Abstract
The incidence of non-small-cell lung cancer among elderly patients has increased; therefore, older patients are increasingly being considered for radical pulmonary resection. However, data regarding the outcome of video-assisted thoracoscopic surgery (VATS) in elderly patients are limited. The aim of this study was to evaluate the safety and feasibility of VATS in elderly patients with non-small-cell lung cancer. From January 2008 to January 2014, a total of 78 patients aged ≥ 70 years (elderly group) undergoing VATS for NSCLC were matched with 78 patients < 70 years (young group) by demographics, tumor characteristics, and details of surgical procedures. The elderly group was characterized by a higher incidence of hypertension (P = 0.001) and diabetes mellitus (P = 0.014), and ≥ 2 comorbidities (P = 0.009). Intraoperative variables, such as surgical duration blood loss, and transfusion rate, were not notably different between the groups. Postoperative 30-day mortality, 30-day complications, and 30-day major complications were comparable between the groups. The 5-year overall survival rates were 69% in the young group and 64% in the elderly group, respectively (P = 0.258). The 5-year disease-free survival rates were 65% in the young group and 60% in the elderly group, respectively (P = 0.327). Our results clearly demonstrated that VATS for non-small-cell lung cancer could be safely and efficacy performed in elderly patients; thus, advanced age itself should not be regarded as a contraindication for VATS.
Keywords: Non-small cell lung cancer, pulmonary resection, video-assisted thoracoscopic surgery, elderly patients
Introduction
Among Eastern Asia countries, the population older than 80 years has continuously increased during the last century. Meanwhile, the rising incidence of non-small-cell lung cancer has also resulted in a dramatic increase in the number of elderly patients considered for pulmonary resection [1-4]. However, elderly patients represent only 10-20% of those considered for radical resection, reflecting a high proportion of patients contraindicated for curative treatment [5]. Indeed, elderly patients present more frequently with comorbidities, particularly cardiovascular and pulmonary diseases; thus, the benefit-to-risk ratio continues to leave many clinicians reluctant to propose radical pulmonary resection for treatment of elderly patients with non-small-cell lung cancer [6]. However, advances in surgical skill and intensive care have reduced age related contraindications for radical pulmonary resection and enabled increasing numbers of patients to undergo radical pulmonary resection. In addition, recent studies have suggested that age did not appear to be a risk factor influencing short- or long-term outcomes after resection of non-small-cell lung cancer [7-9]. In light of these findings, elderly patients with non-small-cell lung cancer are increasingly being considered for treatment strategies similar to those of their younger counterparts. Video-assisted thoracoscopic surgery (VATS) pulmonary resection has greatly evolved during the past several years. Several reports indicated that VATS pulmonary resection is associated with less blood loss, lower use of narcotics, and shorter hospital stay, with no difference in complication rates or oncological outcomes compared with open resection [10-16]. However, to date, there exists very limited evidence on the short and long-term outcomes after VATS pulmonary resection in elderly patients (≥ 70 years). Therefore, the objective of this retrospective study was to assess the influence of age on postoperative and oncological outcomes after VATS pulmonary resection.
Patients and methods
Study population
This study complied with the Declaration of Helsinki. This retrospective research was approved by our local ethics committees. The need for informed consent from patients was waived because of its retrospective nature.
Data of all consecutive patients who underwent VATS pulmonary resection for non-small-cell lung cancer at our institution from January 2008 to January 2014 were retrieved from a prospective database for this retrospective study. Patients with an incomplete clinical data or insufficient follow-up (< 6 months) were excluded from analysis, and those that met the inclusion criteria were divided into two age groups: ≥ 70 years (elderly group, n = 78) and < 70 years (young group n = 78). For comparisons, the 78 elderly patients undergoing VATS pulmonary resection were individually matched to one control patient according to demographics data, tumor characteristics, and details of surgical procedures based on the following criteria: gender, American Society of Anesthesiologists (ASA) score, tumor location, clinical tumor TNM stage, and extent of pulmonary resection.
Preoperative evaluation
All patients underwent bronchoscopy, endobronchial ultrasound, computed tomographic scans of brain, chest, and upper abdomen to determine the clinical stage. Mediastinoscopy was not routinely performed except positive mediastinal or hilar lymph node on chest computed tomographic scan. Positron emission tomography-computerized tomography (PET-CT) and bone scanning was performed when necessary. The tumor TNM stage was based on the 7th edition of the TNM [17] classification of lung cancer, which was proposed by International Association for the Study of Lung Cancer (IASLC), Union for International Cancer Control (UICC) and American Joint Committee on Cancer (AJCC), and the mediastinal lymph node staging was based on the newest lymph node map proposed by IASLC [18]. For those of the patients operated before 2010, their TNM stage was recalculated to match the 7th edition of TNM classification of lung cancer proposed by UICC, AJCC and IASLC [19].
Surgical technique
All resections were performed with curative intent. The surgical procedure has been described elsewhere [11]. The indications of VATS pulmonary resection were as follows: (1) tumors located in the peripheral area; (2) no neoadjuvant therapy; (3) patients with clinical stage I non-small-cell lung cancer before operation; and (4) no extended resection. All surgical data, including type of resection, blood loss, blood transfusion, duration of resection and intraoperative complications were reviewed in the medical chart.
Short-term outcomes
Postoperative complications were classified according to the Clavien-Dindo classification [20], which defines major complications by a score of ≥ 3 [20]. If the patient had two or more complications, the most severe was selected for analysis. Both postoperative complications and mortality were considered as those occurring within 30 days of VATS pulmonary resection. Pathological resection margin was classified as R0 or R1 according to protocol by UICC [17].
Long-term data
After discharge, patients underwent blood test every 3 months and chest CT scan every 6 months. If chest symptoms or susceptible cancer recurrence occurred, an additional examination was carried out. The overall survival was assessed from the date of VATS pulmonary resection until the last follow up or death of any cause. The disease-free survival was calculated from the date of VATS pulmonary resection until the date of cancer recurrence or death from any cause. Tumor recurrence was diagnosed by history, physical examination, endoscopic evaluation, radiologic investigations or pathology when available. The last follow up was January 2015.
Statistical analysis
Data were analyzed by t test. For data following non-normal distribution, results were expressed as median and range and were compared by nonparametric test. Differences of semiquantitative results were analyzed by Mann-Whitney U-test. Differences of qualitative results were analyzed by chi-square tests or Fisher exact test as appropriate. P < 0.05 was considered statistically significant. SPSS 14.0 (SPSS Inc., Chicago, IL, USA) was applied.
Results
Clinicopathologic characteristics
Clinical characteristics are detailed in Table 1. There were 78 and 78 patients in the elderly and young groups, respectively. The groups were well matched for gender, ASA grade, tumor location, clinical tumor TNM stage, and extent of pulmonary resection. However, the elderly group included patients characterized by more hypertension (P = 0.001) and diabetes mellitus (P = 0.014). Moreover, the proportion of patients with two or more comorbidities was significantly higher in the elderly group than in the young group (P = 0.009). There was no significant difference in tumor location, clinical tumor TNM stage, and extent of pulmonary resection. There were no significant differences in blood loss, surgical duration, and transfusion rate. In addition, there was no notable difference in the incidence of R0 resection between the groups (Table 2).
Table 1.
Clinicopathologic characteristics of the two groups
| Young (n = 78) | Elderly (n = 78) | P value | |
|---|---|---|---|
| Age (y) | 58 (41-68) | 73 (70-82) | 0.000 |
| Gender (Male: Female) | 74:49 | 51:38 | 0.676 |
| Comorbidity | |||
| Copd | 1 | 2 | 1.000 |
| Hypertension | 3 | 16 | 0.001 |
| Diabetes Mellitus | 2 | 10 | 0.014 |
| Stable angina | 1 | 2 | 1.000 |
| Chronic atrial fibrillation | 3 | 1 | 0.612 |
| Chronic renal inadequacy | 2 | 1 | 1.000 |
| FEV1 (observed to predicted) % | 87 (77-97) | 85 (75-95) | 0.321 |
| Two or more comorbidities | 2 | 11 | 0.009 |
| Clinical stage | 0.632 | ||
| IA | 41 | 38 | |
| IB | 37 | 40 | |
| ASA score | 0.181 | ||
| I | 65 | 58 | |
| II | 8 | 13 | |
| III | 5 | 7 | |
| Tumor location | 0.972 | ||
| Left upper lobe | 25 | 23 | |
| Left lower lobe | 23 | 25 | |
| Right upper lobe | 16 | 17 | |
| Right lower lobe | 14 | 13 | |
| Extent of pulmonary resection | 0.822 | ||
| Lobectomy | 35 | 32 | |
| Segmentectomy | 27 | 27 | |
| Wedge resection | 16 | 19 | |
| Blood loss (ml) | 160 (120-450) | 170 (130-420) | 0.258 |
| Surgical duration (min) | 210 (170-260) | 200 (160-270) | 0.354 |
| Transfusion rate (%) | 5 (6.4) | 8 (10.3) | 0.385 |
Table 2.
Pathological data of the two groups
| Young (n = 78) | Elderly (n = 78) | P value | |
|---|---|---|---|
| Histological type | 0.862 | ||
| Adenocarcinoma | 47 | 45 | |
| Squamous cell carcinoma | 21 | 24 | |
| Large cell carcinoma | 10 | 9 | |
| Pathological stage | 0.392 | ||
| IA | 23 | 26 | |
| IB | 36 | 32 | |
| IIA | 9 | 10 | |
| IIB | 5 | 6 | |
| IIIA | 5 | 4 | |
| Residual tumor | 0.156 | ||
| R0 | 78 | 76 | |
| R1 | 0 | 2 | |
| R2 | 0 | 0 |
Mortality and morbidity
No patient died within 30 days after the pulmonary resection. Postoperative 30-day complications occurred in 21.8% of patients aged ≥ 70 years and in 18.0% of the young patients (P = 0.547). We did not observe difference between the elderly and young groups in terms of postoperative hospital stay (Table 3). The severity of 30-day complications, according to Clavien-Dindo criteria, was comparable between the two groups (Table 3).
Table 3.
Post-operative course of the two groups
| Young (n = 78) | Elderly (n = 78) | P value | |
|---|---|---|---|
| Post-operative complications | 14 | 17 | 0.547 |
| Severity of complications | 0.710 | ||
| Major (3, 4 and 5) | 3 | 5 | |
| Minor (1 and 2) | 11 | 12 | |
| Major | - | ||
| Pulmonary embolism | 0 | 1 | |
| Acute coronary syndrome | 1 | 2 | |
| Respiratory insufficiency | 1 | 1 | |
| Acute heart failure | 1 | 0 | |
| Minor | - | ||
| Pneumonia | 3 | 5 | |
| Acute renal failure | 1 | 2 | |
| Urinary tract infection | 1 | 1 | |
| Atrial fibrillation | 1 | 1 | |
| Chylothorax | 1 | 1 | |
| Recurrent nerve palsy | 1 | 1 | |
| Prolonged air leak (more than 5 days) | 3 | 1 | |
| postoperative hospital stay (d) | 10 (8-21) | 12 (11-25) | 0.368 |
Tumor recurrence and survival
After a median follow-up period of 39 months for the entire study population, 28.2% of the patients developed tumor recurrence in the elderly group compared with 21.8% in the young group (P = 0.355) (Table 4). The location of the recurrence and the time to recurrence were not significantly different between the two groups (Table 4). The 5-year overall survival rate was 64% in the elderly group and 69% in the young group (P = 0.258) (Figure 1). A 5-year disease-free survival rate of 60% was achieved in patients aged ≥ 70 years, which was not significantly different from that in the young patients (65%; P = 0.327) (Figure 2). Multivariate Cox regression analysis of overall survival showed that significant predictors of worse overall survival were pathologic N2 disease, lymphatic invasion and poor tumor differentiation (Table 5). Significant predictors of worse disease-free survival were pathologic N1/N2 disease, and tumors with poor differentiation (Table 6).
Table 4.
Tumor recurrence pattern and site of the two groups
| Young (n = 78) | Elderly (n = 78) | P value | |
|---|---|---|---|
| Overall recurrence n (%) | 17 (21.8) | 22 (28.2) | 0.355 |
| Locoregional n (%) | 11 (14.1) | 14 (17.9) | - |
| Mediastinal lymph node | 5 | 6 | |
| Pleura | 2 | 5 | |
| Ipsilateral lung | 4 | 3 | |
| Distant n (%) | 6 (7.7) | 8 (10.3) | - |
| Brain | 3 | 3 | |
| Liver | 2 | 3 | |
| Adrenal | 1 | 2 | |
| Time to recurrence (median) | 21 months | 17 months | 0.082 |
Figure 1.
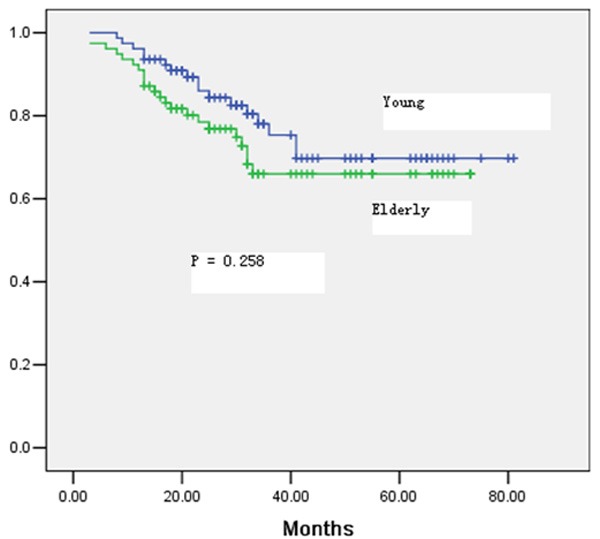
Overall survival for all patients in the elderly and young groups.
Figure 2.
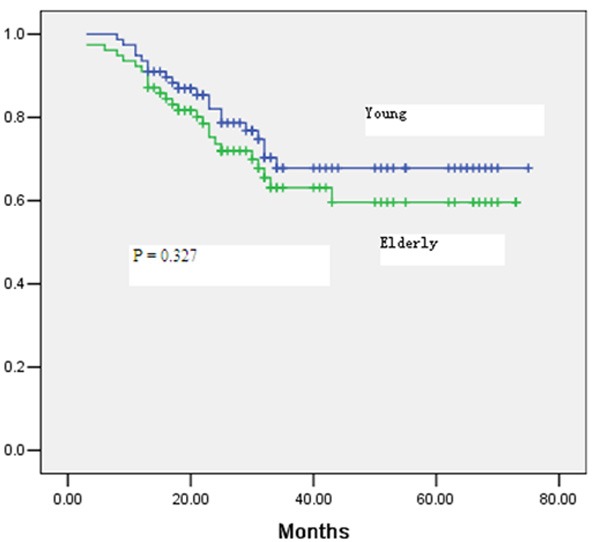
Disease-free survival of all patients in the elderly and young groups.
Table 5.
Multivariate Cox regression analyses of overall survival
| Regression variables | Adjusted hazard ratio | 95% CI | P value |
|---|---|---|---|
| Lymphatic invasion | |||
| No | 1.00 | ||
| Yes | 1.69 | 1.25-3.58 | 0.010 |
| Pathological N stage | |||
| N0 | 1.00 | ||
| N1 | 1.31 | 0.78-2.20 | 0.099 |
| N2 | 2.58 | 1.87-3.58 | 0.018 |
| Differentiation grade | |||
| Good | 1.00 | ||
| Moderate | 1.58 | 0.37-1.98 | 0.436 |
| Poor | 2.35 | 1.77-4.02 | 0.031 |
Table 6.
Multivariate Cox regression analyses of disease-free survival
| Regression variables | Adjusted hazard ratio | 95% CI | P value |
|---|---|---|---|
| Pathological N stage | |||
| N0 | 1.00 | ||
| N1 | 1.65 | 1.19-1.88 | 0.038 |
| N2 | 2.58 | 2.14-3.87 | 0.003 |
| Differentiation grade | |||
| Good | 1.00 | ||
| Moderate | 1.25 | 0.55-2.00 | 0.258 |
| Poor | 2.55 | 1.44-3.35 | 0.005 |
Discussion
Until now, elderly patients have been shown to have worse long-term outcomes compared to younger patients due to limited life expectancy and some comorbidities. In particular, elderly patients with comorbid conditions often have difficulty with anesthesia and postoperative recovery. For this reason, patients and their physicians are often reluctant to treat operable non-small-cell lung cancer surgically and tend to choose conservative or palliative management [21-23]. However, minimally invasive surgery has introduced in the field of pulmonary resection because of advances in surgical techniques. Patients treated with minimally invasive surgery have faster recovery because of smaller incisions, decreased pain, and less blood loss [24-26].
The progress of VATS has been very slow in Eastern Asia because of its technical difficulties due to less training in resident team, fear of inadequate lymphadenectomy, and suspicion of oncological inadequacy; however, VATS has been increasingly reported by several high-volume medical centers [24-26]. Our study was to design to evaluate short and long-term outcomes of elderly patients undergoing VATS for non-small-cell lung cancer. Our study clearly suggests that VATS pulmonary resection for non-small-cell lung cancer in elderly patients is safe and does not compromise oncological outcomes. Our results showed a similar mortality and morbidity rate and long-term outcomes compared with those of previous series [27-29].
Even though nearly 10% of the elderly patients were classified as ASA score III, the present series confirms that postoperative 30-day overall complication and major complication rates were similar between young and elderly patients. We did not observe any difference in postoperative respiratory failure between the two groups.
Pulmonary resection via thoracotomy approach may increase the risk of cardiopulmonary complications through several mechanisms. This surgical approach may result in a 40% reduction of the vital capacity and a 50% reduction in functional residual capacity. On the opposite, the VATS approach resulted in dramatically decreased surgical trauma since only 4 or 6 port incisions are performed, and the resected specimen is extracted through limited incisions. In this context, decreased postoperative pain and early postoperative recovery may therefore provide improved cardiopulmonary function recovery.
Regarding long-term outcomes, our study showed no difference in overall survival and disease-free survival between the two groups. In previous studies, the 5-year overall survival rates and disease-free survival rates in patients ≥ 70 years with clinical stage I non-small-cell lung cancer undergoing radical pulmonary resection were 54%-89% and 43-80%, respectively [30-32]. In some literatures, long-term outcomes of elderly patients was slightly less than that of young patients, but still acceptable [33-35]. The authors hypothesized that this difference might be due to more limited survival expectancy of the elderly patients and higher prevalence of comorbidity, which may due to no cancer-related death. In our series, the overall and disease-free survival was similar between the two groups. The reason was that clinical stage I non-small-cell lung cancer had acceptable prognosis and the follow up period was not very long, so some death related to cancer recurrence did not detected.
This study has several limitations. Firstly, this study is based on a single-center, not multiple-center and based on retrospective non-randomized analysis, not prospective randomized analysis. Secondly, the size of sample is small and the follow up period was very long, which should be taken into account when interpreting the results.
In conclusion, VATS pulmonary resection for non-small-cell lung cancer in elderly patients is feasible and results in acceptable perioperative complications and long-term outcomes that are similar to those in young patients, suggesting that advanced age itself should not be regarded as a contraindication for VATS pulmonary resection.
Acknowledgements
We sincerely thank the patients, their families and our hospital colleagues who participated in this research.
Discourse of conflict of interest
None.
References
- 1.Tan Q, Huang J, Ding Z, Lin H, Lu S, Luo Q. Meta-analysis for curative effect of lobectomy and segmentectomy on non-small cell lung cancer. Int J Clin Exp Med. 2014;7:2599–2604. [PMC free article] [PubMed] [Google Scholar]
- 2.Wang L, Liu Y, Xu S. Prognostic factors for surgically managed patients with stage II non-small cell lung cancer. Int J Clin Exp Med. 2015;8:862–868. [PMC free article] [PubMed] [Google Scholar]
- 3.Luo X, Luo W, Lin C, Zhang L, Li Y. Andrographolide inhibits proliferation of human lung cancer cells and the related mechanisms. Int J Clin Exp Med. 2014;7:4220–4225. [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Z. Selection of chemotherapy for non-small cell lung cancer is facilitated by new therapeutic strategies. Int J Clin Exp Med. 2014;7:3833–3742. [PMC free article] [PubMed] [Google Scholar]
- 5.Froesch P, Martucci F, Györik S, Dutly AE, Cafarotti S. Management of non-small cell lung cancer in the elderly. Eur J Intern Med. 2014;25:888–894. doi: 10.1016/j.ejim.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 6.Bravo-Iñiguez C, Perez Martinez M, Armstrong KW, Jaklitsch MT. Surgical resection of lung cancer in the elderly. Thorac Surg Clin. 2014;24:371–381. doi: 10.1016/j.thorsurg.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Qiao D, Wang Z, Lu Y, Wen X, Li H, Zhao H. A retrospective study of risk and prognostic factors in relation to lower respiratory tract infection in elderly lung cancer patients. Am J Cancer Res. 2014;5:423–432. [PMC free article] [PubMed] [Google Scholar]
- 8.Miyazaki T, Yamasaki N, Tsuchiya T, Matsumoto K, Kunizaki M, Taniguchi D, Nagayasu T. Inflammation-based scoring is a useful prognostic predictor of pulmonary resection for elderly patients with clinical stage I non-small-cell lung cancer. Eur J Cardiothorac Surg. 2015;47:e140–145. doi: 10.1093/ejcts/ezu514. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z, Zhang J, Cheng Z, Li X, Wang Z, Liu C, Xie Z. Factors affecting major morbidity after video-assisted thoracic surgery for lung cancer. J Surg Res. 2014;192:628–634. doi: 10.1016/j.jss.2014.07.051. [DOI] [PubMed] [Google Scholar]
- 10.Lee HS, Jang HJ. Thoracoscopic mediastinal lymph node dissection for lung cancer. Semin Thorac Cardiovasc Surg. 2012;24:131–141. doi: 10.1053/j.semtcvs.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Ramos R, Girard P, Masuet C, Validire P, Gossot D. Mediastinal lymph node dissection in early-stage non-small cell lung cancer: totally thoracoscopic vs thoracotomy. Eur J Cardiothorac Surg. 2012;41:1342–1348. doi: 10.1093/ejcts/ezr220. discussion 1348. [DOI] [PubMed] [Google Scholar]
- 12.Yang H, Li XD, Lai RC, She KL, Luo MH, Li ZX, Lin YB. Complete mediastinal lymph node dissection in video-assisted thoracoscopic lobectomy versus lobectomy by thoracotomy. Thorac Cardiovasc Surg. 2013;61:116–123. doi: 10.1055/s-0031-1299589. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe A, Koyanagi T, Obama T, Ohsawa H, Mawatari T, Takahashi N, Ichimiya Y, Abe T. Systematic node dissection by VATS is not inferior to that through an open thoracotomy: a comparative clinicopathologic retrospective study. Surgery. 2005;27:745–752. doi: 10.1016/j.surg.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Palade E, Passlick B, Osei-Agyemang T, Günter J, Wiesemann S. Video-assisted vs open mediastinal lymphadenectomy for Stage I non-small-cell lung cancer: results of a prospective randomized trial. Eur J Cardiothorac Surg. 2013;44:244–249. doi: 10.1093/ejcts/ezs668. [DOI] [PubMed] [Google Scholar]
- 15.Fan X, Zhang Z, Liu Y. Therapeutic efficacy and prognosis in the treatment of lung cancer by video-assisted thoracoscopic surgery. Chin Med J (Engl) 2014;127:2096. [PubMed] [Google Scholar]
- 16.Battoo A, Jahan A, Yang Z, Nwogu CE, Yendamuri SS, Dexter EU, Hennon MW, Picone AL, Demmy TL. Thoracoscopic pneumonectomy: an 11-year experience. Chest. 2014;146:1300–1309. doi: 10.1378/chest.14-0058. [DOI] [PubMed] [Google Scholar]
- 17.Reck M, Heigener DF, Mok T, Soria JC, Rabe KF. Management of non-small-cell lung cancer: recent developments. Lancet. 2013;382:709–719. doi: 10.1016/S0140-6736(13)61502-0. [DOI] [PubMed] [Google Scholar]
- 18.Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P Members of IASLC Staging Committee. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2009;4:568–577. doi: 10.1097/JTO.0b013e3181a0d82e. [DOI] [PubMed] [Google Scholar]
- 19.Suda K, Sato K, Mizuuchi H, Kobayashi Y, Shimoji M, Tomizawa K, Takemoto T, Iwasaki T, Sakaguchi M, Mitsudomi T. Recent evidence, advances, and current practices in surgical treatment of lung cancer. Respir Investig. 2014;52:322–329. doi: 10.1016/j.resinv.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 21.Pei G, Zhou S, Han Y, Liu Z, Xu S. Risk factors for postoperative complications after lung resection for non-small cell lung cancer in elderly patients at a single institution in China. J Thorac Dis. 2014;6:1230–1238. doi: 10.3978/j.issn.2072-1439.2014.07.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sihoe AD, Van Schil P. Non-small cell lung cancer: when to offer sublobar resection. Lung Cancer. 2014;86:115–120. doi: 10.1016/j.lungcan.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Caprario LC, Strauss GM. The benefit of chemotherapy in elderly patients with small cell lung cancer. Expert Rev Anticancer Ther. 2014;14:645–657. doi: 10.1586/14737140.2014.901171. [DOI] [PubMed] [Google Scholar]
- 24.Murthy S. Video-assisted thoracoscopic surgery for the treatment of lung cancer. Cleve Clin J Med. 2012;79(Electronic Suppl 1):eS23–eS25. doi: 10.3949/ccjm.79.s2.05. [DOI] [PubMed] [Google Scholar]
- 25.Papiashvilli M, Stav D, Cyjon A, Haitov Z, Gofman V, Bar I. Lobectomy for non-small cell lung cancer: differences in morbidity and mortality between thoracotomy and thoracoscopy. Innovations (Phila) 2012;7:15–22. doi: 10.1097/IMI.0b013e3182566221. [DOI] [PubMed] [Google Scholar]
- 26.Zhong C, Yao F, Zhao H. Clinical outcomes of thoracoscopic lobectomy for patients with clinical N0 and pathologic N2 non-small cell lung cancer. Ann Thorac Surg. 2013;95:987–992. doi: 10.1016/j.athoracsur.2012.10.083. [DOI] [PubMed] [Google Scholar]
- 27.Edwards MA, Naunheim KS. Lobectomy by VATS: taking the plunge. Chest. 2014;146:246–2418. doi: 10.1378/chest.14-0349. [DOI] [PubMed] [Google Scholar]
- 28.Gajra A, Jatoi A. Non–small-cell lung cancer in elderly patients: a discussion of treatment options. J. Clin. Oncol. 2014;32:2562–2569. doi: 10.1200/JCO.2014.55.3099. [DOI] [PubMed] [Google Scholar]
- 29.Ogawa F, Miyata S, Nakashima H, Matsui Y, Shiomi K, Iyoda A, Satoh Y. Clinical impact of lung age on postoperative complications in non-small cell lung cancer patients aged > 70 y. J Surg Res. 2014;188:373–380. doi: 10.1016/j.jss.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 30.Ogawa F, Wang G, Matsui Y, Hara H, Iyoda A, Satoh Y. Risk factors for postoperative complications in the elderly with lung cancer. Asian Cardiovasc Thorac Ann. 2013;21:313–318. doi: 10.1177/0218492312457359. [DOI] [PubMed] [Google Scholar]
- 31.Beckett P, Tata LJ, Hubbard RB. Risk factors and survival outcome for non-elective referral in non-small cell lung cancer patients--analysis based on the National Lung Cancer Audit. Lung Cancer. 2014;83:396–400. doi: 10.1016/j.lungcan.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 32.Rueth NM, Parsons HM, Habermann EB, Groth SS, Virnig BA, Tuttle TM, Andrade RS, Maddaus MA, D’Cunha J. Surgical treatment of lung cancer: predicting postoperative morbidity in the elderly population. J Thorac Cardiovasc Surg. 2012;143:1314–1323. doi: 10.1016/j.jtcvs.2011.09.072. [DOI] [PubMed] [Google Scholar]
- 33.Yazgan S, Gürsoy S, Yaldiz S, Basok O. Outcome of surgery for lung cancer in young and elderly patients. Surg Today. 2005;35:823–827. doi: 10.1007/s00595-004-3035-7. [DOI] [PubMed] [Google Scholar]
- 34.Dexter EU, Jahangir N, Kohman LJ. Resection for lung cancer in the elderly patient. Thorac Surg Clin. 2004;14:163–171. doi: 10.1016/S1547-4127(04)00007-6. [DOI] [PubMed] [Google Scholar]
- 35.Chambers A, Routledge T, Pilling J, Scarci M. In elderly patients with lung cancer is resection justified in terms of morbidity, mortality and residual quality of life? Interact Cardiovasc Thorac Surg. 2010;10:1015–1021. doi: 10.1510/icvts.2010.233189. [DOI] [PubMed] [Google Scholar]


