Abstract
The effects of carnosic acid (CA) were investigated on the acute myeloid leukemia (AML) cell growth in vivo. A NOD/SCID AML mouse model, which was set up by inoculation with K562/A02 cells, was used to study whether tumor growth in vivo can be inhibited by CA combined with adriamycin. After being inoculated with K562/A02 cells, the NOD/SCID mice were expressed positive human mdr1 and bcr/abl genes. This result indicates that the K562/A02/SCID leukemia mouse model is successfully established. The mice treated with CA combined with adriamycin exhibit a significant lower number of leukemia cells (20%) than that of untreated animals (32.5%) (P<0.05), in particular with higher percentages of apoptotic cells than the mice treated by single adriamycin (control) group. The median of 95% CI survival time is 19 (10.0-44.2) and 33 (29.4-36.6) days for the control group and the CA-treated group, respectively. The difference is statistically significant (P<0.05). It is illustrated that the natural compound CA, combined with Adriamycin, has high potential to inhibit the growth of malignant cells in vivo, and is a promising adjuvant anti-cancer drug. Prospective studies should be conducted to understand the functional mechanism of CA at the molecular level.
Keywords: Carnosic acid, K562/A02 cells, leukemia mouse model, adriamycin, in vivo
Introduction
The treatment outcome of acute myeloid leukemia (AML) has achieved great improvement, however it still faces up with a tremendous challenge. The performance of current chemotherapeutic drugs with highly toxic and poorly tolerated is the major obstacle in the treatment, particularly for the treatment of elder patients [1-3]. The drug resistance is also an obstacle in the treatment of cancer, notably for acute leukemia. Previous literature has shown that the resistance may be mediated by the multidrug resistance (MDR-1/P-glycoprotein) gene. A recent review indicates that there is a substantial body of research on P-gp inhibition as a means of improving the efficacy of therapeutic agents that are ATP-binding cassette transporter substrates [4]. Many compounds have been reported to be capable of modulating the MDR phenotype, however, few can be clinically applied due to their unacceptable side effects or toxicity at the doses required to be effective. Therefore, an alternative approach of developing novel agents with reduced efflux properties may prove to be the most promising way to improve the efficacy of existing agents for AML [4].
Polyphenolic antioxidants are a group of relatively safe compounds that can be used as a component of the diet or herbal medicinal remedies, among a plenty of agents tested in combination with natural plant-derived compounds. Rosemary (Rosmarinus officinalis L.) and its extracts such as caffeic acid, rosmarinic acid (RA), ursolic acid (UA), carnosic acid (CA), and carnosol, have been studied for the antitumor or antineoplastic behavior on different types of cancer cell lines/rat or mouse models [5]. The anticancer effects of rosemary extracts were found to be statistically significant in animal models including colonic cancer [6,7], mammary tumors [8,9], leukemia [10], and skin tumors [11]. In general, the potential anticancer mechanism of rosemary is either the induction of cancer protective markers/factors (apoptosis or carcinogen metabolic enzymes) or the inhibition of tumor promoting events (cell growth and proliferation, DNA adduct/free radical formation, carcinogen activating enzymes, or lipid peroxidation) [12-16].
In our previous work we have demonstrated that CA can reverse MDR of K562/AO2 cells in vitro by increasing intracellular adriamycin concentration, down-regulating expression of MDR-l, and inhibiting the function of P-gp. CA can decrease viability of the human promyelocytic leukaemia cell line HL-60, in dose- and time-dependent manners [17,18].
In the article the antileukemic activity of CA combined with adriamycin was investigated in vivo by a K562/A02/SCID mouse model of human AML.
Materials and methods
Cell culture
Human leukemic K562/A02 cells (obtained from the laboratory of Immunology of the Institute of Basic Medical, Shandong Academy of Medical Sciences) were cultured in RPMI 1640 medium (Gibco, Los Angeles, CA, USA) supplemented with 10% heat inactivated FBS, 100 µg/ml penicillin, 100 µg/ml streptomycin, and 2 mM L-glutamine, and maintained in a humidified atmosphere with 5% CO2 at 37°C. The cells were sub-cultured twice each week, and the exponentially growing cells were used in all treatments. Before treatment, compound cells were washed with PBS and fresh medium was added. At the time of treatment, working solutions were diluted accordingly in RPMI 1640.
Experimental animals
Establishment of K562/A02/SCID mouse model
Experiments were carried out in the animal facility of Animal Experimental Center of Shandong University in accordance with Shandong University Animal Care Committee approved protocol. Male NOD/SCID (non-obese diabetic/severe combined immune deficient) mice age 4 weeks, were purchased from Experimental Animal Center of Shanghai Academy of Medical Sciences. Mice were housed in sterilized cages and provided with autoclaved water and a standard powdered rodent diet ad libitum. The experimental protocols were initiated following a 7-day acclimatization period.
All NOD/SCID mice were given cyclophosphamide 2 mg/mouse for three days. Using an adjusted K562/A02 cell concentration of 5×107/ml, each mouse was inoculated with 1×107/ml of the cells through their tail vein [19].
K562/A02/SCID leukemia model validation experiments
After inoculation of K562/A02 cells in NOD/SCID mice for two weeks, six animals were killed to determinate whether the K562/A02/SCID leukemia model was successful established. White blood cells were separated from the blood. Expressions of human mdr and bcr/abl mRNA were performed using RT-PCR. The visible leukemia cells were determined from peripheral blood, bone marrow smears, and slices of liver and neck lymph nodes.
Human mdr and bcr/abl mRNA detection by RT-PCR
In vitro total mRNA was extracted from the cells with Trizol reagent (Invitrogen Co. California, USA) according to the manufacturer’s instructions. Single-stranded cDNA was synthesized by reverse transcription from 1 μg of the total RNA using Reverse Transcriptase RNAse (RT-PCR) M-MLV (Invitrogen Co. LA, USA) and oligo-dT. The amplification was performed in a final volume of 50 μL, containing 5 μL cDNA, 0.5 μL of each oligonucleotide primer, 1 μL of each dNTP, and 1 unit of Taq DNA polymerase. Amplification was carried out in a Thermal Cycler. The PCR primers and expected product size were as follows:
mdr1, forward: 5’-CCCATCATTGCAATAGCAGG-3’ and reverse: 3’-ACTCCTCGTCTTCAAACTTG-3’ (157 bp); bcr/abl, forward: 5’-AGCATTCCGCTGACCATCA-3’ and reverse: 5’-GCGTGATGTAGTTGCTTGGGAC-3’ (200 bp); β-actin, forward: 5’-GTGGGGCGCCCCAGGCACCA-3’ and reverse: 5’-CTCCTTAATGTCACGCACGATTTC-3’ (539 bp).
Circulating conditions were 94°C one min, 57°C one min, 72°C one min, 30 cycles, and 72°C for 10 min. The end products were identified by electrophoresis using 1.5% agarose gel. The positive control was P210 bcr/abl cDNA, and the negative control was the reverse transcription product of total RNA in normal human peripheral blood cells.
The increasing curative effect treatment with CA combined with adriamycin in the K562/A02/SCID leukemia model
Twenty K562/A02/SCID male were randomly divided into 2 groups, and each group had ten mice. The experimental group was fed with 1% (v/v) CA powder (purity ≥91%, Sigma, USA) feed sterilized by Co-60 radiation. After 2 weeks of feeding, a solution of 1 mg/kg adriamycin (the purity higher than 98.0 (HPLC, Sigma, USA) was injected by intraperitoneal injection, with an interval time of two days, and a total of 3 injections for the animals in the two groups. Clinical manifestations of all the animals were observed and recorded daily. After feeding for 4 weeks, tail vein blood was collected, the number of the leukemia cells was calculated in the blood smears, and expressions of human mdr and bcr/abl mRNA were determined by RT-PCR (the same method as above). Protein expressions of the mdr and bcr/abl genes were also detected by Western Blot. The percentages of apoptotic cells were determined for the two groups (the mice treated with adriamycin group and the mice treated with CA combined with adriamycin group) by flow cytometry analysis. Each dead animal’s organs including lung, liver, kidney and bone marrow were fixed using 10% formalin and histopathologically detected. Overall animal survival was estimated by Kaplan-Meier analysis.
Statistical analysis
Quantitative data in the study fit the normal distribution. Continuous variables were expressed as mean ± SD (standard deviation). Comparing with those in the control group, the difference in mean of apoptosis cells in the experiment group was analyzed by Student’s t test. The percent of leukemia cells in peripheral blood smears between the two groups tested by Chi-square. A log-rank (Wilcoxon) test was used to determine significant differences between survival curves generated by the Kaplan-Meier method. P values of <0.05 (two-sided) were considered statistically significant. All data analysis was performed using SPSS19.0 (SPSS Inc., USA).
Results
Determination of the established K562/A02/SCID leukemia mouse model
Two weeks after inoculation of K562/A02 cells in the NOD/SCID mice, a large number of infiltrated K562/A02 cells were found in the peripheral blood, bone marrow smears, and slices of neck lymph nodes and liver of mice (Figure 1).
Figure 1.
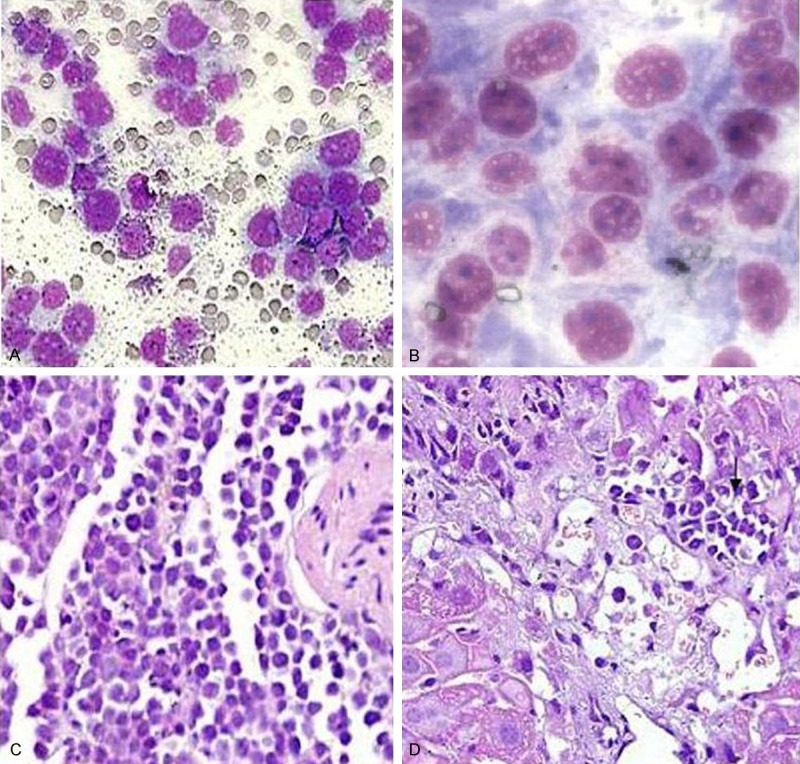
The human K562/A02 cells of mice tissue biopsies. Note: A: Peripheral blood smear; B: Bone marrow smear (×1000); C: Lymph node; D: Liver (Same magnificence in A, C and D, ×400).
Expressions of human mdr1 and bcr/abl mRNA in the experimental animals were nearly at the same levels as in the positive control (Figure 2). These specific markers of human leukemia cells indicated that human K562/AO2 leukemia cells grew in the mouse model.
Figure 2.
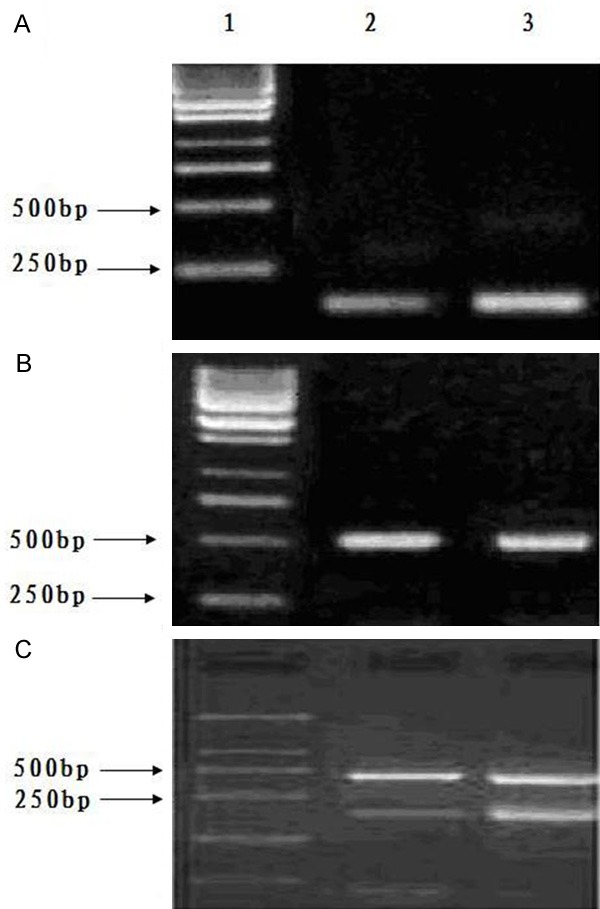
Expressions of mdr1, bcr/abl mRNA, and β-actin. A: mdr1; B: β-actin; C: β-actin and bcr/abl; 1. Marker; 2. Experimental model group; 3. Positive control group. Note: Expressions of mdr1 and bcr/abl mRNA were at the same basic levels for the experimental model group and the positive control group.
Animal assay of increasing curative effect of leukemia with treatment with CA combined with adriamycin
Much more leukemia cells were in the peripheral blood and bone marrow smears. In peripheral blood smears the percentages of visible leukemia cells in 200 white cell counts were 20.0% and 32.5% for the CA-treated and the control groups respectively, and the difference was statistically significant.
The percentages of apoptotic cells of mice treated with single adriamycin (15.5±1.56) and the mice treated with CA combined with adriamycin (11.2±1.80) have a significant difference (P<0.05) (Figure 3).
Figure 3.
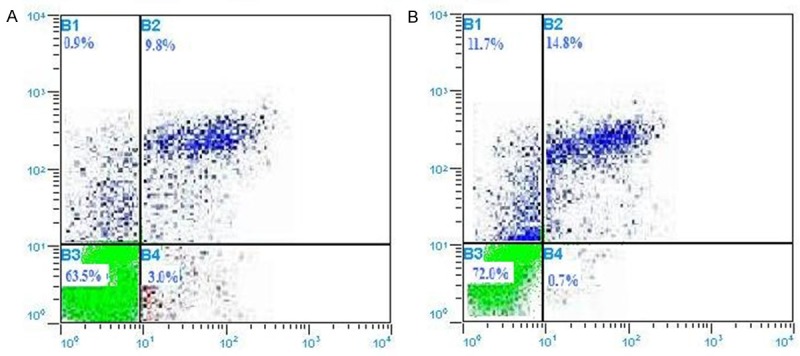
The distribution of percentages of apoptosis (A) for mice treated with adriamycin (control group); (B) for the mice treated with CA combined with adriamycin (experimental group) tested by flow cytometry. The percentages of apoptotic cells of A group (15.5±1.56) and B group (11.2±1.80) has a significant difference (P<0.05).
Expressions levels of mdr1 and bcr-abl mRNA and protein in the experimental animals were significantly lower than that of the control animals (Figures 4 and 5).
Figure 4.
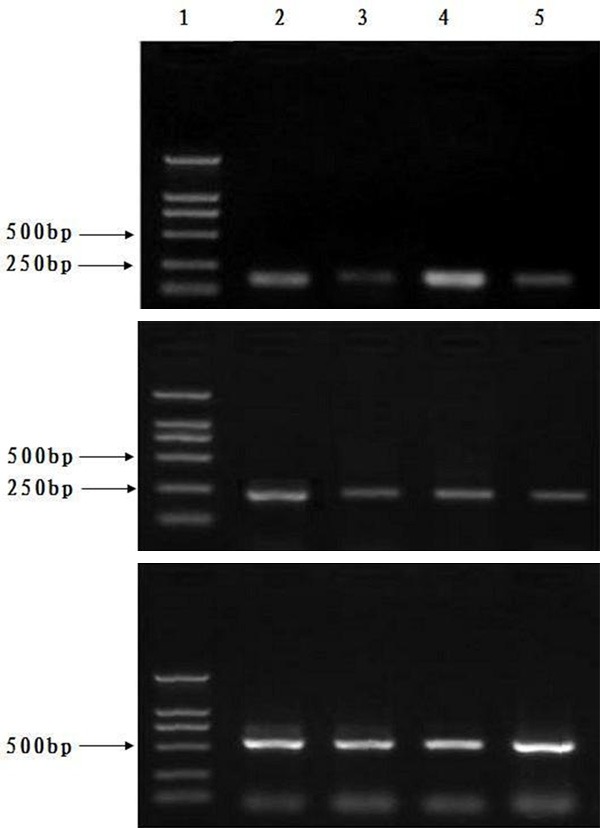
Expressions levels of mdr1 and bcr-abl mRNA in the experimental animals were significantly lower than in the control animals. A: mdr1; B: bcr/abl; C: β-actin; line 1, Marker; line 2 and 4, control animal group; line 3 and 5, treated animal group. Note: Compared with the mice treated with adriamycin (control group), expression levels of human mdr1 and bcr/abl mRNA declined in mice treated with CA combined with adriamycin (experimental group).
Figure 5.
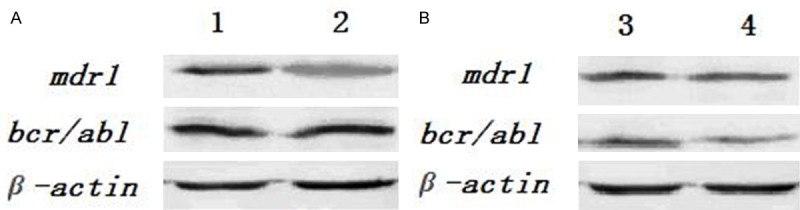
Protein expression of mdr1, bcr/abl, and β-actin genes by Western blot determination in the experimental animals were significantly lower than in the control animals. Note: A: Line 1 for the established animal model, line 2 for the positive control; B: Line 3 for mice treated by adriamycin (control group), line 4 for mice treated by CA combined with adriamycin (experimental group).
In the slices of lung, liver, kidney and bone marrow, the pathological changes have no significant difference, but micro-vessels show much more leukemia cells in the control animals than those in the CA-treated animals (Figure 6).
Figure 6.
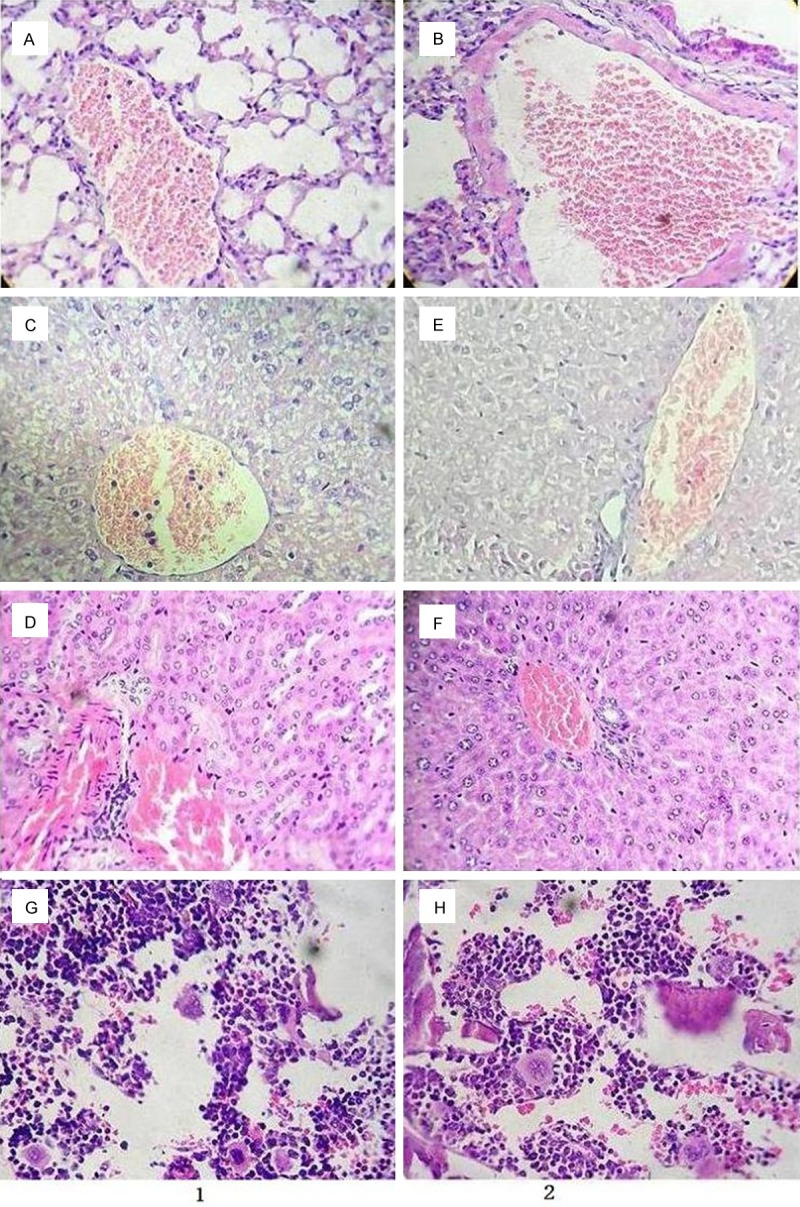
The outcome of pathological slices examination for the treatment and control groups. Note: Line 1 for the mice treated with adriamycin (control group); A: Lung; C: Liver; D: Kidney; G: Bone marrow; Line 2 for the mice treated with CA combined with adriamycin (experimental group): B: Lung; E: Liver; F: Kidney; H: Bone marrow (Same magnificence in A-H, ×400).
The median of 95% CI survival time is 19 (10.0-44.2) and 33 (29.4-36.6) days for the control group and the CA-treated group, respectively. The difference is statistically significant (P<0.05) (Table 1; Figure 7).
Table 1.
Survival analysis for the treatment and control groups*
| Group | Mean (day) | 95% CI | Median (day) | 95% CI |
|---|---|---|---|---|
| Control | 19.5 | 9.3-29.6 | 19.0 | 10.0-44.2 |
| Treatment | 33.0 | 31.5-34.5 | 33.0 | 29.4-36.6 |
CI, confidence interval; Generalized Wilcoxon test: Breslow=5.60, P=0.018.
Figure 7.
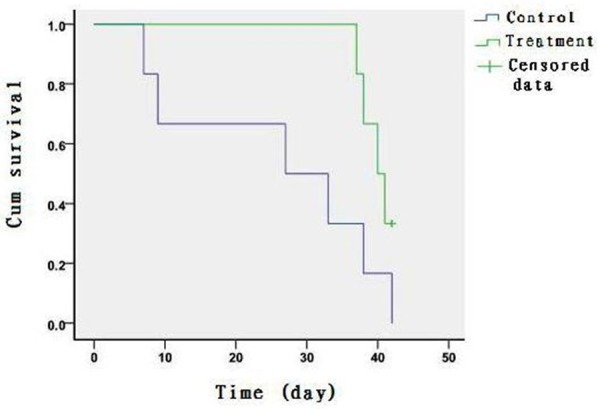
Kaplan-Meier curves of the survival time (day) in the mice treated with adriamycin or CA combined with adriamycin.
Discussion
The K562 cell line is of human chronic myeloid leukemia cells with positive expression of the bcr-abl gene. The multidrug-resistance (MDR) leukemia cell line K562/AO2 is established through in vitro selection of K562 cells with an increasing concentration of adriamycin. The cells with the characteristic of over-expressing the mdr1 gene are a tool for in vitro research of MDR [19]. In this work the K562/A02/SCID mouse model is established on the basis of the previous method [20]. Positive expression of mdr and bcr/abl mRNA and the outcome of pathological tests confirm that the animal model is successfully established.
CA is an extract from rosemary (Rosmarinus officinalis L.). Its antitumor or antineoplastic activities on different types of cancer cell lines/rat or mice models have been studied [5]. However, the anticancer mechanism of CA is not clear yet. From research of outcomes of all rosemary’s extracts, the mechanism involves probably the apoptosis, carcinogen metabolic enzymes, or inhibition of tumor promoting events [15,16]. In our previous work, we found that in vitro assay CA decreased the viability of the human promyelocytic leukemia cell line HL-60, in dose- and time-dependent manners, and induced G1 arrest and apoptosis. And these effects of CA were augmented when induced by a low (physiological) concentration of As2O3, which was associated with the up-regulation of p27 and activation of caspase-9. The mechanism was found to be mediated by the induction of phosphatase and tensin homologue (PTEN) expression [18]. The results of in vivo study in this work are consistent with the previous in vitro experimental results. Therefore, it is reasonable to conclude from both in vitro and in vivo results that CA is a promising adjuvant chemical drug candidate in AML therapy.
MDR is mainly caused by a cancer cell membrane of P-glycoprotein (P-gp), which can pump the anti-cancer drug out of the cell, and therefore reduce the intracellular drug concentration and trigger MDR. The basic strategy to reverse MDR is adopting the low toxicity or non-toxic compounds to bind P-gp and block its transport function [21].
Increasing interest in CA is due to its pharmacological properties. However, no report was found on its safety as a medical drug. In our previous work we evaluated the medical security of CA. The results showed that the oral lethal dose (LD50) for mice was 7100 mg/kg of body weight in the acute toxicity study. Histopathological changes were observed in the heart and liver of all surviving mice treated with single-dose CA, and in the kidney with inflammatory cell infiltration for the surviving mice treated with doses larger than 7500 mg/kg and over of CA. For the sub-chronic toxicity study, CA was administered for 30 days to produce slight reductions in the weight gain pattern, which did not reach a significant level comparing with the control values. For blood and serum biochemistry tests and histopathological examination of the animal organs, the effects of low-dose CA exposure on the experimental rats have no observable adverseness. The results suggest that the clinical dose of CA has relatively very low toxicity [22].
The results of the present study indicate that CA can prevent the growth of tumors with minor side effects. It is demonstrated that rosemary extracts inhibit the glycolysis and ATP synthesis in tumor cells in culture, except normal cells [23]. The molecular mechanism of this effect is not understood completely yet. In fact, the study of the mechanism on how CA differentially affects the normal and tumor cell energy metabolism may help to explain the basic differences, and suggest a potential new pathway to fight cancers without negative effects on normal cells [24]. CA can improve a protective immune reaction. Natural killer (NK) cells and type I interferons (IFNab), tumor necrosis factor (TNF), IL-12, IL-10, and other cytokines play a major role in the innate control of immune-system replication. Notably, enhancing induction of inflammation factor over-expression is via a nuclear factor-kappa B (NF-κB) dependent mechanism [25]. Recently, studies have found that CA inhibits the prostaglandin E (2) (PGE (2)), an inflammatory and tumorigenic agent, formation [26]. Our previous work determines that the strong synergistic effect of inhibiting leukemia cell activities in CA and As2O3 combined treatment was associated with high expressions of cleaved caspase-3, PTEN, and p27 gene proteins [17,18].
As compared with all chemotherapy drugs, adriamycin is highly toxic and poorly tolerated by patients. The present work indicates that CA combined with the current chemotherapeutic drugs could reverse MDR and reduce the dose of clinical AML therapy. However, the limitation of evidence does not fit the preclinical study. Further research on the mechanism for CA combined with other chemical drugs in the treatment of leukemia is necessary.
Conclusion
In the present work we confirmed that CA combined with adriamycin can significantly extend the survival time in the K562/AO2 leukemia animal model. CA is a promising adjuvant chemical drug candidate for clinical AML therapy.
Acknowledgements
Thanks to Dr. Edward C. Mignot (Shandong University) for linguistic advice. This work was supported by grants from the 2006 Doctor Fund Projects of Shandong Province of China (No. 2006BS03061) and a major research project of Shandong Province in 2010 (No. 2010GSF10260).
Disclosure of conflict of interest
None.
References
- 1.Lengfelder E, Hanfstein B, Haferlach C, Braess J, Krug U, Spiekermann K, Haferlach T, Kreuzer KA, Serve H, Horst HA, Schnittger S, Aul C, Schultheis B, Erben P, Schneider S, Müller-Tidow C, Wörmann B, Berdel WE, Sauerland C, Heinecke A, Hehlmann R, Hofmann WK, Hiddemann W, Büchner T German Acute Myeloid Leukemia Cooperative Group (AMLCG) Outcome of elderly patients with acute promyelocytic leukemia: results of the German Acute Myeloid Leukemia Cooperative Group. Ann Hematol. 2013;92:41–52. doi: 10.1007/s00277-012-1597-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Latagliata R, Breccia M, Fazi P, Vignetti M, Di Raimondo F, Sborgia M, Vincelli D, Candoni A, Salvi F, Rupoli S, Martinelli G, Kropp MG, Tonso A, Venditti A, Melillo L, Cimino G, Petti MC, Avvisati G, Lo-Coco F, Mandelli F GIMEMA Acute Leukaemia Working Party. GIMEMA AIDA 0493 amended protocol for elderly patients with acute promyelocytic leukaemia. Long-term results and prognostic factors. Br J Haematol. 2011;154:564–568. doi: 10.1111/j.1365-2141.2011.08593.x. [DOI] [PubMed] [Google Scholar]
- 3.Ono T, Takeshita A, Kishimoto Y, Kiyoi H, Okada M, Yamauchi T, Tsuzuki M, Horikawa K, Matsuda M, Shinagawa K, Monma F, Ohtake S, Nakaseko C, Takahashi M, Kimura Y, Iwanaga M, Asou N, Naoe T Japan Adult Leukemia Study Group. Long-term outcome and prognostic factors of elderly patients with acute promyelocytic leukemia. Cancer Sci. 2012;103:1974–1978. doi: 10.1111/j.1349-7006.2012.02390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xia CQ, Smith PG. Drug efflux transporters and multidrug resistance in acute leukemia: therapeutic impact and novel approaches to mediation. Mol Pharmacol. 2012;82:1008–1021. doi: 10.1124/mol.112.079129. [DOI] [PubMed] [Google Scholar]
- 5.Ngo SN, Williams DB, Head RJ. Rosemary and cancer prevention: preclinical perspectives. Crit Rev Food Sci Nutr. 2011;51:946–954. doi: 10.1080/10408398.2010.490883. [DOI] [PubMed] [Google Scholar]
- 6.Anderson D, Cheng Y, Duan RD. Ursolic acid inhibits the formation of aberrant crypt foci and affects colonic sphingomyelin hydrolyzing enzymes in azoxymethane-treated rats. J Cancer Res Clin Oncol. 2008;134:101–107. doi: 10.1007/s00432-007-0255-4. [DOI] [PubMed] [Google Scholar]
- 7.Moran AE, Carothers AM, Weyant MJ, Redston M, Bertagnolli MM. Carnosol inhibits beta-catenin tyrosine phosphorylation and prevents adenoma formation in the C57BL/6J/Min/ + (Min/+) mouse. Cancer Res. 2005;65:1097–1104. [PubMed] [Google Scholar]
- 8.Singletary K, MacDonald C, Wallig M. Inhibition by rosemary and carnosol of 7, 12-dimethylbenz[a] anthracene (DMBA)-induced rat mammary tumorigenesis and in vivo DMBA-DNA adduct formation. Cancer Lett. 1996;104:43–48. doi: 10.1016/0304-3835(96)04227-9. [DOI] [PubMed] [Google Scholar]
- 9.Amagase H, Sakamoto K, Segal ER, Milner JA. Dietary rosemary suppresses 7, 12-dimethylbenz(a)anthracene binding to rat mammary cell DNA. J Nutr. 1996;126:1475–1480. doi: 10.1093/jn/126.5.1475. [DOI] [PubMed] [Google Scholar]
- 10.Sharabani H, Izumchenko E, Wang Q, Kreinin R, Steiner M, Barvish Z, Kafka M, Sharoni Y, Levy J, Uskokovic M, Studzinski GP, Danilenko M. Cooperative antitumor effects of vitamin D3 derivatives and rosemary preparations in a mouse model of myeloid leukemia. Int J Cancer. 2006;118:3012–3021. doi: 10.1002/ijc.21736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang MT, Ho CT, Wang ZY, Ferraro T, Lou YR, Stauber K, Ma W, Georgiadis C, Laskin JD, Conney AH. Inhibition of skin tumorigenesis by rosemary and its constituents carnosol and ursolic acid. Cancer Res. 1994;54:701–708. [PubMed] [Google Scholar]
- 12.Huang SC, Ho CT, Lin-Shiau SY, Lin JK. Carnosol inhibits the invasion of B16/F10 mouse melanoma cells by suppressing metalloproteinase-9 through down-regulating nuclear factor-kappaB and c-Jun. Biochem Pharmacol. 2005;69:221–232. doi: 10.1016/j.bcp.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 13.Huang HC, Huang CY, Lin-Shiau SY, Lin JK. Ursolic acid inhibits IL-1beta or TNF-alpha-induced C6 glioma invasion through suppressing the association ZIP/p62 with PKC-zeta and downregulating the MMP-9 expression. Mol Carcinog. 2009;48:517–531. doi: 10.1002/mc.20490. [DOI] [PubMed] [Google Scholar]
- 14.Cheung S, Tai J. Anti-proliferative and antioxidant properties of rosemary Rosmarinus officinalis. Oncol Rep. 2007;17:1525–1531. [PubMed] [Google Scholar]
- 15.Costa S, Utan A, Speroni E, Cervellati R, Piva G, Prandini A, Guerra MC. Carnosic acid from rosemary extracts: a potential chemoprotective agent against aflatoxin B1. An in vitro study. J Appl Toxicol. 2007;27:152–159. doi: 10.1002/jat.1186. [DOI] [PubMed] [Google Scholar]
- 16.Thomas X, Pigneux A, Raffoux E, Huguet F, Caillot D, Fenaux P. Superiority of an arsenic trioxide-based regimen over a historic control combined all-trans retinoic acid plus intensive chemotherapy in the treatment of relapsed acute promyelocytic leukemia. Haematologica. 2006;91:996–997. [PubMed] [Google Scholar]
- 17.Yu XN, Chen XL, Li H, Li XX, Li HQ, Jin WR. Reversion of P-glycoprotein-mediated multidrug resistance in human leukemic cell line by carnosic acid. Chin J Physiol. 2008;51:348–356. [PubMed] [Google Scholar]
- 18.Wang R, Li H, Guo G, Li X, Yu X, Li H, Wang J, Liu F, Chen X. Augmentation by carnosic acid of apoptosis in human leukaemia cells induced by arsenic trioxide via upregulation of the tumour suppressor PTEN. J Int Med Res. 2008;36:682–690. doi: 10.1177/147323000803600409. [DOI] [PubMed] [Google Scholar]
- 19.Heaney NB, Pellicano F, Zhang B, Crawford L, Chu S, Kazmi SM, Allan EK, Jorgensen HG, Irvine AE, Bhatia R, Holyoake TL. Bortezomib induces apoptosis in primitive chronic myeloid leukemia cells including LTC-IC and NOD/SCID repopulating cells. Blood. 2010;115:2241–2250. doi: 10.1182/blood-2008-06-164582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park J, Kim KI, Koh Y, Wond NH, Oh JM, Leee DS, Byoung Kim K, Ahn KS, Yoon SS. Establishment of a new Glivec-resistant chronic myeloid leukemia cell line, SNUCML-02, using an in vivo model. Exp Hematol. 2010;38:773–781. doi: 10.1016/j.exphem.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 21.Ford JM, Hait WN. Pharmacology of drugs that alter multidrug resistance in cancer. Pharmacol Rev. 1990;42:155–199. [PubMed] [Google Scholar]
- 22.Wang QL, Li H, Li XX, Cui CY, Wang R, Yu NX, Chen XL. Acute and 30-day oral toxicity studies of administered carnosic acid. Food Chem Toxicol. 2012;50:4348–4355. doi: 10.1016/j.fct.2012.08.057. [DOI] [PubMed] [Google Scholar]
- 23.Renner C, Asperger A, Seyffarth A, Meixensberger J, Gebhardt R, Gaunitz F. Carnosine inhibits ATP production in cells from malignant glioma. Neurol Res. 2010;32:101–105. doi: 10.1179/016164109X12518779082237. [DOI] [PubMed] [Google Scholar]
- 24.Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25:4633–4646. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
- 25.Eickhoff J, Hanke M, Stein-Gerlach M, Kiang TP, Herzberger K, Habenberger P, Müller S, Klebl B, Marschall M, Stamminger T, Cotten M. RICK activates a NF-kappaB-dependent anti-human cytomegalovirus response. J Biol Chem. 2004;279:9642–9652. doi: 10.1074/jbc.M312893200. [DOI] [PubMed] [Google Scholar]
- 26.Barni MV, Carlini MJ, Cafferata EG, Puricelli L, Moreno S. Carnosic acid inhibits the proliferation and migration capacity of human colorectal cancer cells. Oncol Rep. 2012;27:1041–1048. doi: 10.3892/or.2012.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]


