Abstract
Objective: The aim of this study was to evaluate the efficacy, safety and sexual life quality outcomes of ultrasound-guided high-intensity focused ultrasound (HIFU) ablations for the treatment of patients with symptomatic adenomyosis and uterine volumes >200 cm3. Methods: In our prospective clinical trial 47 patients with uterine volumes >200 cm3 and symptomatic adenomyosis were treated with single treatment sessions of ultrasound-guided HIFU ablations. Beside uterus and adenomyosis lesion size reductions, outcome measures were symptom severity score (SSS), visual analogue scale (VAS) of dysmenorrhea, female sexual function index (FSFI) scores and the incidence of complications. Results: In all 47 patients, the adenomyosis lesions sizes were significantly reduced 12 months after the interventions (P<0.01). The SSS and dysmenorrhea VAS scores were significantly reduced 12 months after the interventions (P<0.01) and the FSFI scores gradually improved during 12 months after the HIFU ablations (P<0.001). No serious complication occurred. Conclusion: Ultrasound-guided HIFU ablation is a safe and effective noninvasive alternative for the treatment of uterine volumes >200 cm3 with symptomatic adenomyosis. Particularly maintaining the integrity of patients’ uteri leads to significant FSFI score improvements, which were essentially reduced before the HIFU treatments.
Keywords: High-intensity focused ultrasound, adenomyosis, symptom severity score, female sexual function index, and dysmenorrhea
Introduction
Adenomyosis is a benign female disease, which is caused by the presence of ectopic endometrial glands and stroma in the myometrium and frequently affects 30-50 years aged women [1]. Approximately 70% of the patients presenting with varying extents of clinical symptoms, such as increased amount of menstrual blood loss, prolonged menstruation and gradually aggravated dysmenorrhea, which may lead to sterility or recurrent pregnancy loss. Adenomyosis related uterus enlargements cause pelvic pain, dysmenorrhea and equally disturbs patients’ micturition and defecation, thereby severely affecting patients quality of life [2]. Common treatments include surgical or medical approaches. Traditional surgeries include laparoscopic myomectomy [3] and hysterectomy [1], the later one merely used for elderly patients without fertility desire. Medical therapies, such as danazol and GnRH-a have been applied in treating young populations with desire for fertility, which lead to temporary alleviation lasting <6 months. However, it has several limitations, such as long period of treatment, severe adverse events and frequent recurrence [4].
Since minimally invasive surgical techniques had limited success in the treatment of adenomyosis, techniques including uterine artery embolization (UAE) and radiofrequency ablation were developed, but there is no common sense on which treatment is most suitable [5].
Since 1990s, HIFU has been introduced into China as a novel noninvasive treatment of hysteromyoma with good clinical efficacy [6]. HIFU utilizes the beam-convergence features, good penetrability in human tissues and sound absorption characteristics of frequency ultrasound. It transforms energy of sound absorption into heat energy raising temperatures to thresholds of protein denaturalization between 60 and 100°C and lead to coagulative necrosis in the target area, whereas does not destroy surrounding tissues. The necrotic tissues after HIFU can be gradually absorbed, which leads to alleviating or allaying of clinical symptoms and physical signs, thereby attaining the purpose of a noninvasive therapy for the disease [7]. In this prospective clinical trial, the outcomes of HIFU treatments for adenomyosis patients with a uterine size exceeding 200 cm3 were analyzed particularly regarding their sexual life qualities.
Patients and methods
Patients
This research was approved by the ethic committee of the Chongqing Medical University and informed consents were provided by each participating patient. A total of 51 adenomyosis patients with a uterine size >200 cm3 admitted to Haifu Hospital of the First Affiliated Hospital of Chongqing Medical University between January and December 2012 were enrolled in this study. All patients were between 25 and 51, (37.43±5.08) years old. Mean body mass index was (21.98±1.68) kg/m2 and pre-interventional MRIs revealed that the median uterus size was 323.5 cm3 (P25 244.48 cm3 and P75 444.56 cm3). The median volume of adenomyotic lesions was 102.16 cm3 (P25 69.1 cm3 and P75 187.47 cm3).
Inclusion criteria were: 1. Adenomyosis diagnosis was confirmed by at least two gynecologists and HIFU physicians according to clinical manifestations and Magnetic Resonance Imaging (MRI). 2. Patients were aged >18 years. 3. Patients received no medical treatments at 3 months prior to surgery. 4. Patients with adenomyosis with a diameter above 3 cm, located at unilateral uterine muscle walls with surgical indications, had desire to receive treatments and complied with 12-month follow-up. 5. Patients could accurately communicate with physicians and nurses during treatment. 6. Patients had no other complications, such as heart disease, hypertension and others. 7 Patients aged <51 years, their spouses were alive and had normal partner relationships (divorced and widowed subjects were excluded).
Exclusion criteria were: 1. Acute pelvic inflammation or acute episodes of chronic pelvic inflammation. 2. Patients during menstruation, pregnancy and lactation periods. 3. Connective tissue illnesses or received abdominal radiotherapy with a large dosage. 4. Patients which had undergone enhanced MRI and had anesthetic contraindications.
Treatment procedures and medical equipment
Pre-treatment preparation
Patients were told to have mild and digestible soft diets for intestine preparation at 3 d before surgery. At 2 d before intervention, patients received 30 g Folium Sennae boiled in water until the incidence of diarrhoea. At 1 d before treatment, patients took orally compound PEG electrolyte cathartics (1500-2000 ml) and had water at the night before treatment and the day of treatment. Before treatment, urine was discharged by a catheter, which was kept in the patients during the procedure. At 30 min before treatment, removal of skin hair as well as degreasing were conducted to avoid the influences on the acoustic pathway.
MRI determinations
Procedures were performed with a Magnetom Symphony 1.5T MRI Tim system (Magnetom Espree, Siemens Medical Solutions, Germany). MRI sequences and scan parameters included T1-weighted spin-echo sequences, T2-weighted spin-echo sequences and enhanced T1-weighted gradient-echo sequences. The pelvis enhanced MRI examinations were performed before and 1 week as well as 12 months after the treatments. Pre-intervention MRIs evaluated the size and location position of lesions (anterior or posterior wall). The volume of lesions and uteri were evaluated by 3D diameters of target lesions including long diameter (D1), anterior and posterior diameters (D2) and horizontal diameters (D3), which were measured by T2-weighted imaging. The volume of the lesion, lesion ablation (Non-Perfused Volume (NPV)) and the uterus was calculated from the ellipsoid formula: V=0.5233×D1×D2×D3.
HIFU application
The Model-JC200 Focused Ultrasound Tumor Therapeutic System (Chongqing Haifu Medical Technology Co., Ltd., Chongqing, China) was used with adjustment to a 1.5 mm ×1.5 mm ×8 mm, spheroid-shaped focal region with a long axis of 8 mm and a short axis of 3 mm. Patients were kept in a prone position, the treated skin was soaked in low temperature water, the urine catheter was retained and bladder perfusion was conducted. Degassed water sacs were pressed on the abdominal wall for pushing away the intestine, ensuring no intestine tissues within the acoustic pathway. Injection of sufentanil citrate and midazolam was performed according to patients’ constitution and weight until a Ramsay score of 2-3 was obtained, which ensured that patients could tolerate the discomforts related to the treatment, maintain adequate cardiopulmonary function, provide suitable responses to verbal stimulus but maintained in an effective analgesia and sedation status.
Ablation was performed in a dot exposure pattern with a therapeutic power of 300-400 W. Intra-interventional real-time ultrasound was used to monitor and evaluate the efficacy. The distance between focus and endometrium was ≥15 mm and ≥15 mm between focus and uterine serosa. The distance between treated layers was 5 mm. The treatment was discontinued in case of lump-shaped gray changes or overall gray changes without significant blood signal within the lesions immediately after color Doppler ultrasound detection.
Outcome evaluations
MRI derived blood perfusion and lesion sizes were observed by 2-3 gynecologists with the same equipment and fixed parameters before and 1 week as well as 12 months following the therapy. All patients filled SSS [8] questionnaires before and 3-, 6- and 12-month following the treatments including 8 items: increased amount of menstrual blood loss, menstrual blood clotting, prolonged menstruation, menstrual disorders, pelvic tightness/pressure, frequent urination during day and night times, fatigue. VAS scores [9] of dysmenorrhea was adopted to compare the alleviation of dysmenorrhea symptom before and after treatment. The Female Sexual Function Index (FSFI) [10] with 19 items was employed as questionnaire including the following 6 domains: desire, subjective arousal, vaginal lubrication, orgasm, satisfaction and pain.
Immediate evaluation of adverse reactions after HIFU included patients’ physical signs, treated skin, the sacrococcygeal region as well as presence of vaginal secretion and limb activities. Subsequent follow-up adverse reactions were monitored at 1 month after intervention.
Statistical analyses
SPSS statistics for windows (Version 17.0. Chicago: SPSS Inc.) was employed for all analyses. Data with normal distribution were expressed as mean ± standard error (x ± SD) and otherwise as median [M (P25, P75)]. Student’s t-tests were used for analyzing the data of the lesion changes and uterine volumes. Independent sample Kruskal-Wallis test was used for the SSS and VAS and FSFI scores. A value of P<0.05 was considered as statistically significant.
Results
Clinical outcomes of HIFU ablations for adenomyosis
Fifty-one adenomyosis patients successfully received HIFU ablations. Two patients were lost during follow-up and 2 became pregnant, whereas 47 patients completed the follow up. Most cases presented with diffusive lesions. The area of lesions was calculated from the uterus wall at which the lesions were located. To guarantee the normal uterus wall function, complete ablation was not performed. All patients were subjected to enhanced MRI at 1 week after HIFU treatments (Figure 1) and no significant blood perfusion within the lesions occurred. Following HIFU ablation, the incidence of coagulative necrosis was 100%, the median NPV was 72.5 cm3 (42.89 cm3 and 137.3 cm3) and the ablation rate achieved (61.36±17.72%).
Figure 1.
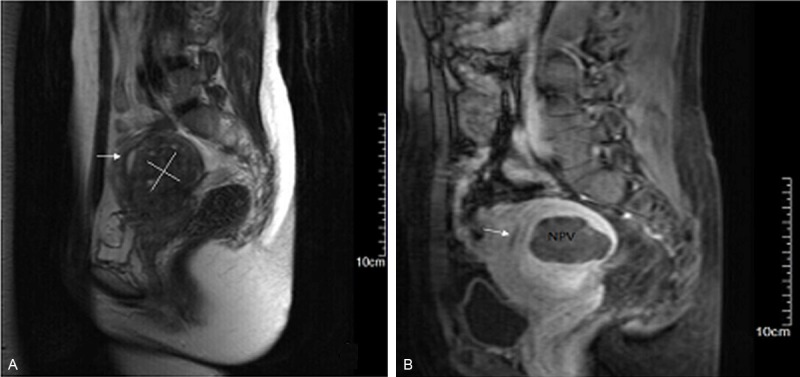
Representative MRI imaging of uterine adenomyosis before and 1 week after HIFU treatment. Arrows = endometrium. A. T2WI at 2 d before HIFU. The area of the white line indicates a lesion. B. Enhanced T1WI at 1 week after HIFU. The dark shadow shows the area of lesion ablation (Non-Perfused Volume (NPV)). The percentage of NPV in this patient was 76%.
Imaging of lesions during follow-up
Color Doppler ultrasounds were conducted immediately after HIFU ablations and revealed no significant blood signal within the lesions. MRI was performed before and 12 months after HIFU treatment to measure and calculate the volume and reduction rates of the lesions. At 12 months after HIFU ablation, the uterine size was decreased by 22% and the volume of lesions declined by 30% with statistical significance (P<0.01), as illustrated in Table 1; Figure 2.
Table 1.
Volume comparisons of uterus and adenomyotic lesion before and 12 months after HIFU
| Before treatment (n=51) | 12 m post-treatment (n=47) | |
|---|---|---|
| Size of uterus (cm3) | 323.5 (P25 244.48, P75 444.56) | 252.33 (P25 192.02, P75 340.15)* |
| Size of adenomyosis lesion (cm3) | 102.16 (P25 69.1, P75 187.47) | 71.5 (P25 48, P75 132.42)** |
P<0.01;
P<0.01.
Figure 2.
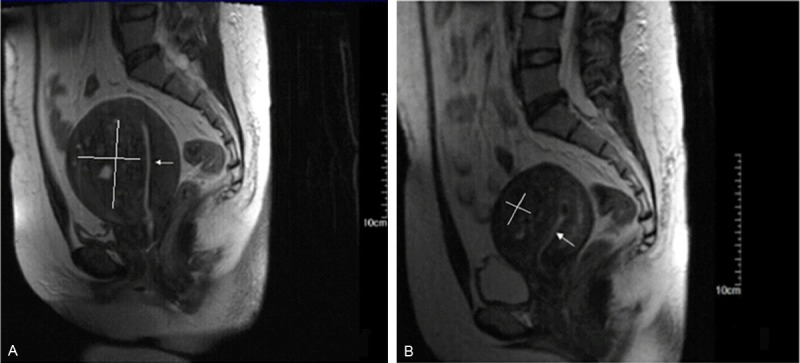
Representative MRI images before and 12 months after HIFU ablation. A. T2WI before HIFU. B. T2WI at 12 months after HIFU, showing 35% and 62% shrinkage in the size of the uterus and adenomyosis lesions, respectively. The area of the white line indicates a lesion.
SSS and VAS grading of dysmenorrhea
SSS of 8 domains including increased amount of menstrual blood loss, menstrual blood clot, prolonged menstruation, menstrual disorders, pelvic tightness/pressure, frequent urination during daytime and night, fatigue and VAS scores of dysmenorrhea before and 3, 6 and 12 month after HIFU intervention are shown in Table 2. SSS scores at 3, 6 and 12 month after HIFU ablation decreased by 33%, 50% and 65%, respectively. Heavy menstrual bleeding and blood clotting decreased significantly by 31% and 36% (P<0.05), whereas pelvic tightness/pressure and dysmenorrheal VAS scores decreased by 40% and 29% (P<0.001) already at 3 month after interventions. All scores reached decreased values with P<0.01 or P<0.001 significance at 6 months after HIFU treatments and at 12 months all scores were significantly less than before treatment with P values <0.001 (Table 2).
Table 2.
SSS and VAS scores of dysmenorrhea before and 3, 6 and 12 months after HIFU ablation
| Pre-treatment (n=51) | 3 m post-treatment (n=51) | 6 m post-treatment (n=47) | 12 m post-treatment (n=47) | |
|---|---|---|---|---|
| Total SSS | 36.66±19.44 | 24.60±13.88 | 18.18±11.63*** | 12.95±10.31*** |
| Heavy menstrual bleeding | 7.25±3.72 | 5.0±2.92* | 3.61±2.52*** | 2.57±2.23*** |
| Blood clotting | 5.99±3.85 | 3.85±2.75* | 2.66±2.18*** | 1.66±1.58*** |
| Fluctuation of menses durations | 4.22±3.92 | 2.76±2.41 | 2.01±1.75** | 1.51±1.4*** |
| Fluctuations of menses intervals | 3.52±3.23 | 2.25±1.93 | 1.66±1.34** | 1.23±1.14*** |
| Pelvic tightness/pressure | 8.34±2.61 | 5.01±2.18*** | 3.44±2.01***,▲ | 2.46±2.04*** |
| Daytime urination | 1.99±1.31 | 1.49±0.7 | 1.35±0.94*** | 0.97±0.6*** |
| Nighttime urination | 1.72±0.46 | 1.42±0.52 | 1.29±0.85*** | 0.93±0.55*** |
| Fatigue | 3.63±2.47 | 2.82±2.08 | 2.16±1.76** | 1.62±1.42*** |
| Dysmenorrheal VAS | 6.67± 1.6 | 4.75 ±1.7*** | 3.10±1.26***,▲▲ | 1.86±1.18*** |
P<0.05 compared to pre-treatment scores;
P<0.01 compared to pre-treatment scores;
P<0.001 compared to pre-treatment scores;
P<0.05 compared to scores of the last previous follow up;
P<0.01 compared to scores of the last previous follow up.
FSFI grading
The scores of desire, subjective arousal, vaginal lubrication, orgasm, satisfaction, pain and overall FSFI score before and 3, 6 and 12 months after HIFU ablation are shown in Table 3. All items improved significantly until the 3rd month after treatments, further improved to P values of <0.001 at the 6th post-interventional month and became slightly better until 12 month after HIFU treatments, but without statistical significance compared to the 6th month scores (Table 3).
Table 3.
FSFI scores before and 3, 6 and 12 months after HIFU ablations
| Pre-treatment (n=51) | 3 m post-treatment (n=51) | 6 m post-treatment (n=47) | 12 m post-treatment (n=47) | |
|---|---|---|---|---|
| Desire | 2.96±1.5 | 3.43±1.3*** | 4.02±1.4***,▲▲▲ | 4.52±1.46*** |
| Arousal | 3.43±2.72 | 4.37±3.18** | 5.01±2.24***,▲▲▲ | 5.57±2.25*** |
| Lubrication | 3.94±3.33 | 4.55±4.11* | 5.14±2.94***,▲▲ | 5.54±2.78*** |
| Orgasm | 3.39±3.03 | 3.72±2.99* | 4.24±1.77***,▲▲▲ | 4.63±1.96*** |
| Global satisfaction | 3.49±3.08 | 3.84±3.11* | 4.47±2.05***,▲▲▲ | 4.95±1.47*** |
| Pain | 3.56±3.15 | 4.02±3.22* | 4.53±2.06***,▲▲ | 5.12±2.67*** |
| Total FSFI | 20.77±1.26 | 23.93±2.19** | 27.41±2.65***,▲▲ | 30.33±2.89*** |
P<0.05 compared to pre-treatment scores;
P<0.01 compared to pre-treatment scores;
P<0.001 compared to pre-treatment scores;
P<0.01 compared to scores of the last previous follow up;
P<0.001 compared to scores of the last previous follow up.
Adverse events
The degree of adverse reactions after adenomyosis treated with HIFU did not exceed the classification B from the society of interventional radiology (SIR) [11]. After the treatments 44 patients (86.28%) had adverse events and 37 cases (72.56%) complained of lower abdominal pain, from which 4 had severe pain in the night after surgery, which was allayed after injection of dolantin. One patient still had abdominal distention at 14 d after surgery. She was left untreated but the symptoms were alleviated after the 1st post-interventional menstruation. Four (7.84%) patients presented with sacrococcygeal pain after the interventions, which was significantly allayed after 3-5 d administration of non-steroidal anti-inflammatory drugs. Twenty-five cases (49.02%) had a slight amount of vaginal hemorrhage secretions, which lasted for 3-7 days. Nine women (17.65%) had mild degree of abnormal movement function of the lower extremity, mainly characterized as lower extremity numbness and hypodynamia, 3 among whom were alleviated after intravenous injection of electrolytes. One patient (1.96%) had urethral irritation after HIFU ablation, which was allayed after 3 days by drinking more water and frequent urination. At 14 d after ablation five patients (9.8%) discharged vaginal fluid without treatment, which was alleviated after the first menstruation. No serious complications, such as nerve injury, bladder injury, intestinal perforation, skin burn or others were observed.
Discussion
Initial reports noted, that HIFU for the treatment of adenomyosis gained favorable clinical efficacy with high safety [12,13]. The results in this study revealed that the mean uterine size and the volume of lesions significantly decreased by 22% (P<0.05) and 30% (P<0.01) at 12 month after HIFU ablations (Table 1). The data are similar to values reported by Fan et al. (23.8% and 26.1%) with MRI-guided HIFU treatments for adenomyosis, but in their setting the pre-interventional uterus and adenomyosis lesion sizes were smaller than in our study [14]. In contrast to the report from Zhang et al. [15] which only noted improvements of menorrhagia and dysmenorrhea after HIFU interventions, we found significant improvements of SSS scores from 8 domains as well as VAS scores of dysmenorrhea at 6 and 12 months after HIFU treatments. This study showed that HIFU ablation could effectively allay particularly dysmenorrhea and other clinical symptoms of adenomyosis patients with a uterus size above 200 cm3. Previous research revealed that female patients diagnosed with myomas and an uterine size >200 cm3 had more pain, less satisfaction and lower FSFI scores during copulation compared with healthy women [16]. In addition, patients with adenomyosis are generally more likely to have copulation difficulties, probably resulting from the damages and expansions of surrounding tissues, which lead to dyspareunia and lower satisfaction [17]. HIFU ablation is an alternative option for adenomyosis treatments, which maintains the integrity of patients’ uteri [15] and we hypothesized that it might also restore sexual live quality, since uterus sparing surgery was reported to be associated with a greater improvement in sexual function after pelvic organ prolapses [18]. Besides, patients with adenomyosis tend to lower self-evaluations and divert more attention to their disease rather than to the sexual life [19].
In this study, we observed that adenomyosis patients with a uterine size >200 cm3 had lower FSFI scores and more sexual function barriers compared with normal women. The scores of desire, subjective arousal, vaginal lubrication, orgasm, satisfaction and pain were all significantly increased at 3, 6 and 12 month after HIFU interventions. The main improvements took place until 6 months and predominantly developed between 3rd and 6th months following the HIFU ablations, whereas did not significantly change between 6 and 12 months after treatments, indicating that disturbances of sexual life was caused by adenomyosis and could be reversed by HIFU interventions in a relative short period.
During HIFU application, most adverse reactions of adenomyosis are caused by hyperthermic effects. To guarantee therapeutic efficacy of a HIFU intervention and prevent the incidence of complications, sound intensity and temperature variability can be utilized to properly reduce treatment power and extend radiation time, which collectively ensure that the total dose keeps unchanged with therapeutic safety and decrease of adverse event incidences. In this study, 37 (72.56%) of the patients complaint lower abdominal pain, which was treated with dolantin in 4 and maintained until the 14th post-interventional day in 1 patient, but also disappeared then after the 1st menstruation following the HIFU ablation. Other adverse events included slight vaginal hemorrhages (49.02%), mild lower extremity numbness and hypodynamia (17.65%), vaginal fluid discharge (9.8%) as well as sacrococcygeal pain (7.8%), which disappeared after conventional treatments or without treatments after the 1st post-interventional menstruation. In this clinical trial, no severe surgical complications, such as nerve injury, bladder injury, intestinal perforation and skin burn occurred, indicating that HIFU ablation is a safe treatment for adenomyosis.
In summary, HIFU ablation for uterine adenomyosis is a safe treatment leading to significant reductions of uterus and adenomyosis lesion sizes, which reflected in substantial mitigation of SSS and dysmenorrheal VAS scores. In addition, the improvements were accompanied by significant augmentations of FSFI scores, which were essentially diminished in the adenomyosis patients before the treatments.
Acknowledgements
This study was supported by National Key Technology Research and Development Program (No. 2011BAI14B01).
Disclosure of conflict of interest
None.
References
- 1.Bergeron C, Amant F, Ferenczy A. Pathology and physiopathology of adenomyosis. Best Pract Res Clin Obstet Gynaecol. 2006;20:511–521. doi: 10.1016/j.bpobgyn.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 2.Yeniel O, Cirpan T, Ulukus M, Ozbal A, Gundem G, Ozsener S, Zekioglu O, Yilmaz H. Adenomyosis: prevalence, risk factors, symptoms and clinical findings. Clin Exp Obstet Gynecol. 2007;34:163–167. [PubMed] [Google Scholar]
- 3.Seinera P, Arisio R, Decko A, Farina C, Crana F. Laparoscopic myomectomy: indications, surgical technique and complications. Hum Reprod. 1997;12:1927–1930. doi: 10.1093/humrep/12.9.1927. [DOI] [PubMed] [Google Scholar]
- 4.Imaoka I, Ascher SM, Sugimura K, Takahashi K, Li H, Cuomo F, Simon J, Arnold LL. MR imaging of diffuse adenomyosis changes after GnRH analog therapy. J Magn Reson Imaging. 2002;15:285–290. doi: 10.1002/jmri.10060. [DOI] [PubMed] [Google Scholar]
- 5.Taran FA, Stewart EA, Brucker S. Adenomyosis: Epidemiology, Risk Factors, Clinical Phenotype and Surgical and Interventional Alternatives to Hysterectomy. Geburtshilfe Frauenheilkd. 2013;73:924–931. doi: 10.1055/s-0033-1350840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart EA, Rabinovici J, Tempany CM, Inbar Y, Regan L, Gostout B, Hesley G, Kim HS, Hengst S, Gedroyc WM. Clinical outcomes of focused ultrasound surgery for the treatment of uterine fibroids. Fertil Steril. 2006;85:22–29. doi: 10.1016/j.fertnstert.2005.04.072. [DOI] [PubMed] [Google Scholar]
- 7.Cline HE, Hynynen K, Watkins RD, Adams WJ, Schenck JF, Ettinger RH, Freund WR, Vetro JP, Jolesz FA. Focused US system for MR imaging-guided tumor ablation. Radiology. 1995;194:731–737. doi: 10.1148/radiology.194.3.7862971. [DOI] [PubMed] [Google Scholar]
- 8.Spies JB, Coyne K, Guaou Guaou N, Boyle D, Skyrnarz-Murphy K, Gonzalves SM. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomyomata. Obstet Gynecol. 2002;99:290–300. doi: 10.1016/s0029-7844(01)01702-1. [DOI] [PubMed] [Google Scholar]
- 9.Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17:45–56. doi: 10.1016/0304-3959(83)90126-4. [DOI] [PubMed] [Google Scholar]
- 10.Rosen R, Brown C, Heiman J, Leiblum S, Meston C, Shabsigh R, Ferguson D, D’Agostino R Jr. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 11.Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14:S199–202. doi: 10.1097/01.rvi.0000094584.83406.3e. [DOI] [PubMed] [Google Scholar]
- 12.Zhou M, Chen JY, Tang LD, Chen WZ, Wang ZB. Ultrasound-guided high-intensity focused ultrasound ablation for adenomyosis: the clinical experience of a single center. Fertil Steril. 2011;95:900–905. doi: 10.1016/j.fertnstert.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 13.Yang Z, Cao YD, Hu LN, Wang ZB. Feasibility of laparoscopic high-intensity focused ultrasound treatment for patients with uterine localized adenomyosis. Fertil Steril. 2009;91:2338–2343. doi: 10.1016/j.fertnstert.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 14.Fan TY, Zhang L, Chen W, Liu Y, He M, Huang X, Orsi F, Wang Z. Feasibility of MRI-guided high intensity focused ultrasound treatment for adenomyosis. Eur J Radiol. 2012;81:3624–3630. doi: 10.1016/j.ejrad.2011.05.036. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Li K, Xie B, He M, He J, Zhang L. Effective ablation therapy of adenomyosis with ultrasound-guided high-intensity focused ultrasound. Int J Gynaecol Obstet. 2014;124:207–211. doi: 10.1016/j.ijgo.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 16.Ertunc D, Uzun R, Tok EC, Doruk A, Dilek S. The effect of myoma uteri and myomectomy on sexual function. J Sex Med. 2009;6:1032–1038. doi: 10.1111/j.1743-6109.2008.01086.x. [DOI] [PubMed] [Google Scholar]
- 17.Long CY, Fang JH, Chen WC, Su JH, Hsu SC. Comparison of total laparoscopic hysterectomy and laparoscopically assisted vaginal hysterectomy. Gynecol Obstet Invest. 2002;53:214–219. doi: 10.1159/000064567. [DOI] [PubMed] [Google Scholar]
- 18.Costantini E, Porena M, Lazzeri M, Mearini L, Bini V, Zucchi A. Changes in female sexual function after pelvic organ prolapse repair: role of hysterectomy. Int Urogynecol J. 2013;24:1481–1487. doi: 10.1007/s00192-012-2041-3. [DOI] [PubMed] [Google Scholar]
- 19.Ekin M, Cengiz H, Ozturk E, Kaya C, Yasar L. Genitourinary symptoms in patients with adenomyosis. Int Urogynecol J. 2013;24:509–512. doi: 10.1007/s00192-012-1903-z. [DOI] [PubMed] [Google Scholar]


