Abstract
Aim: This study was to investigate the E-cadherin expression patterns in primary breast cancers and metastatic lymph node. Methods: Only lymph nodes which were pathologically identified as metastases were included in this study to pair up the primary tumors. E-cadherin RNA expression levels in invasive ductal breast cancer subjects were detected. E-cadherin gene copies were normalized using beta-actin gene copies. ER, PR, cerbB2 expressions in the primary tumor were routinely examined by immunohistochemistry method. Tumor characteristics and number of metastatic lymph nodes were gathered from the pathology reports. Results: We tried to explore the relationship between E-cadherin expression in 21 primary tumors and their corresponding metastatic lymph nodes. However, the Q-RT-PCR data show that an aberrant expression existed in both primary tumors and the corresponding lymph nodes (P=0.115), in which metastatic lymph nodes showed slight higher gene copies compared with primary sites (77.77±94.74 vs 43.35±40.03, respectively). It is noteworthy that nodal E-cadherin expression was closely but negatively correlated with tumor size (P<0.01, r=-0.775) and number of metastasized lymph nodes (P<0.05, r=-0.519), as tumor size and number of metastasized lymph nodes were already clinically proven to be important prognostic factors. There was no correlation between ER, PR, cerbB2 status in primary tumors and the nodal E-cadherin expression (P>0.05). Conclusions: It is indicated that E-cadherin expression is aberrant in invasive ductal cancers and their corresponding metastatic lymph nodes. E-cadherin expression in the metastasized lymph node is closely related to tumor size and number of metastasized lymph nodes.
Keywords: E-cadherin, primary breast cancer, metastatic lymph node, real-time PCR
Introduction
Breast cancer is one of most common malignant tumors in the world and ranked first as a cause of cancer death for females in Shanghai. Tumor invasion with subsequent metastases is one of major cause of morbidity and mortality in patients with breast cancer. The development of metastases is the most important prognostic factor, as almost all patients with distant metastasis succumb to the disease [1].
Detachment of cell-cell adhesion is indispensable for the first step of invasion and metastasis of cancer. This mechanism is frequently associated with the impairment of either E-cadherin expression or function [2]. E-cadherin is one of calcium-dependent transmembrane glycoprotein mediating cell-cell adhesion, specifically involved in epithelial cell-to-cell adhesion [3]. It is mainly localized in adherent junctions and is mediated by extracellular domain cell-cell adhesion through calcium dependent interactions. The E-cadherin gene, located on chromosome 16q22.1, is also an important regulator of morphogenesis [4-6].
Transfection and expression E-cadherin cDNA inhibited the invasiveness of epithelial tumor cell lines. Other numerous studies have linked aberrant expression of E-cadherin with the development of metastases in breast cancer and other cancers [7]. In cancer, decreased E-cadherin expression is one of the alterations that characterize the invasive phenotype, and the data support its role as a tumor suppressor gene [8,9]. Although in vitro cell lines studies have provided evidence of an association between reduced E-cadherin and invasion, this association has not consistently been shown in vivo [7,10-12]. Few studies have specifically looked at the expression pattern of E-cadherin in breast primary and lymph node or distant metastasis [7,13]. However, only immunocytochemical staining for detection of E-cadherin limited the value of these studies.
In the present study, we used the quantitative real-time PCR (Q-RT-PCR) technique to assess the E-cadherin RNA expression levels in invasive ductal breast cancer subjects and their corresponding metastatic lymph nodes [14]. We further studied the relationship among E-cadherin expression levels and tumor size, ER, PR and numbers of metastatic lymph node.
Materials and methods
Patient selection and specimens
A total of 21 patients with operable breast tumors and metastatic lymph nodes who underwent Modified Radical Mastectomy for Breast Cancer between 2003 and 2004 were included in this study. Patients were diagnosed by core-tissue biopsy (CTB) using Bard-Magnum Gun (MG1522, Bard Magnum Biopsy Instrument, CR Bard, Inc.) with 14-gauge 13-cm-long biopsy needles (Bard Magnum Core Tissue Biopsy Needle, CR Bard, Inc.) [15]. The excisional primary tumor tissues and axillary lymph nodes were kept in liquid nitrogen. The axillary lymph nodes were pathologically identified as the metastases. ER, PR, cerbB2 expressions on the primary tumors were routinely examined by immunohistochemistry method [16]. Tumor characteristics and number of metastatic lymph nodes were gathered from the pathology reports.
RNA isolation and cDNA synthesis
Total RNA was isolated from 15-20 mg liquid-nitrogen-frozen breast cancer tissue and metastatic lymph node tissue using Total RNA Extraction Miniprep System (Cat. No.: GR1001, VIOGENE Inc.) [17]. The performance was according to the manufacturer’s protocols. Tumor and lymph node sample RNA was diluted in DEPC-treated RNase-free ultra-pure water (DEPC; Sigma-Aldrich, the Netherlands) and stored at -80°C.
Reverse Transcription is carried out with the SuperScript First-Strand Synthesis System (Shinegene inc.) for RT-PCR. The following procedure is based on manufacture’s protocol. Prepare the RNA/primer mixture mix including total RNA 5 mg, random primers (50 ng/ml) 3 ml, 10 mM dNTP 1 ml in each tube. Incubate the samples at 65°C for 5 min and then on ice for at least 1 min. Prepare reaction master mixture. For each reaction: 10× RT buffer 2 ml,25 mM MgCl2 4 ml, 0.1 M DTT2 ml, RNAaseOUT1 ml. Add the reaction mixture to the RNA/primer mixture, mix briefly, and then place at room temperature for 2 min. Add 1 ml (50 units) of SuperScript II RT to each tube, mix and incubate at 25°C for 10 min.Incubate the tubes at 42°C for 50 min, heat inactivate at 70°C for 15 min, and then chill on ice. Add 1 ml RNase H and incubate at 37°C for 20 min. Store the 1st strand cDNA at -20°C until use for real-time PCR.
Quantitative real-time RT-PCR
The mRNA level of E-cadherin was measured by quantitive real-time RT-PCR method using a Hot Start Fluorescent PCR Core Reagent Kit for SYBR Green I. Quantitive real-time RT-PCR (FTC2000 Detect System, Funglyn Biotech, Toronto, ON, Canada) was carried out with 5 ml cDNA in a 30 ml PCR reaction system. In addition, we used control gene β-actin to normalize the mRNA level of E-cadherin gene. Primer pairs (h e-cadherin forward, 5’-TGCTCACATTTCCCAACTCC-3’ reverse, 5’-CCTTGCCTTCTTTGTCTTTGTT-3’) were designed by Oligo 6.0 primer analysis software (Medprobe, Oslo, Norway). Real-time PCR was performed in 50 ml of reaction mixture system, including 25 ml 1× Hotstart Fluo-PCR mix, about 300 nM forward and reverse primers, and containing about 2 ml of tumor sample cDNA as a template. Reaction conditions were as follows: 50°C for 2 min for UNG activation and 94°C for 4 min for TaqDNA polymerase activation, followed by 35 cycles of 94°C for 30 s for denaturation, 55°C for 30 s for annealing and 72°C for 1 min for extension. For every transcript measured, serial dilutions (1:10, 1:100, 1:1000, 1:10 000) of standard-concentration sample were used to generate a standard curve. Data were analyzed with FTC2000 software (Funglyn Biotech, Toronto, ON, Canada) according to the above standard curve.
Immunohistochemistry
The primary cancer breast cancers tissue and metastatic lymph nodes was collected from each patient, fixed in 10% formalin, embedded in paraffin, and sectioned (3 μm). Immunohistochemistry was performed to detect E-cadherin (mouse anti-human polyclonal antibody, 1:100; Shanghai Changdao Biotech Co., Ltd) according to manufacturer’s instructions. Immunohistochemical features were assessed using independent evaluation by a pathologist.For interpretation of the IHC stain results, the IHC tests were categorized as negative (0), “1+,” “2+,” or “3+” in high-power fields (40× magnification) according to the intensity of cytoplasmic staining in every case.
Statistical analysis
A two-sample t test was performed to compare the difference of Expression of E-cadherin between primary breast cancer and their corresponding lymph node. The correlation between E-cadherin expression and pathological variables were analyzed by correlation analysis using SPSS 14. P<0.05 was considered significant.
Results
Clinical and pathological features
All patients were females, and all the pathological types were the invasive ductal breast cancers. The tumor characteristics are presented in Table 1. The media age at diagnosis of primary invasive breast cancer was 50.8 years (range, 35-79).
Table 1.
Clinical and pathological characteristics
| Item | Data |
|---|---|
| Number of patients | 21 |
| Pathological stage at diagnosis [n (%)] | |
| Stage II (T2N1M0) | 12 (57) |
| Stage III (T2N2M0) | 9 (43) |
| Tumor size (range) (cm) | 3.4 (2-5) |
| Histological type | |
| Ductal | 21 |
| Number of invaded lymph node (range) | 8.8 (1-18) |
| Estrogen receptor status [n (%)] | 11 (52) |
| Negative | 10 (48) |
| Positive | |
| Progesterone receptor status [n (%)] | 8 (38) |
| Negative | 13 (62) |
| Positive | |
| CerbB2 receptor status [n (%)] | |
| Negative | 9 (43) |
| Positive | 12 (57) |
E-cadherin mRNA in primary breast cancers and metastatic lymph nodes
The quantitative real-time PCR (Q-RT-PCR) was used to assess the mRNA level of E-cadherin in primary breast cancers and their corresponding metastatic lymph nodes. The data are shown in Figure 1.
Figure 1.
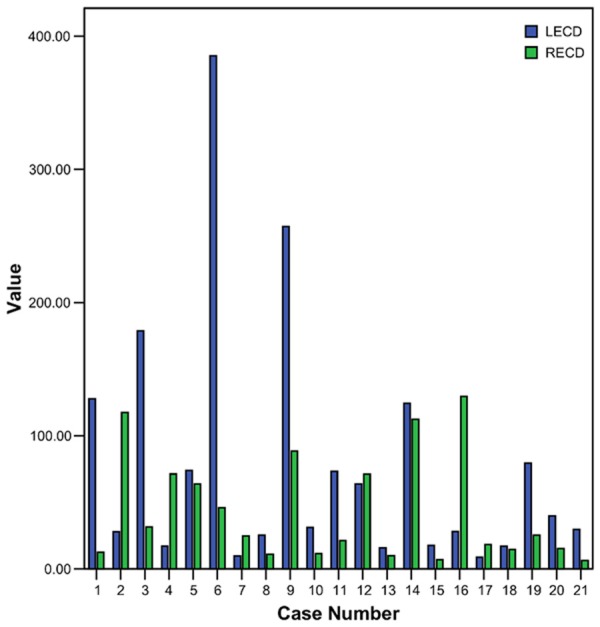
E-cadherin gene expression levels in paired samples. Blue bars represent metastasis samples, and green bars represent primary tumors. Vertical axes show normalized densities for the indicated genes.
An aberrant expression exists in both primary tumors and the corresponding lymph nodes, in which metastatic lymph nodes showed slight higher gene copies comparing to primary sites (77.77±94.74 vs 43.35±40.03, respectively). But the statistical analysis showed that there was no significant difference (P=0.15) (Table 2).
Table 2.
E-cadherin expression in primary breast cancer and corresponding metastatic lymph node
| Gene copy (x̅±SD) | t-test (t) | P correlation (r) | P | |
|---|---|---|---|---|
| Primary breast cancer | 43.349±4.028 | 1.694 | >0.05 | >0.05 |
| Metastatic lymph node | 77.767±9.472 |
Immunohistochemical expression of E-cadherin in primary breast cancers tissue and metastatic lymph nodes
Immunohistochemistry was performed to detect E-cadherin in the primary breast cancers tissue and metastatic lymph nodes. Representative images were taken from the results of Immunohistochemistry (Figure 2). Statistically, E-cadherin was expressed in 85.7% (18/21) of metastatic lymph nodes tissue, which was higher than the 66.7% (14/21) in Primary breast cancers tissue, while the difference was not significant (P>0.05) (Table 3).
Figure 2.
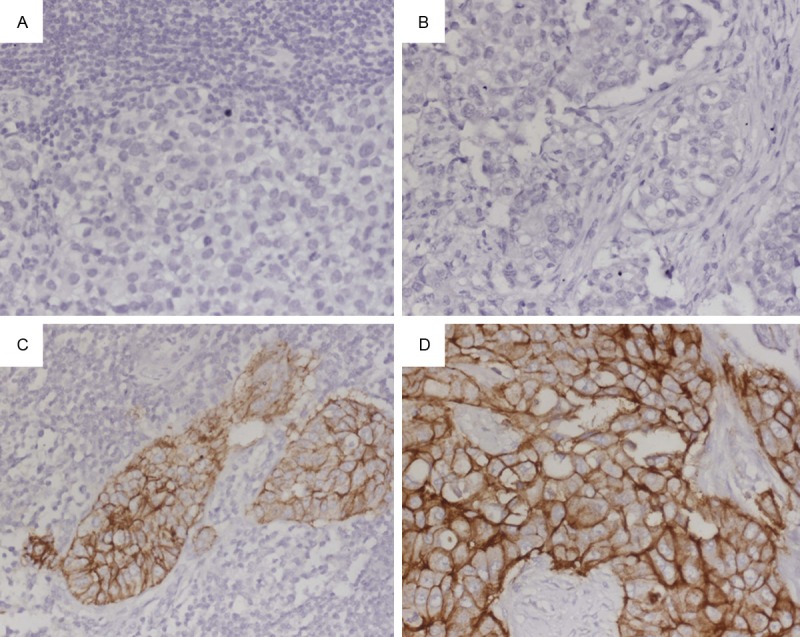
Immunohistochemical analysis of E-cadherin. E-cadherin expression was evaluated at high-power field (×40 magnification) by an experienced pathologist. A. Negative for E-cadherin in Primary Breast cancers tissue. B. Negative for E-cadherin in metastatic lymph nodes. C. Three positive for E-cadherin in Primary Breast cancers tissue. D. Three positive for E-cadherin in metastatic lymph nodes.
Table 3.
Immunohistochemical expression of E-cadherin in Primary breast cancers tissue and metastatic lymph nodes
| Group | Case | Expression of E-cadherin | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| - | + | ++ | +++ | χ2 | P value | ||
| Primary breast cancer tissue | 21 | 7 | 6 | 4 | 4 | 2.108 | 0.550 |
| Lymph node | 21 | 3 | 8 | 5 | 5 | ||
Relationship between expression of E-cadherin and tumor characteristics
The relationships between the E-cadherin expression and clinicopathologic parameters were summarized in Table 4. It is noteworthy that nodal E-cadherin expression was closely but negatively correlated with tumor size (P<0.01, r=-0.775) and number of metastasized lymph nodes (P<0.05, r=-0.519), as tumor size and number of metastasized lymph nodes were already clinically proven to be important prognostic factors. There was no correlation between ER, PR, cerbB2 status in primary tumors and the nodal E-cadherin expression (P>0.05).
Table 4.
Relationships between the E-cadherin expression and clinicopathologic parameters
| T E-cadherin (r) | P | L E-cadherin (r) | P | |
|---|---|---|---|---|
| Tumor Size | 0.177 | 0.443 | -0.775 | 0.0001 |
| N lymph node | 0.029 | 0.902 | -0.519 | 0.016 |
| ER | 0.100 | 0.665 | 0.009 | 0.97 |
| PR | 0.188 | 0.415 | -0.08 | 0.729 |
| CerbB2 | 0.278 | 0.223 | -0.085 | 0.715 |
Discussion
The expression of E-cadherin in breast cancer metastases is largely unknown and, to our knowledge, few studies specifically investigate the expression of E-cadherin in primary breast cancer in relationship to their corresponding metastatic lymph nodes [13,18].
We performed the Real-time RT-PCR method to study the relationship between E-cadherin expression in node-positive patients, with matched primary tumors and metastatic lymph nodes. We found that aberrant E-cadherin expression is a common event in primary invasive ductal breast cancer [7].
We also compared E-cadherin expression in primary invasive ductal tumors and their corresponding metastatic lymph nodes, and found no significant difference in mean expression. However, in metastatic lymph nodes, E-cadherin expression is slight higher gene copies comparing to primary sites (77.77±94.74 vs. 43.35±40.03, respectively). According to these findings, one of the possible explanations is that cancer cells may re-expression E-cadherin protein once they reach distant sites [12]. Previous studies in breast cancer also have shown that loss of E-cadherin in primary tumors may be a transient phenomenon, enabling cells to break away and to be subsequently re-expressed in metastatic sites, possibly by facilitating lymphatic tumor emboli [7,19]. Bukholm et al [12] also found that 19 of 20 lymph node metastases strongly expressed E-cadherin protein. Kowalski et al [7] specifically evaluated paired primary breast tumors and matched distant metastases, and found that in a subset of patients the metastases had stronger E-cadherin expression than primary specimens. Park et al [20] also found that abnormal expression of the adhesion molecules in the primary tumors with re-expression in corresponding nodal metastases is a common event in breast ductal carcinomas and may play a central role in establishing metastasis. However, the mechanism and biologic role of E-cadherin re-expression at the metastatic site has not been elucidated, although it appears that translational regulation and post-translational events are probable mechanisms of E-cadherin re-expression [21].
Interestingly, a lot of evidence showed that decrease or loss of E-cadherin expression can enhance the invasive ability of cancer cells. Some researchers found that chemotherapy can enhance cancer invasion/metastasis, and both in vitro and in vivo studies have shown that metastases can be more invasive than primary tumors [22-26]. The mechanism that is still unknown and need further researches. Our data showed that E-cadherin expression is higher in metastatic lymph nodes than primary tumors, the metastases were proved to be more invasive. This finding indicated that E-cadherin might not play an important role in the metastases’ invasiveness. However, Dr. Ando and his colleagues [27] be-lieve that it is reasonable to suggest that the tumor suppressor E-cadherin may serve as a tumor enhancer when exposed to leptin and estradiol, that its ability to help cells aggregate then enhances the transformation of normal cells to cancerous cones, stimulating the growth of tumor mass. When the researchers used an E-cadherin antibody or a calcium-chelating agent to block E-cadherin function in the present of estradiol, this enhanced cell growth stopped [27].
In breast cancer, a relationship between E-cadherin expression and ER expression has been noted previously [10]. ER-positive tumors have been demonstrated to express normal amounts of E-cadherin protein, and loss of E-cadherin and ER genes has been linked to disease progression in invasive carcinomas of the breast. Nass and colleagues [28] found an association between coincident methylation of E-cadherin and the ER gene during breast cancer progression, probably not attributable to coincidence of methylation for two genes. In our study, however, we did not find an association between E-cadherin expression and the ER, PR, HER-2/neu status. We further studied the relationship between E-cadherin expression and tumor size. It is noteworthy that nodal E-cadherin expression was closely but negatively correlated with tumor size (P<0.01, r=-0.775) and number of metastasized lymph nodes (P<0.05, r=-0.519), as tumor size and number of metastasized lymph nodes were already clinically proven to be important prognostic factors.
In summary, the present study provides evidence that aberrant E-cadherin expression is a common event in primary invasive ductal breast cancer. E-cadherin is expression or re-expression at metastatic lymph nodes of invasive ductal breast cancer, supporting the hypothesis that re-expression of E-cadherin may play a role in the establishment of the metastatic cells at distant sites. Because of the difficulty in collecting fresh tumor tissues and matched invasive lymph nodes, we only collected 21 pairs of specimens, which may be too small to give a definite conclusion. But it still provides some evidence in E-cadherin expression in metastatic lymph nodes and E-cadherin may play a role in metastatic sites.
Acknowledgements
This work was supported by the foundation of Shanghai Municipal Commission of Health and Family Planning (No: 20124293).
Disclosure of conflict of interest
None.
References
- 1.Ansquer Y, Mandelbrot L, Lehy T, Salomon L, Dhainaut C, Madelenat P, Feldmann G, Walker F. Expression of BRCA1, HER-1 (EGFR) and HER-2 in sporadic breast cancer and relationships to other clinicopathological prognostic features. Anticancer Res. 2005;25:4535–4541. [PubMed] [Google Scholar]
- 2.Kawanishi J, Kato J, Sasaki K, Fujii S, Watanabe N, Niitsu Y. Loss of E-cadherin-dependent cell-cell adhesion due to mutation of the beta-catenin gene in a human cancer cell line, HSC-39. Mol Cell Biol. 1995;15:1175–1181. doi: 10.1128/mcb.15.3.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wijnhoven BP, Dinjens WN, Pignatelli M. E-cadherin-catenin cell-cell adhesion complex and human cancer. Br J Surg. 2000;87:992–1005. doi: 10.1046/j.1365-2168.2000.01513.x. [DOI] [PubMed] [Google Scholar]
- 4.Thoreson MA, Anastasiadis PZ, Daniel JM, Ireton RC, Wheelock MJ, Johnson KR, Hummingbird DK, Reynolds AB. Selective uncoupling of p120(ctn) from E-cadherin disrupts strong adhesion. J Cell Biol. 2000;148:189–202. doi: 10.1083/jcb.148.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taraszka KS, Higgins JM, Tan K, Mandelbrot DA, Wang JH, Brenner MB. Molecular basis for leukocyte integrin alpha(E)beta(7) adhesion to epithelial (E)-cadherin. J Exp Med. 2000;191:1555–1567. doi: 10.1084/jem.191.9.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lickert H, Bauer A, Kemler R, Stappert J. Casein kinase II phosphorylation of E-cadherin increases E-cadherin/beta-catenin interaction and strengthens cell-cell adhesion. J Biol Chem. 2000;275:5090–5095. doi: 10.1074/jbc.275.7.5090. [DOI] [PubMed] [Google Scholar]
- 7.Kowalski PJ, Rubin MA, Kleer CG. E-cadherin expression in primary carcinomas of the breast and its distant metastases. Breast Cancer Res. 2003;5:R217–222. doi: 10.1186/bcr651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gamallo C, Palacios J, Suarez A, Pizarro A, Navarro P, Quintanilla M, Cano A. Correlation of E-cadherin expression with differentiation grade and histological type in breast carcinoma. Am J Pathol. 1993;142:987–993. [PMC free article] [PubMed] [Google Scholar]
- 9.Frixen UH, Behrens J, Sachs M, Eberle G, Voss B, Warda A, Lochner D, Birchmeier W. E-cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol. 1991;113:173–185. doi: 10.1083/jcb.113.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipponen P, Saarelainen E, Ji H, Aaltomaa S, Syrjanen K. Expression of E-cadherin (E-CD) as related to other prognostic factors and survival in breast cancer. J Pathol. 1994;174:101–109. doi: 10.1002/path.1711740206. [DOI] [PubMed] [Google Scholar]
- 11.Moll R, Mitze M, Frixen UH, Birchmeier W. Differential loss of E-cadherin expression in infiltrating ductal and lobular breast carcinomas. Am J Pathol. 1993;143:1731–1742. [PMC free article] [PubMed] [Google Scholar]
- 12.Bukholm IK, Nesland JM, Borresen-Dale AL. Re-expression of E-cadherin, alpha-catenin and beta-catenin, but not of gamma-catenin, in metastatic tissue from breast cancer patients [seecomments] . J Pathol. 2000;190:15–19. doi: 10.1002/(SICI)1096-9896(200001)190:1<15::AID-PATH489>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 13.Hunt NC, Douglas-Jones AG, Jasani B, Morgan JM, Pignatelli M. Loss of E-cadherin expression associated with lymph node metastases in small breast carcinomas. Virchows Arch. 1997;430:285–289. doi: 10.1007/BF01092751. [DOI] [PubMed] [Google Scholar]
- 14.Lay MJ, Wittwer CT. Real-time fluorescence genotyping of factor V Leiden during rapid-cycle PCR. Clin Chem. 1997;43:2262–2267. [PubMed] [Google Scholar]
- 15.Kreuzer G, Boquoi E. [Significance of fine needle biopsy in the diagnosis of breast cancer. 1: Indications, methodology and malignancy criteria of puncture cytology] . Fortschr Med. 1985;103:381–384. [PubMed] [Google Scholar]
- 16.Gaiser T, Bernhards J. Tyramide signal amplification: an enhanced method for immunohistochemistry on methyl-methacrylate-embedded bone marrow trephine sections. Acta Haematol. 2007;117:122–127. doi: 10.1159/000097458. [DOI] [PubMed] [Google Scholar]
- 17.Chomczynski P, Sacchi N. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: twenty-something years on. Nat Protoc. 2006;1:581–585. doi: 10.1038/nprot.2006.83. [DOI] [PubMed] [Google Scholar]
- 18.Harigopal M, Berger AJ, Camp RL, Rimm DL, Kluger HM. Automated quantitative analysis of E-cadherin expression in lymph node metastases is predictive of survival in invasive ductal breast cancer. Clin Cancer Res. 2005;11:4083–4089. doi: 10.1158/1078-0432.CCR-04-2191. [DOI] [PubMed] [Google Scholar]
- 19.Gupta A, Deshpande CG, Badve S. Role of E-cadherins in development of lymphatic tumor emboli. Cancer. 2003;97:2341–2347. doi: 10.1002/cncr.11332. [DOI] [PubMed] [Google Scholar]
- 20.Park D, Karesen R, Axcrona U, Noren T, Sauer T. Expression pattern of adhesion molecules (E-cadherin, alpha-, beta-, gamma-catenin and claudin-7), their influence on survival in primary breast carcinoma, and their corresponding axillary lymph node metastasis. Apmis. 2007;115:52–65. doi: 10.1111/j.1600-0463.2007.apm_524.x. [DOI] [PubMed] [Google Scholar]
- 21.Rashid MG, Sanda MG, Vallorosi CJ, Rios-Doria J, Rubin MA, Day ML. Posttranslational truncation and inactivation of human E-cadherin distinguishes prostate cancer from matched normal prostate. Cancer Res. 2001;61:489–492. [PubMed] [Google Scholar]
- 22.Liang Y, McDonnell S, Clynes M. Examining the relationship between cancer invasion/metastasis and drug resistance. Curr Cancer Drug Targets. 2002;2:257–277. doi: 10.2174/1568009023333872. [DOI] [PubMed] [Google Scholar]
- 23.Cleton-Jansen AM. E-cadherin and loss of heterozygosity at chromosome 16 in breast carcinogenesis: different genetic pathways in ductal and lobular breast cancer? Breast Cancer Res. 2002;4:5–8. doi: 10.1186/bcr416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshida R, Kimura N, Harada Y, Ohuchi N. The loss of E-cadherin, alpha-and beta-catenin expression is associated with metastasis and poor prognosis in invasive breast cancer. Int J Oncol. 2001;18:513–520. [PubMed] [Google Scholar]
- 25.De Leeuw WJ, Berx G, Vos CB, Peterse JL, Van de Vijver MJ, Litvinov S, Van Roy F, Cornelisse CJ, Cleton-Jansen AM. Simultaneous loss of E-cadherin and catenins in invasive lobular breast cancer and lobular carcinoma in situ. J Pathol. 1997;183:404–411. doi: 10.1002/(SICI)1096-9896(199712)183:4<404::AID-PATH1148>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 26.Ji X, Woodard AS, Rimm DL, Fearon ER. Transcriptional defects underlie loss of E-cadherin expression in breast cancer. Cell Growth Differ. 1997;8:773–778. [PubMed] [Google Scholar]
- 27.Mauro L, Catalano S, Bossi G, Pellegrino M, Barone I, Morales S, Giordano C, Bartella V, Casaburi I, Ando S. Evidences that leptin up-regulates E-cadherin expression in breast cancer: effects on tumor growth and progression. Cancer Res. 2007;67:3412–3421. doi: 10.1158/0008-5472.CAN-06-2890. [DOI] [PubMed] [Google Scholar]
- 28.Nass SJ, Herman JG, Gabrielson E, Iversen PW, Parl FF, Davidson NE, Graff JR. Aberrant methylation of the estrogen receptor and E-cadherin 5’ CpG islands increases with malignant progression in human breast cancer. Cancer Res. 2000;60:4346–4348. [PubMed] [Google Scholar]


