Abstract
Objective: To compare the platelet to lymphocyte ratio (PLR) in normal people, benign prostatic hyperplasia (BPH) patients and prostate cancer (PCA) patients, and to explore the prognostic role of PLR in PCA. Methods: 155 normal people, 168 BPH patients and 103 PCA patients were enrolled. PCA patients were divided into PLR low value group (PLR<150) and PLR high value group (PLR≥150), and the difference of patients’ clinical characteristics between high value group and low value group was comparative studied.Results: The differences of PLR among normal people, BPH patients and PCA patients were statistically significant. In addition, platelet counts, neutrophil counts, PSA level, LDH level, AKP level, CRP level and alkaline phosphatase level were also significantly increased in PLR high value group, while the hemoglobin level was decreased. Besides, serious events such as coma during hospitalization were also more likely to appear in PLR high value group. PCA patients had an average follow-up of 3 years, and a total of 25 cases of patients died, including 11 (16.4%) cases in the PLR low value group, and 14 (38.9%) cases in PLR high value group with. Three years survival rate of patients in high value group was significantly reduced. Additionally, PLR was a possible risk factor associated with mortality, and an independent predictor of all-cause mortality during follow-up. Conclusion: PLR is significantly increased in PCA patients, and it is an independent predictor of 3-year mortality in PCA patients.
Keywords: Platelet to lymphocyte ratio, prostate cancer, prognosis, mortality, clinical significance
Introduction
Prostate cancer (PCA) is one of the most common malignant cancers, and is also the second biggest killer for males up-to-date [1]. According to statistics, the prevalence of PCA in Europe is as high as 214/100,000, and number of newly diagnosed cases each year is 2.6 million. In China, the prevalence of PCA is much lower than that of Europe and America, however with the prolonging of life expectancy and westernized diet spread, the prevalence and mortality of PCA are significantly rising [2,3]. Current treatments of PCA are: radical prostatectomy, castration treatment, castration plus anti-androgen treatment, endocrine treatment etc [4]. However, for undeveloped medical environment of China now, and the insignificant clinical manifestations of PCA in early stage, most of patients are developed to medium to late stage cancer when diagnosed. Thus looking for an early diagnostic marker of PCA has been one of the hot topics in the field. Currently, commonly used screening markers of PCA are serum prostate specific antigen (PSA) and its subtype, human kallikrein (HK), prostate specific membrane antigen (PSMA), early prostate cancer antigen (EPCA) and RNA markers etc [5-8], but those are not PCA specific tumor markers, and the sensitivity and specificity in early diagnosis are not satisfactory. Pathological, epidemiological and clinical evidences show that inflammatory plays an important role in cancer genesis. Rudolf Virchow first discovered leukocytes in tumor tissues in 1863, and made the connection of cancer and inflammatory reactions [9]. With the development of molecular biology technology, researchers further discovered tumor related inflammatory cells can secrete a number of inflammatory mediators and cytokines to promote tumor growth, invasion and metastasis, and influence patients’ prognosis. Also, these kinds of inflammatory reaction could be reflected through some blood indicators [10,11]. Sonpavdeet al. [12] discovered neutrophil to lymphocyte ratio (NLR) was closely related to the metastasis of PCA, that NLR≥5 is an important marker of PCA metastasis, decreasing follow-up survival rate. But Turkmen et al. [13] found platelet to lymphocyte ratio(PLR) was superior to NLR in predicting severity of inflammation, and Neofytou et al. [14] found PLR was better than NLR in the diagnosis of colorectal cancer. So, whether PLR has a better performance in predicting PCA or not, there are currently no confirmed reports yet.
This study aimed to compare the difference of PLR between healthy individuals, benign prostatic hyperplasia (BPH) patients and PCA patients, and further discussing the relationship between the level of PLR and clinical data of PCA patients, finding new evident indicators for PCA examination and prognosis.
Subjects and methods
Subjects
Patients visited our hospital during Dec. 2009 and Jun. 2012 were enrolled: 168 cases of BPH patients with the mean age of 63.7±7.1 years old (50-80 years), 103 PCA cases with mean age of 66.1±6.9 years (55-82 years), and 155 matching healthy cases with the mean age of 41.1±6.5 years (31-48 years). The inclusion criteria of PCA were accorded to 2011 PCA diagnostic criteria published by the Ministry of Health, the inclusion criteria of BPH were that: the size of prostate met the following conditions including left-right diameter ≥ 4.4 cm, anteroposterior diameter ≥ 3.6 cm, and superioinferior diameter ≥ 4.0 cm; and the inclusion criteria for healthy individuals were that those examination indicators of the prostate were normal and their age were younger than 50. The exclusion criteria for PCA, BPH and healthy individuals were: acute infection, chronic inflammation, other cancers and abnormal lymphocyte proliferation. General data (except age) differences between groups were statistically insignificant, so it was comparable between groups.
Data collection
General information: sex, age. History: frequent urination, dysuria, hematuria, diabetes mellitus, smoking and drinking history, PCA family history. Clinical data analysis: diagnosis records, digital rectal examination information; blood pressure, heart rate, blood routine test, renal function, liver function, electrolytes results, blood glucose, and blood lipid etc.
Sample collection and process
Peripheral venous blood was drawn in test tubes without anticoagulant, and sent to a clinical laboratory for examination with Sysmex XE-2001 automatic blood cell analyzer. PSA, LDH, AKP, CRP, Alkaline Phosphatase level and hemoglobin level were tested as well.
Statistical analysis
Data were processed with SPSS 13.0. Quantitative data were presented in (mean ± standard error) form, and categorical data were presented in percentage form. P value less than 0.05 was considered statistically significant. Difference among three groups was analyzed with ANNOVA, relationship between risk factors and mortality was analyzed with Cox regression, survival analysis of different groups of patients was analyzed by Kaplan-Meier method with the survival curve and P<0.05 was considered statistically significant.
Results
PLR in healthy individuals, BPH patients and PCA patients
PLR of healthy individuals, BPH patients and PCA patients was calculated according to blood routine test. The result showed that PLR in healthy individuals, BPH patients and PCA patients was 114.3±48.1, 120.2±61.3 and 151.2±68.9 respectively. PLR in PCA patients was significantly higher than that of healthy individuals and BPH patients. It implies that PLR may be closely related to PCA (Figure 1).
Figure 1.
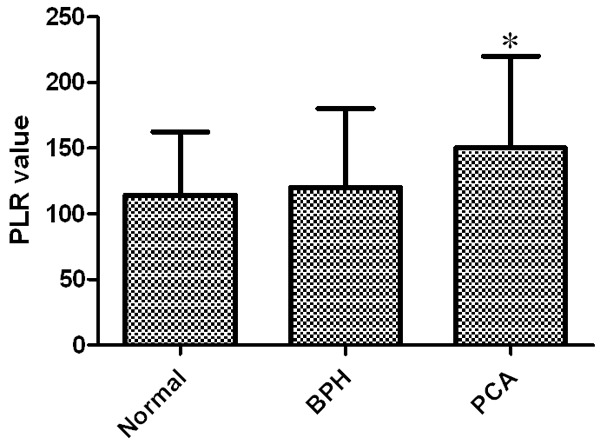
Comparison of the PLR in normal people, BPH patients and PCA patients. *P<0.05 compared with Normal group and BPH group.
Clinical data of PCA patients
103 PCA patients were divided into PLR low value group (PLR<150) and PLR high value group (PLR≥150) according to PLR results. 67 were in the low value group and 36 were in the high value group. The clinical comparison between low value and high value group shows, comparing to low value group, high value group had older age, more patients belonging to Gleason Score = 8-10, organ involvement > 2 and tumor stage > III subgroups, P<0.05 (Table 1).
Table 1.
Baseline characteristics of PCA patients
| Low value group (n=67) | High value group (n=36) | P value | |
|---|---|---|---|
| Age | 64.7±7.5 | 67.5±7.1 | 0.01 |
| Gleason Score = 8-10 | 36 (53.7%) | 21 (58.3%) | 0.02 |
| Pain Score | 4.6±2.1 | 5.0±2.3 | 0.14 |
| Organ Involvement > 2 | 62 (50.7%) | 25 (69.4%) | 0.04 |
| Tumor stage > III | 28 (41.8%) | 27 (75.0%) | <0.001 |
PCA patients’ laboratory results
Comparing the laboratory results of two groups, the results showed platelet count and neutrophil count were significantly higher in the PLR high value group than the PLR low value group, however the lymphocyte count was significantly lower. Besides, in the PLR high value group, PAS, LDH, AKP, CRP and alkaline phosphatase level were significantly elevated, and hemoglobin significantly lowered, P<0.05. Other laboratory tests yielded no difference (Table 2).
Table 2.
Laboratory results of PCA patients
| Low value group (n=67) | High value group (n=36) | P value | |
|---|---|---|---|
| Platelet (×109/L) | 201±62.5 | 250±86.5 | <0.001 |
| Lymphocyte (×109/L) | 2.0±0.75 | 1.20±0.62 | <0.001 |
| Neutrophil (×109/L) | 9.6±2.5 | 11.5±8.2 | <0.001 |
| WBC (×109/L) | 12.9±2.4 | 11.9±2.5 | 0.67 |
| PSA level (μg/L) | 95±42.6 | 166 ±72.5 | <0.001 |
| Urine NTx level (U/L) | 11.1±3.1 | 11.9±3.6 | 0.25 |
| LDH level (U/L) | 204±72.5 | 255±88.1 | 0.03 |
| AKP level (IU/L) | 184±81.5 | 324±128.1 | <0.001 |
| Hemoglobin level (g/dL) | 12.5±2.7 | 11.2±3.1 | 0.04 |
| CRP level (μg/L) | 2.53±1.1 | 3.63±1.7 | 0.04 |
| Alkaline Phosphatase level (U/L) | 104±52.7 | 168±60.6 | <0.001 |
General information during hospitalization of PCA patients
During hospitalization, 3 PCA patients died, 2 were in the low PLR value group with the rate of 2.9% and 1 was in the high PLR value group with the rate of 2.8%. The difference was non-significant. Besides, severe events such as coma were more frequent in PLR high value group, P<0.05 (Table 3).
Table 3.
Cardiac events and complications in-hospital
| Low value group (n=67) | High value group (n=36) | P value | |
|---|---|---|---|
| In-hospital mortality | 2 (2.9%) | 1 (2.8%) | 0.60 |
| Coma | 1 (1.5%) | 3 (8.3%) | 0.04 |
| Days in hospital | 8.7±3 | 9.0±5 | 0.73 |
Mortality of PCA patients during follow-up and Kaplan-Meier curve
The mean follow-up observation of PCA patients was 3 years, and 25 patients died during follow-up period. 11 cases were in the PLR low value group with the mortality rate of 16.4%, and 14 cases were in the PLR high value group with the mortality rate of 38.9%. The Kaplan-Meier curve showed that comparing to low value group, the 3-year survival rate of high value group patient was significantly lower, accompany with log-rank Chi square value was 6.171, P<0.05 (Figure 2).
Figure 2.
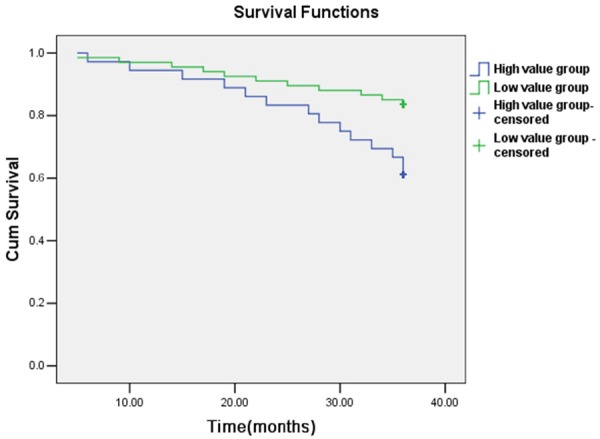
Kaplan-Meier survival curves of PCA patients over 3 years of follow-up. Prediction value of PLR to mortality of PCA patients during follow-up.
According to Cox regression, after correcting possible risk factors related to mortality, PLR was still an independent risk factor of death during follow-up. Comparing to PLR low value group, the ratio of hazardous death during follow-up of PLR high value group patients was 2.41 (95% CI: 1.61-4.73, P<0.001, Table 4).
Table 4.
COX regression analysis of the prediction value of PLR in PCA patients
| Observation groups | Uncorrected HR (95% CI) | P-value | Corrected HR (95% CI) | P-value |
|---|---|---|---|---|
| Low value group | 1 | 1 | ||
| High value group | 2.67 (1.73-4.92) | <0.001 | 2.41 (1.61-4.73) | <0.001 |
Discussion
Early stage clinical manifestations of prostate cancer are not significant or specific. Thus, looking for an early stage diagnostic marker is of great significance. In this study, we found that comparing to healthy normal individuals and BPH patients, PCA patients have significantly elevated PLR. Further study shows, PCA patients whose PLA are above 150 have significantly a higher mortality rate during three year follow-up than those whose PLR are lower than 150, and relevant clinical data and laboratory tests results show great differences too. This gives new evidences for PCA diagnosis and prognosis.
Currently, commonly used diagnostic approaches of PCA are digital rectal examination, prostate specific antigen test, transrectal ultrasonography, aspiration biopsy, and image studies such as CT and MRI. However, the histological morphology and biological behavior of prostate cancer are complicated. So, there are still difficulties in early diagnosis. As advancements achieved in modern molecular biology, researchers started to search for relevant markers [15,16]. Some researchers found dozens of miRNAs’ (e.g. miRNA-10, miRNA-25) expression levels were higher in PCA patients than healthy population through miRNA chips [17,18]. Other researchers discovered multiple proteins (eg. Apolipoprotein A2I, serum amyloid protein A and cytokeratin 1) expression level in PCA patients’ serum had significant differences with healthy individuals with proteomics technology [19]. But these markers still need further large and standardized clinical trials to get more reliable data to guide clinical practice. Thus it is of great importance to look for markers that have higher sensitivity and specificity.
Total blood cell count is a simple and cheap routine test, which gives us all information about blood formation, including the counts and dimensions of erythrocytes, leukocytes, and platelets, and some parameters such as NLR. In these parameters, leukocytes and platelets can directly reflect inflammation, which was known closely related to tumor genesis. Sonpavde et al. [12] discovered NLR was closely related to PCA metastasis, and they found NLR≥5 was an important marker of decreased survival during follow-up of PCA. Whereas Turkmen et al. [13] discovered PLR was a better marker in predicting inflammation severity, and Neofytou et al. [14] found PLR performed better in diagnosing colorectal cancer than NLR. Besides, Templetonet al. [20] did a meta-analysis and found PLR’s significant role in the diagnosis of various kinds of solid tumors (eg. Gastric cancer, lung cancer, metrocarcinoma, and hepatic carcinoma), and PLR was generally high in tumor patients, and patients with higher value of PLR had lower survival. Therefore, discussion of elevated PLR as an independent predictor of PCA is of good clinical significance and feasibility.
In our study, we found the PLR of healthy individuals, BPH patients and PCA patients are 114.3±48.1, 120.2±61.3 and 151.2±68.9 respectively, in which PCA group was significantly higher than others. It implied PLR had close relationship with PCA, and was consistent with reports in other cancers. By further analyzing clinical data, results showed comparing to PLR low value group, PLR high value group had older age, more patients belonging to Gleason Score = 8-10, organ involvement > 2 and tumor stage > III. It implied that metastasis and other organ involvement were more likely to happen in PLR high value group. Besides, in PLR high value group, PSA, LDH, AKP, CRP and alkaline phosphatase levels were significantly elevated, and PSA was an important clinical marker in diagnosing PCA, hence the correlation between PLR and PSA further demonstrated the reliability of diagnosing PCA with PLR. More interestingly, when we had followed up PCA patients for 3 years, the results showed during follow-up 25 patients died, 11 of which belonged to PLR low value group (mortality rate 16.4%), and 14 belonged to PLR high value group (mortality rate 38.9%). Kaplan-Meier survival curve showed, comparing to low value group, the three-year survival rate of high value group patients was significantly low, which was consistent with reports in other cancers. Cox regression showed, the ratio of hazardous death during follow up of PLR high value group was 2.41 (95%, CI: 1.45-4.51), and was an independent predictor. The results of this study further confirmed that PLR’s role in tumor genesis and development, providing more evidence for studies concerning inflammation and tumor.
However, there are limitations of this study, for example, the sample size was not big enough, and researchers didn’t compare PLR variation during early and advanced PCA, and researchers didn’t compare the sensitivity and specificity of PLR and NLR in predicting PCA. So the next step is to enlarge sample size to ensure the certainty of data; to compare the PLR variation during early and advanced PCA process, to further explore the role of PLR, to compare the sensitivity and specificity of PLR and NLR in predicting PCA, providing more reliable evidences for clinical examination of PCA.
Overall, our study confirms, PLR is significantly elevated in PCA patients, is closely related to PCA patients’ clinical data, is an independent risk factor of PCA patients’ death during follow up, and is of great clinical significance in PCA diagnosis and prognosis.
Disclosure of conflict of interest
None.
References
- 1.Loblaw DA, Virgo KS, Nam R, Somerfield MR, Ben-Josef E, Mendelson DS, Middleton R, Sharp SA, Smith TJ, Talcott J, Taplin M, Vogelzang NJ, Wade JL 3rd, Bennett CL, Scher HI. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology practice guideline. J. Clin. Oncol. 2007;25:1596–1605. doi: 10.1200/JCO.2006.10.1949. [DOI] [PubMed] [Google Scholar]
- 2.Eisenberger MA, Blumenstein BA, Crawford ED, Miller G, McLeod DG, Loehrer PJ, Wilding G, Sears K, Culkin DJ, Thompson IM Jr, Bueschen AJ, Lowe BA. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med. 1998;339:1036–1042. doi: 10.1056/NEJM199810083391504. [DOI] [PubMed] [Google Scholar]
- 3.Makarov DV, Humphreys EB, Mangold LA, Carducci MA, Partin AW, Eisenberger MA, Walsh PC, Trock BJ. The natural history of men treated with deferred androgen deprivation therapy in whom metastatic prostate cancer developed following radical prostatectomy. J Urol. 2008;179:156–161. doi: 10.1016/j.juro.2007.08.133. discussion 161-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, True LD, Nelson PS. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srivastava A, Goldberger H, Afzal Z, Suy S, Collins SP, Kumar D. Detection of circulatory microRNAs in prostate cancer. Methods Mol Biol. 2015;1238:523–538. doi: 10.1007/978-1-4939-1804-1_27. [DOI] [PubMed] [Google Scholar]
- 6.Katz B, Reis ST, Viana NI, Morais DR, Moura CM, Dip N, Silva IA, Iscaife A, Srougi M, Leite KR. Comprehensive study of gene and microRNA expression related to epithelial-mesenchymal transition in prostate cancer. PLoS One. 2014;9:e113700. doi: 10.1371/journal.pone.0113700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thakur V, Talwar M, Singh PP. Low free to total PSA ratio is not a good discriminator of chronic prostatitis and prostate cancer: An Indian experience. Indian J Cancer. 2014;51:335–337. doi: 10.4103/0019-509X.146790. [DOI] [PubMed] [Google Scholar]
- 8.Moradi Sardareh H, Goodarzi MT, Yadegar-Azari R, Poorolajal J, Mousavi-Bahar SH, Saidijam M. Prostate cancer antigen 3 gene expression in peripheral blood and urine sediments from prostate cancer and benign prostatic hyperplasia patients versus healthy individuals. Urol J. 2014;11:1952–1958. [PubMed] [Google Scholar]
- 9.Kao SC, Pavlakis N, Harvie R, Vardy JL, Boyer MJ, van Zandwijk N, Clarke SJ. High blood neutrophil-to-lymphocyte ratio is an indicator of poor prognosis in malignant mesothelioma patients undergoing systemic therapy. Clin Cancer Res. 2010;16:5805–5813. doi: 10.1158/1078-0432.CCR-10-2245. [DOI] [PubMed] [Google Scholar]
- 10.Gondo T, Nakashima J, Ohno Y, Choichiro O, Horiguchi Y, Namiki K, Yoshioka K, Ohori M, Hatano T, Tachibana M. Prognostic value of neutrophil-to-lymphocyte ratio and establishment of novel preoperative risk stratification model in bladder cancer patients treated with radical cystectomy. Urology. 2012;79:1085–1091. doi: 10.1016/j.urology.2011.11.070. [DOI] [PubMed] [Google Scholar]
- 11.Ohno Y, Nakashima J, Ohori M, Gondo T, Hatano T, Tachibana M. Followup of neutrophil- to-lymphocyte ratio and recurrence of clear cell renal cell carcinoma. J Urol. 2012;187:411–417. doi: 10.1016/j.juro.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Sonpavde G, Pond GR, Armstrong AJ, Clarke SJ, Vardy JL, Templeton AJ, Wang SL, Paolini J, Chen I, Chow-Maneval E, Lechuga M, Smith MR, Michaelson MD. Prognostic impact of the neutrophil-to-lymphocyte ratio in men with metastatic castration-resistant prostate cancer. Clin Genitourin Cancer. 2014;12:317–324. doi: 10.1016/j.clgc.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Turkmen K, Erdur FM, Ozcicek F, Ozcicek A, Akbas EM, Ozbicer A, Demirtas L, Turk S, Tonbul HZ. Platelet-to-lymphocyte ratio better predicts inflammation than neutrophil-to-lymphocyte ratio in end-stage renal disease patients. Hemodial Int. 2013;17:391–396. doi: 10.1111/hdi.12040. [DOI] [PubMed] [Google Scholar]
- 14.Neofytou K, Smyth EC, Giakoustidis A, Khan AZ, Cunningham D, Mudan S. Elevated platelet to lymphocyte ratio predicts poor prognosis after hepatectomy for liver-only colorectal metastases, and it is superior to neutrophil to lymphocyte ratio as an adverse prognostic factor. Med Oncol. 2014;31:239. doi: 10.1007/s12032-014-0239-6. [DOI] [PubMed] [Google Scholar]
- 15.Niu WB, Gui SL, Lin YL, Fu XL, Ma JG, Li WP. Promoter methylation of protocadherin8 is an independent prognostic factor for biochemical recurrence of early-stage prostate cancer. Med Sci Monit. 2014;20:2584–2589. doi: 10.12659/MSM.893083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsaur I, Noack A, Makarevic J, Oppermann E, Waaga-Gasser AM, Gasser M, Borgmann H, Huesch T, Gust KM, Reiter M, Schilling D, Bartsch G, Haferkamp A, Blaheta RA. CCL2 Chemokine as a Potential Biomarker for Prostate Cancer: A Pilot Study. Cancer Res Treat. 2015;47:306–12. doi: 10.4143/crt.2014.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohammad AA, Rahbar A, Lui WO, Davoudi B, Catrina A, Stragliotto G, Mellbin L, Hamsten A, Ryden L, Yaiw KC, Soderberg-Naucler C. Detection of circulating hcmv-miR-UL112-3p in patients with glioblastoma, rheumatoid arthritis, diabetes mellitus and healthy controls. PLoS One. 2014;9:e113740. doi: 10.1371/journal.pone.0113740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly BD, Miller N, Healy NA, Walsh K, Kerin MJ. A review of expression profiling of circulating microRNAs in men with prostate cancer. BJU Int. 2013;111:17–21. doi: 10.1111/j.1464-410X.2012.11244.x. [DOI] [PubMed] [Google Scholar]
- 19.Li WX, Wu H. Screening for serum markers with protein fingerprint technique in patients with prostate cancer. Zhejiang Med J. 2012;37:512–514. [Google Scholar]
- 20.Templeton AJ, Ace O, McNamara MG, Al-Mubarak M, Vera-Badillo FE, Hermanns T, Seruga B, Ocana A, Tannock IF, Amir E. Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2014;23:1204–1212. doi: 10.1158/1055-9965.EPI-14-0146. [DOI] [PubMed] [Google Scholar]


