died 9 days after surgery (patient 4). Similar as patient 4, patient 5 was diagnosed with type A 20 days after her delivery, and she was treated with artificial vascular graft (AVG) and DASI along with coronary artery by pass surgery (CABS) successfully. Unlike the above described type A patients, patient 10 was diagnosed during 12 weeks of gestation (Figure 1). She was arranged for abortion first, and was then treated with Bentall, CABS, and mitral valve replacement (MVR) along with tricuspid valve plasty (TVP). Because of ventricular fibrillation (VF) along with the development of LCOS, she died soon after the surgery. Patient 3 was diagnosed with onset of type A 12 days after delivery of her baby, but she refused surgical treatment and died in a local hospital. Together, 3 out of 5 type A patients were survived after surgical treatment, while the other 2 patients died of complications (Table 2).
Figure 1.
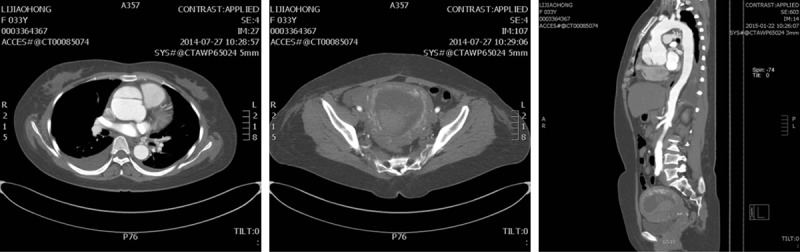
Aortic dissection (Type A) associated with early trimester pregnancy in patient 10.
Table 2.
Therapeutic approaches and outcomes
| Patent | Treatment | Way of delivery | Outcome of gravida | Cause of death | Outcome of fetus | 1 year follow-up |
|---|---|---|---|---|---|---|
| 1 | TEVAR | PV | Alive | Alive | Mother and child alive and well | |
| 2 | TEVAR | PV | Alive | Alive | Mother and child alive and well | |
| 3 | Refuse surgery | CS | Death | Unknown | Alive | Child alive and well |
| 4 | Bentall+AVG+DASI | PV | Death | LCOS | Alive | Child alive and well |
| 5 | AVG+DASI+CABS | CS | Alive | Alive | Mother and child alive and well | |
| 6 | Bentall | PV | Alive | Alive | Mother and child alive and well | |
| 7 | Bentall | PV | Alive | Alive | Mother and child alive and well | |
| 8 | TEVAR | CS | Alive | Alive | Mother and child alive and well | |
| 9 | AVG | Abortion | Alive | Death | Mother alive and well | |
| 10 | Bentall+CABS+MVR+TVP | Abortion | Death | VF+LCOS | Death | |
| 11 | AVG+DASI | PV | Alive | Alive | Mother and child alive and well |
TEVAR = thoracic endovascular aortic repair; AVG = artificial vascular graft; DASI = descending aorta stent implantation; CABS = coronary artery bypass surgery; MVR = mitral valve replacement; TVP = tricuspid valve plasty; VF = ventricular fibrillation; LCOS = low cardiac output syndrome; CS = cesarean section; PV = per vaginam.
Two type B patients, patients 1 and 2 (Figure 2), were diagnosed at 37 weeks and 36 weeks of gestation, respectively. They were first subjected to surgical repair of the aorta by covered stent, followed by cesarean sections for delivery of their babies successfully. In contrast, the other two type B patients, patient 8 and 11, manifested onset of aortic dissection after days 7 and 9 of delivery, respectively. Patient 8 was treated with thoracic endovascular aortic repair (TEVAR), while patient 11 was treated with AVG along with DASI. Collectively, all type B patients were survived from surgical treatment (Table 2). Patient 9 was diagnosed as aorta aneurysm at 10 weeks of gestation. She was advised to terminate pregnancy first, and she was then scheduled for ascending aorta replacement by implanting artificial vascular graft (AVG) successfully (Table 2).
Figure 2.
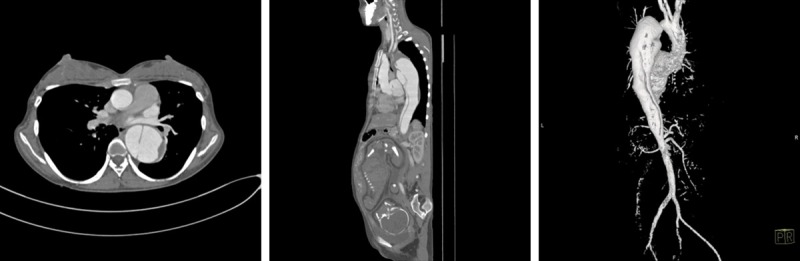
Aortic dissection (Type B) associated with late trimester pregnancy in patient 2.
Discussion
The aorta acts as a second pump during diastole via its elasticity and, therefore, it plays an important role in the control of systemic vascular resistance and heart rate. The histological composition of aortic wall includes three layers, the tunica intima, media and adventitia. Aortic dissection is manifested by the disruption of the medial layer due to intramural bleeding, which results in the separation of the aortic wall layers and subsequent formation of a true lumen and a false lumen [2]. Other than hypertension and Marfan’s syndrome, the most common risk associated with aortic dissection is pregnancy [3]. Although aortic dissection in pregnant women is rare, it could be a deadly event [4]. During pregnancy the heart rate, left ventricular mass, stroke volume, and cardiac output are usually increased, which may cause a hemodynamic stress on the aortic wall [5]. Hormonal changes relevant to pregnancy such as the increase of estrogen and progestogen in late trimester of pregnancy may add additional risk since aorta expresses the estrogen and progestogen receptors [6]. Therefore, high levels of estrogen and progestogen have been suggested relevant to aorta degeneration including hyperplasia of aortic smooth muscle, decrease of acid mucopolysaccharides and disintegrity of elastic fibers [7]. Furthermore, as the termination of puerperal uteroplacenta circulation and uterine contraction along with interstitial fluid absorption, the circulating volume in a postpartum woman could be increased by 15-25% within 72 h [8,9]. Therefore, aortic dissection usually occurs during the third trimester of pregnancy or immediate postpartum stage. Indeed, 3 out of the 11 characterized patients in our report were noted with manifestation of aortic dissection after 33 weeks of gestation (patients 1, 2 and 6), and 6 patients were found with onset of aortic dissection during the postpartum stage. Patients 9 and 10 with early onset of aortic dissection (10 weeks and 12 weeks of gestation, respectively) were actually associated with Marfan’s syndrome.
It is worthy of note that Marfan’s syndrome is likely to be the highest risk for pregnant women with onset of aortic dissection [11]. Five out of 11 patients in our current report actually manifested acute aortic dissection secondary to Marfan’s syndrome. Specifically, 3 patients showed onset of aortic dissection during gestation, and aortic dissection occurred in 1 patient 9 days after giving birth, while 1 patient manifested aorta aneurysm during her 12-week of gestation. The major clinical manifestations for Marfan’s syndrome include a family history of this disease, arachnodactyly, eye disease and cardiovascular disease such as aneurysm. Its diagnosis can be reached based on clinical manifestations and genetic testing. Particularly, mutations in the FBN1 gene have been characterized in patients with Marfan’s syndrome, and altered FBN1 function is associated with aortic elastic fiber fracture and damage [10].
Interestingly, our patients manifested onset of aortic dissection during the stage of pregnancy or puerperium without special symptoms, and the most frequent symptom was chest/back pain which occurred suddenly. The feature of this particular type of pain could be sharp, ripping, tearing or knife-like. Even morphine was administered the pain sometimes, however, could not be relieved. Other than chest (back) pain, 2 of our patients manifested dyspnea. The frequent objective signs are hypertension, weak pulse or asphygmia, and a significant difference for blood pressure of both upper extremities [12].
Pregnant women associated with aortic dissection are a special group of patients, and effective surgical approach should be taken timely according to the circumstance of fetal and maternal conditions. Particularly, surgical approaches should be appropriately selected for those patients with type A aortic dissection based on different stage of gestation [13,14]. For example, aortic dissection occurs in the early stage of pregnancy (the first 28 weeks of gestation), surgical repair of aortic dissection should be selected to minimize the potential damaging effect on the fetus and surgery should be taken once the pregnancy reaches fetal maturity. In contrast, for those patients diagnosed in the late stage of pregnancy (after 32 weeks of gestation) cesarean section is generally advised upon the diagnosis. In general, the priority is to ensure the safety of pregnant women, and then the safety of the fetus. In the current report, 1 patient diagnosed in the late stage of pregnancy received cesarean section along with Bentall surgery in the setting of low temperature and general anesthesia, and both mother and the baby were survived after surgery. Based on our experience women with type A aortic dissection usually manifest higher risk than that of type B during the course of pregnancy.
In summary, aortic dissection is a devastating event in women during the course of pregnancy or stage of puerperium, which renders both mother and fetus on a very dangerous condition. A quick and accurate diagnosis along with appropriate surgical treatments would be necessary to save the lives for both mother and baby. Particularly, a team with multidisciplinary expertise would be important to ensure the best clinical decisions for treatment of this special group of patients.
Acknowledgements
Our research was supported by a National Natural Science Foundation of China (81471896).
Disclosure of conflict of interest
None.
References
- 1.Thalmann M, Sodeck GH, Domanovits H, Grassberger M, Loewe C, Grimm M, Czerny M. Acute type A aortic dissection and pregnancy: a population-based study. Eur J Cardiothorac Surg. 2011;39:e159–e163. doi: 10.1016/j.ejcts.2010.12.070. [DOI] [PubMed] [Google Scholar]
- 2.Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, Evangelista A, Falk V, Frank H, Gaemperli O, Grabenwöger M, Haverich A, Iung B, Manolis AJ, Meijboom F, Nienaber CA, Roffi M, Rousseau H, Sechtem U, Sirnes PA, Allmen RS, Vrints CJ ESC Committee for Practice Guidelines. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC) Eur Heart J. 2014;35:2873–2926. doi: 10.1093/eurheartj/ehu281. [DOI] [PubMed] [Google Scholar]
- 3.Golledge J, Eagle KA. Acute aortic dissection. Lancet. 2008;372:55–66. doi: 10.1016/S0140-6736(08)60994-0. [DOI] [PubMed] [Google Scholar]
- 4.Smok DA. Aortopathy in pregnancy. Semin Perinatol. 2014;38:295–303. doi: 10.1053/j.semperi.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 5.Goland S, Elkayam U. Cardiovascular problems in pregnant women with marfan syndrome. Circulation. 2009;119:619–623. doi: 10.1161/CIRCULATIONAHA.104.493569. [DOI] [PubMed] [Google Scholar]
- 6.van Hagen IM, Roos-Hesselink JW. Aorta pathology and pregnancy. Best Pract Res Clin Obstet Gynaecol. 2014;28:537–550. doi: 10.1016/j.bpobgyn.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 7.European Society of Gynecology (ESG); Association for European Paediatric Cardiology (AEPC); German Society for Gender Medicine (DGesGM); Regitz-Zagrosek V, Blomstrom Lundqvist C, Borghi C, Cifkova R, Ferreira R, Foidart JM, Gibbs JS, Gohlke-Baerwolf C, Gorenek B, Iung B, Kirby M, Maas AH, Morais J, Nihoyannopoulos P, Pieper PG, Presbitero P, Roos-Hesselink JW, Schaufelberger M, Seeland U, Torracca L ESC Committee for Practice Guidelines. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the task force on the management of cardiovascular diseases during pregnancy of the European Society of Cardiology (ESC) Eur Heart J. 2011;32:3147–3197. doi: 10.1093/eurheartj/ehr218. [DOI] [PubMed] [Google Scholar]
- 8.Tan EK, Tan EL. Alterations in physiology and anatomy during pregnancy. Best Pract Res Clin Obstet Gynaecol. 2013;27:791–802. doi: 10.1016/j.bpobgyn.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Yuan SM. Postpartum aortic dissection. Taiwan J Obstet Gynecol. 2013;52:318–322. doi: 10.1016/j.tjog.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Lindsay ME, Dietz HC. Lessons on the pathogenesis of aneurysm from heritable conditions. Nature. 2011;473:308–316. doi: 10.1038/nature10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sahni S, Bhatia SK. Ascending aortic curvature as an independent risk factor for aortic dissection: the mathematical model and underlying equations. Eur J Cardiothorac Surg. 2012;42:755–756. doi: 10.1093/ejcts/ezs228. [DOI] [PubMed] [Google Scholar]
- 12.Ranasinghe AM, Strong D, Boland B, Bonser RS. Acute aortic dissection. BMJ. 2011;343:d4487. doi: 10.1136/bmj.d4487. [DOI] [PubMed] [Google Scholar]
- 13.Rajagopalan S, Nwazota N, Chandrasekhar S. Outcomes in pregnant women with acute aortic dissections: a review of the literature from 2003 to 2013. Int J Obstet Anesth. 2014;23:348–356. doi: 10.1016/j.ijoa.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Sterner D, Probst C, Mellert F, Schiller W. Surgical treatment and thoracic endovascular aortic repair in type A aortic dissection in a pregnant patient with Marfan syndrome. Ann Vasc Surg. 2014;28:1317. doi: 10.1016/j.avsg.2013.10.011. [DOI] [PubMed] [Google Scholar]


