Abstract
Objective: To study whether miR-23 is regulated in coronary artery disease (CAD) patients and what is the possible mechanism of miR-23 in regulating CAD progression. Method Three different cohorts (including 13 AMI patients, 176 angina pectoris patients and 127 control subjects) were enrolled to investigate the expression levels of circulating miR-23 in patients with myocardial ischemia and also the relationship between plasma miR-23 and severity of coronary stenosis. Plasma miR-23 levels of participants were examined by real-time quantitative PCR. We further detected the correlation of miR-23 and VEGF by molecular and animal assays. Result miR-23 was enriched in not only diseased endothelial progenitor cells (EPCs) but also the plasma of CAD patients. Besides, we found out miR-23 was able to suppress VEGF expression and EPC activities. Reporter assays confirmed the direct binding and repression of miR-23 to the 3’-UTR of VEGF mRNA. Knock down of miR-23 not only restored VEGF levels and angiogenic activities of diseased EPCs in vitro, but further promoted blood flow recovery in ischemic limbs of mice. Conclusion Circulating miR-23 may be a new biomarker for CAD and as a potential diagnostic tool. And increased miR-23 level may be used to predict the presence and severity of coronary lesions in CAD patients.
Keywords: miR-23, VEGF, endothelial progenitor cells (EPCs), coronary artery disease (CAD), disease marker
Introduction
More than 80% of sudden cardiac deaths in the world are caused by atherosclerotic coronary artery disease (CAD), and the remaining 20% of cases are caused by other diseases including cardiomyopathies, congenital heart disease, left ventricular hypertrophy, aortic valve disease, and other cardiac disorders [1,2]. Refurbishment of damaged microcirculation depends largely on bone-marrow-derived circulating endothelial progenitor cells (EPCs), which involve in both angiogenesis and vasculogenesis [3-5]. So, the dysfunction EPCs also contributes in CVD pathogenesis. Reports showed that the number of EPCs is inversely correlated to progression of coronary heart disease [6]. On the other hand, angiogenic activities in patients are also reduced. Patients at cardiovascular risk with both low EPC counts and impaired EPC activities have a higher incidence for cardiovascular events and higher mortality [7]. These studies also lead to the rationale for autologous stem cell therapy in different clinical settings.
MicroRNAs (miRNAs) are endogenous small non-coding RNAs with 21-25 nucleotides in length. By pairing with the 3’ UTR of target mRNAs, miRNAs can regulate protein-coding genes at the posttranscriptional level via degradation of mRNAs or repression of protein translation [7]. At present, About 700 human miRNAs have been identified, and most of them were found to be tissue-/cell-specific [8]. Mounting evidences suggest that miRNAs play crucial roles in various physiological and pathologic processes, and the dysfunctions of miRNAs are associated with various diseases and pathophysiologies [9-11]. Recently, studies showed that miRNAs are abundantly present in body fluid and can be used as biomarkers for some diseases [12-14]. MiR-23 is a muscle specific miRNA and is expressed abundantly in myocardial cells [15]. It has been established that miR-23 plays important roles in myogenesis, cardiac development and hypertrophy. Previous studies demonstrated that miR-23 had a low level presence in the plasma of healthy people [11], and it was expressed differentially in different cardiovascular diseases [7,9]. Recently, it has been reported that the elevated miR-23 is released into peripheral circulation from the injured myocardium after Ca2 + stimulation [15]. Although these studies demonstrated that the expression of circulating miR-23 increased in patients with CAD (including AMI and angina pectoris) and circulating miR-23 can be used as a marker for cardiomyocyte death, few clinical studies have reported on the dynamic change in circulating miR-23 level in the early phase of AMI, and also the correlation between miR-23 concentration and the severity of coronary stenosis in CAD patients is not clear.
In the present work, we aimed to confirm the role of plasma miR-23 as a biomarker for CAD. Furthermore, we investigated the mechanism of the levels of circulating miR-23 in the pathogenesis of coronary heart disease patients to further explore the mechanism involved in the vasculogenesis.
Materials and methods
Characteristics of patients
Experiments were conducted in accordance with the Declaration of Helsinki. Three cohorts participated in this study. The first cohort included 13 patients of AMI and 27 healthy volunteers. The inclusion criteria for AMI patients were based on the third Universal Definition of Myocardial Infarction. Briefly, AMI patients were clinically diagnosed by the following criteria: 1) acute ischemic chest pain within 24 hours; 2) electrocardiogram change of acute myocardial infarction (pathological Q wave, ST-segment elevation or depression); 3) plasma cTnI >0.1 ng/mL. The initial blood sample (denoted by T0) was collected immediately after the AMI patient was admitted to Tongji hospital. Other 5 subsequent blood samples were obtained at 4, 12, 24, 48, 72 hours after the first collection, denoted by 4 h, 12 h, 24 h, 48 h and 72 h, respectively. The second cohort included 22 CAD patients with chest pain having single lesion of the left anterior descending coronary artery and 8 non-CAD patients with negative results of coronary angiography. The third cohort contained 246 patients with acute chest pain. Further, coronary angiography showed that 154 of them were CAD patients with complex lesions of coronary artery, and the remaining 92 patients were non-CAD patients with no coronary artery stenosis. A single blood sample from each participant in both second and third cohorts was obtained immediately after admission, and coronary angiography was used to confirm CAD and define the coronary artery lesions. Blood samples were collected via venous puncture. After isolation by centrifugation, the plasma were transferred to RNase-free tubes and stored at -80°C until further processing.
Participants were selected from inpatients or outpatients departments of Tongji hospital between October 2012 and June 2014 in Wuhan, China. The study was approved by the Medical Ethics Committee in Tongji Hospital and written informed consents were obtained from all the participants.
RNA isolation
Total RNAs were isolated by TRIzol LS Reagent (Invitrogen) according to the manufacturer’s protocol as described previously [27]. In brief, total RNA was purified from 500 μL of plasma and ultimately eluted into 25 μL of RNase-free water.
Detection and quantification of miRNAs by real-time PCR
Two microgram of total RNA was reverse-transcribed using Transcript First-strand cDNA synthesis SuperMix (TransGen Biotech, Beijing, China) according to the manufacturer’s protocol. The Bulge-Loop™ miRNA qRTPCR Detection Kit (Ribobio Co., Guangzhou, China) and SYBR Green PCR SuperMix Kit (TransGen Biotech, Beijing, China) were used in real-time PCR for examining the relative quantification of miR-23 according to the manufacturer’s protocol with the Rotor-Gene 6000 system (Corbett Life Science, QIAGEN, Hilden, Germany), and U6 was measured as endogenous control for normalizing the data of experimental qRT-PCR. Each specimen was measured in triplicate. The threshold cycle (Ct) was defined as the fractional cycle number at which fluorescence exceed the threshold. In our experiment the detection limit of Ct value was defined as 40. The Ct values from qRT-PCR assays over 40 were treated as 40 [15,25,28,29].
microRNA quantitative RT-PCR
RNA extraction and RT-qPCR were performed as described [12]. Total RNA was extracted using Tri Reagent (Sigma-Aldrich Co., St. Louis, USA) according to the manufacturer’s instructions. For miRNA qPCR, the expression levels of specific miRNAs were detected using stem-loop RT-PCR [21]. The universal PCR reverse primer for the miRNAs was 5’-GTGCAGGGTCCGAGGT-3’. Amplification of marker genes was performed using specific primers, Maxima SYBR Green qPCR Master Mix (K0222, Fermentas) and a StepOne sequence detector (Applied Biosystems, USA). The VEGF mRNA expression data were normalized to the average value of GAPDH and beta-actin.
Transfection of microRNA oligonucleotides and plasmids
The lentiviral expression vector expressing miR-23 precursor sequence was constructed using the following primer pairs: forward: 5’-TTgggCATATgTgACCATCA-3’; reverse: 5’-ggAgCTCAACCATACCAggA-3’. MicrON agomir and micrOFF antagomir (RiboBio Co., Guangzhou, China) for miR-23 is commercial synthetic RNA molecules with several chemical modifications for direct transfection without transfect reagents. To over-express or knock down miR-23 in EPCs, agomir or antagomir was added into culture medium at a concentration of 50 nM at 70% to 80% cell confluence. The expression level of transfected microRNAs were monitored and measured by quantitative RT-PCR.
EPC tube formation, transwell cell migration and cell proliferation assays
In vitro tube formation assay was performed on EPCs for assessing the capacity of neovascularization as described [12]. Thawed Basement Membrane Extract (BME, 3433-005-01, Trevigen Inc.) were plated in 96-well at 37uC for up to 1 hour to form a reconstituted basement membrane. EPCs, less than six passage of cultivation, were collected by trypsin/EDTA, and 16104 cells in 100 ml medium were seeded on Matrigel then incubated at 37°C for 6 hours. Tube structures were inspected under an inverted light microscope (100×). To evaluate the tube formation capacity, five representative fields were captured and analyzed by calculating total tube length in each group. All data were obtained from three independent experiments with triplication. For easily interpreting the significance of the experiments, the total tube length were further normalized to control group and presented into relative tube length. For Transwell cell migration assay, 600 ml medium with 10% FBS were added to the lower chamber, while 56104 EPCs in 100 ml medium were subjected to upper chamber of Costar Transwell Polycarbonate Permeable Supports (Corning, NY, USA). After 3 hours incubation at 37°C, cell suspensions were removed from upper chamber and the 8 mm permeable membranes were fixed with 4% paraformaldehyde for at least 15 minutes at room temperature. Migrated cells were then stained with Hochest 33342 reagents (Sigma-Aldrich) for 30 minutes and counted under fluorescent microscope by five representative fields. The degree of cell proliferation was examined by the MTT assay system (Invitrogen, USA) according to the manufacturer’s instructions.
Reporter assays
For luciferase reporter plasmids, the predicted microRNA-binding site was cloned into the XbaI site of the pGL3-Basic plasmid with the following primers: VEGF-UTR-F, 5’-CCgTCTAgATCTTTTgCTCTCTCTTgCTCTC-3’; VEGF-UTR-R, 5’-AgCTCTAgAACggATAAACAg-TAgCACCAA-3. The luciferase reporter plasmids containing VEGF mutant binding site was created using the QuikChange Site-Directed Mutagenesis Kit (Stratagene, USA). For 3’-UTR reporter assays, miRNA and reporter plasmids were analyzed by the measurement of ratio between firefly and rellina luciferase activities.
Mouse ischemic hind-limb model and EPC transplantation
Nude mice ranging from 6 to 8 weeks were purchased from the National Laboratory Animal Center (Taiwan) and kept in micro-isolator cages on a 12-h day/night cycle for 2 weeks before operation. After two-week stabilization, mice received right femoral artery excision for inducing unilateral hind-limb ischemia as previously described [22]. Briefly, mice were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg). Both proximal and distal portion of right femoral artery were ligated, as well as distal portion of saphenous artery. Mice were randomly allocated to three groups (n = 6) with different treatments: EGM2 medium, CAD-EPC with scramble oligonucleotides, and CAD-EPC with miR-23 antagomirs (micrOFF, RiboBio Co., Guangzhou, China). CAD-EPCs from same donors were pre-stained with PKH26 (SigmaAldrich), a tracking dye for staining cell membrane, before transplantation. After 72 hours, a total volume of 200 ml medium with 2.56105 EPCs were injected intramuscularly at six different sites of ischemic limb distal to the arterial occlusion site. Blood perfusion was monitored by Laser Doppler Perfusion Imager (LDPI) system (Moor Instruments Limited, Devon, UK) before and after the surgery, and was then measured weekly. To prevent individual difference, the results were indicated as the ratio of perfusion in the ischemic (right) versus non-ischemic (left) limb.
Statistical analysis
SPSS 20.0 was used for the statistical analysis. Kruskal-Wallis H test and Chi square test were used to analyze the expression rate in all groups. One-way analysis of variance (ANOVA) was used to analyze the differences between groups. The LSD method of multiple comparisons was used when the probability for ANOVA was statistically significant. Statistical significance was set at P<0.05.
Results
Plasma miR-23 levels in CAD patients versus control group
As shown in Figure 1A, all the CAD subgroups had significantly increased expression of miR-23 when compared with the control group. Within the CAD subgroups, patients with AMI appeared to have the lowest level of circulation miR-23, although there were no statistical differences among the three subgroups. In addition, the syntax score was correlated with plasma miR-23 level (Spearman’s rank correlation coefficient; r = −0.468, P < 0.01) (Figure 1B).
Figure 1.

Expression pattern of circulating miR-23 in CAD patients. A. Circulating miR-23 levels in controls and CAD sub-groups. miR-23 was up-regulated in CAD patients (all P<0.05 as compared to controls; P>0.05 when compared among SAP, UAP and AMI sub-groups). B. Correlation between the Syntax score and plasma level of miR-23. (Spearman’s r = 0.468, P<0.01). C. Plasma levels of PLGF in CAD patients and controls. Compared to controls, patients with UAP and AMI had significantly lower level of plasma PLGF. (P = 0.86, SAP vs. Control; P = 0.012, UAP vs. Control; P = 0.005, AMI vs. Control). AMI: acute myocardial infarction; CAD: coronary artery disease patients; PLGF: placental growth factor; SAP: stable angina pectoris; UAP: unstable angina pectoris.
Plasma PLGF levels in CAD patients versus control group
The mean level of plasma PLGF was 32.3 ± 9.3 pg/mL in patients with SAP, which was not significantly different from the control group (28.9 ± 6.6 pg/mL, P = 0.86); However, compared with controls, patients with UAP (38.6 ± 9.1 pg/mL) and AMI (46.3 ± 13.4 pg/mL) had a significantly lower level of plasma PLGF (P = 0.012, UAP vs. Control; P = 0.005, AMI vs. Control). These results were depicted in Figure 1C.
Correlation of plasma miR-23 and PLGF levels in CAD patients and controls
In patients with AMI, the miR-23 level was correlated with the PLGF expression (Figure 2), but correlation was not identified in other individual group.
Figure 2.

Correlation between PLGF and miR-23 in CAD patients. (Pearson r = 0.669, P<0.01). AMI: acute myocardial infarction; CAD: coronary artery disease patients; NS: no significant; PLGF: placental growth factor; SAP: stable angina pectoris; UAP: unstable angina pectoris.
Isolation and characterization of human EPCs from the peripheral blood of CAD patients and healthy subjects
EPCs with a cobblestone-like morphology similar to mature endothelial cells were obtained from peripheral blood of CAD patients (CAD-EPCs) or healthy subjects (PB-EPCs) as described (Figure 3A). FACS analyses showed that both EPCs were negative for the hematopoietic marker CD45 yet the CD34 precursor gene (Figure 3B). They also expressed endothelial markers VEGFR2, VE-cadherin, and PECAM (CD31). Cultured EPCs were subjected into Transwell cell migration and tube formation assays, and clearly PBEPC migrated faster and formed microvasculature structure more efficient than diseased EPCs (Figure 3C). PBEPCs also proliferate faster than CAD-EPCs (Figure 3D).
Figure 3.
Reduced VEGF levels and angiogenic activities in EPCs from CAD patients. A. Morphology of healthy and diseased EPCs. B. Expression of indicated molecules in EPCs by flow cytometry analyses. C. Different angiogenic abilities between healthy and diseased EPCs. EPCs from peripheral blood of healthy individuals (PB-EPCs) migrate faster and form better microvasculature structures in vitro than those from patients with CAD (CAD-EPCs). EPCs from different sources were subjected to Transwell cell migration assays or onto Matrigel for tube formation assays. Migrated cells were stained (representative pictures are shown, left lower panel) and counted (right panel, n = 3). Tube lengths of formed microvascular structure were also measured (middle panel, n = 3). *: P<0.05 by Student’s T test. D. Cell proliferation assays show PB-EPCs grow faster in vitro. Cultured EPCs were subjected into MTT assays for monitoring cell proliferation rate. *: P<0.05 by Student’s T test.
miR-23 regulates EPC function via targeting VEGF
Knock down of miR-23 in CAD EPCs to a level compatible to that in healthy EPCs by oligonucleotide antagomirs restored VEGF expression (Figure 4A). More importantly, angiogenic-related abilities, including the migratory and microtubule formation activities, of diseased EPCs were rescued to levels similar to those of healthy controls (Figure 4B, 4C).
Figure 4.
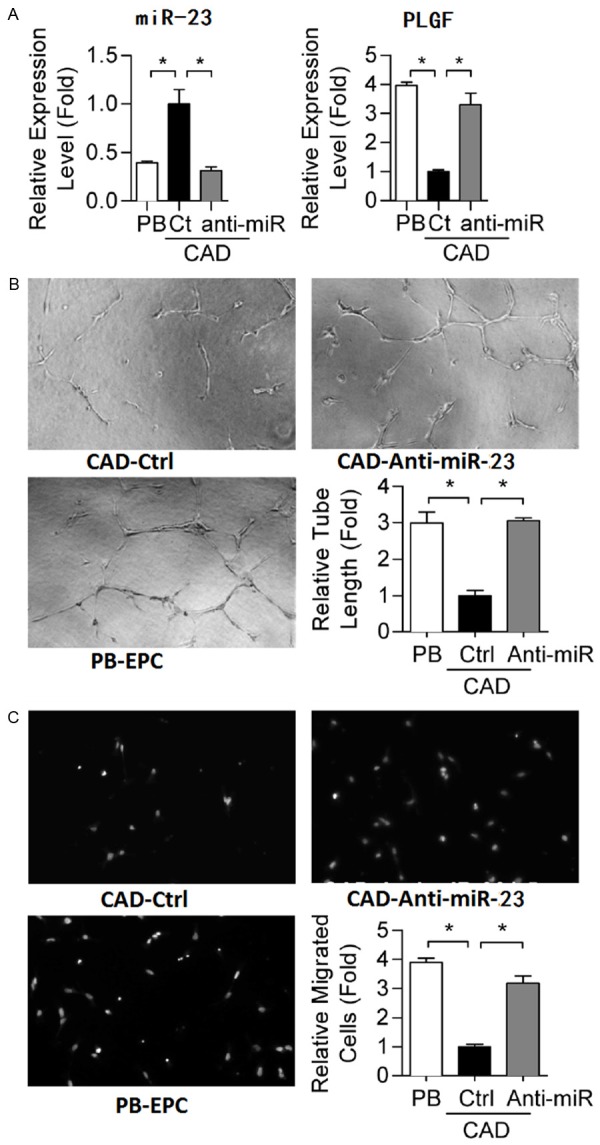
Restoring miR-23 level in CAD-EPCs repairs EPC functions. (A) RT-qPCR shows that miR-23 oligonucleotide antagomir transfection represses CAD-EPC miR-23 to a level similar to that in normal PB-EPC controls. EPC vasculogenesis (B) and migration (C) abilities were also measured. *: P<0.05 by one-way ANOVA test followed by Tukey’s post-hoc test.
miR-23 promotes blood flow recovery in ischemic limbs in mice
miR-23 antagomirs were transfected into diseased EPCs 24 hours before transplantation, and the repression of miR-23 levels in transplanted EPCs at day 0 of injection were verified by RTqPCR (Figure 5A). Local EPC injection was given at the ischemic hind limb distal to the arterial occlusion site intramuscularly 3 days after the surgery, and mice were followed for 2 weeks. As illustrated in Figure 5B, mice without EPC transplantation (the EGM2 group) showed delayed blood flow recovery after ischemia surgery compared with that in mice receiving CAD-EPC (the “Scr” group, CAD-EPCs transfected with scramble oligonucleotides), as determined by Laser Doppler imaging. Meanwhile, knockdown of miR-23 in diseased EPCs significantly improved blood flow recovery by 90% in the ischemic limbs in mice (Figure 5B; n = 6 per group). To further evaluate the effect of miR-23 antagomirs on the homing and differentiation to endothelial cells of the injected EPCs, as well as neovascularization/angiogenesis in mice, immunofluorescence staining was conducted on mouse tissue sample at 7 days after CAD-EPC injection. Capillaries in the ischemic muscles were visualized using anti-CD31 immunostaining (green, Figure 5C), while injected human EPCs were detected by PKH-26 fluorescence (red, Figure 5C). Mice that had received antagomirs transfectants showed the presence of more CD31+/PKH-26+ double-positive cells (white arrowheads) in the capillaries of the ischemic muscle compared to the medium and Scr control mice (Figure 5C; quantitative data in Figure 5D). The limb ischemia model showed that blocking miR-23 restored the defective angiogenic activities of CAD-EPCs.
Figure 5.
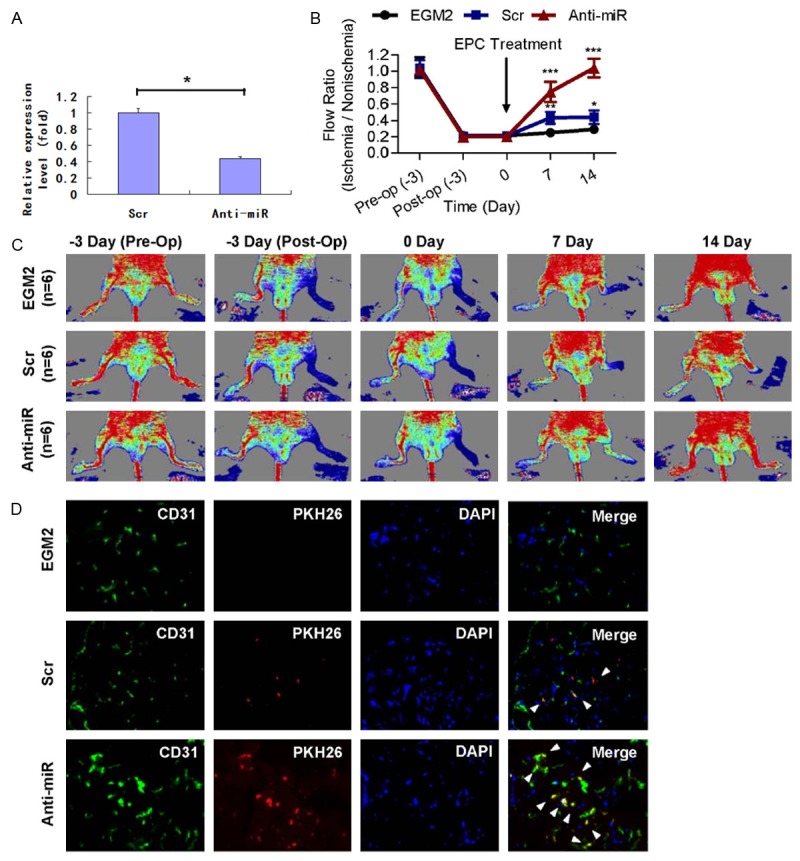
Transplantation of miR-23low CAD-EPCs improves blood perfusion in the ischemic hindlimb. A. miR-23 levels in transfected CAD-EPCs determined by RT-qPCR. *: P<0.05 by Student’s T test. B. Representative images of hind limb blood flow measured by laser Doppler before operation (Pre-Op), immediately after hind limb ischemia surgery (Post-Op), and 2 weeks after intramuscular injection of culture medium (EGM2), peripheral blood EPC-transfected with scramble oligonucleotides (Scr), or CAD-EPC-transfected with miR-23 oligonucleotide antagomirs (Anti-miR). C. Quantitative analysis of capillary densities and CD31+/PKH-26+ double-positive cells in ischemic muscle of mice hind limb ischemia surgery. HPF: high power field; N.D: not detectable; *: P<0.05 by one-way ANOVA test followed by Tukey’s post-hoc test. D. Immunofluorescence staining on nude mice tissues 7 days after injection with PKH-26-labeled CAD-EPCs. Capillaries in the ischemic muscles were visualized by anti-CD31 immunostaining (green), and injected human EPCs were monitored by PKH-26 fluorescence (red). Mice receiving miR-23repressed EPCs had more CD31+/PKH-26+ double-positive cells (white arrowheads) in ischemic muscle than another 2 control mice groups (Scr and medium). DAPI: nuclear staining of live cells (blue).
Discussion
Previous studies demonstrated that miRNAs are abundantly present in a remarkably stable form and they can be detected in peripheral circulation [12-16]. Recently, more and more circulating miRNAs, including heart-, vascular- and muscle- specific miRNAs, have been reported as new biomarkers in multiple cardiovascular diseases [17]. For example, circulating miR-23 is suggested as a biomarker for heart failure [18]. And additionally, cardiac-related miRNAs (miR-208, miR-499 and miR-1) and stress-related miRNAs (miR-21 and miR-146a) may be potential biomarkers for acute coronary syndrome [19]. Moreover, a recent study had reported that circulating miR-126, miR-223 and miR-197 were consistently and significantly related to incidence of myocardial infarction [20]. These observations suggest that circulating miRNAs may be useful not only for prediction of cardiovascular events, but also serve as sensitive biomarkers for improving the diagnostic accuracy of cardiovascular diseases. The present study demonstrated dynamic change in circulating miR-23 expression in the early phase of acute myocardial infarction. Furthermore, our data is the first to demonstrate a positive correlation between circulating miR-23 and the severity of coronary stenosis in CAD patients.
It is believed that the majority of EPCs, which may derived from the CD133+ hemangioblast stem cell population, reside in the bone marrow in a quiescent state and are mobilized into the circulation by specific stimuli. In addition to VEGF, chemotaxis factors such as granulocyte-macrophage colony-stimulating factor (GM-CSF) [21-23], certain drugs such as statin, ischemia, and exercise training can all liberate EPCs from the bone marrow, thereby increasing the number of circulating EPCs [24]. On top of the autocrine stimulation scenario, serum paracrine VEGF, which is released by hypoxic/damaged tissues or cancer cells, also plays a critical role in EPC mobilization and activation. Mechanistically, circulating VEGF protein mobilizes EPCs by activating matrix metalloproteinase-9 (MMP-9), which cleaves membrane bound c-Kit ligand to release soluble c-Kit ligand (also known as stem cell factor) [25,26]. This then stimulates c-Kit+ stem cells, including EPCs, to migrate from a quiescent bone marrow niche to the vascular zone, thereby translocating the cells into a proliferative state [27]. Thus, anti-VEGF microRNAs identified in this study may contribute only in part of the reduced EPC number and activity in CAD patients. It will help to further elucidate CAD pathogenesis by identifying deregulated miRNAs suppressing cKit/CD117 and VEGF receptors (including VEGFR1/FLT1 and VEGFR2/KDR) in CAD-EPCs. For example, miR-221 and miR-222, whose expression levels are up-regulated in CAD-EPCs [28-30], also modulate angiogenesis by targeting c-Kit [31].
Furthermore, circulating miRNAs have been considered as biomarkers for CVDs, diabetes mellitus and cancers, and the distinct modifications in the profile of miRNAs in the blood may sometimes be detectable several years before the disease manifests [32]. Recently, microRNAs are known to actively or passively released in the circulation, and the transfer of RNA via CD63+/CD81+ exosomes functions as a novel mode of intercellular communication [16,33,34]. Most of circulating microRNAs are present in human plasma and serum cofractionate with the Argonaute2 (Ago2) protein. However, circulating microRNAs have also been found in membrane-bound vesicles such as exosomes [35]. Recent evidences point out that exosomal RNAs (exoRNAs) can be used to evaluate health status and disease progression. Moreover, circulating levels of certain miRNAs seem to be predictive of long-term complications. Tumor cells also communicate with endothelial cell via exosomal miRNAs [36]. Here we show that miR-23 secreted by CAD-EPCs are more abundant in patient plasma. Accordingly, it is possible to design new biomarker panels consisting of circulating miR-23 for monitoring EPC activities in vitro or in vivo during the therapeutic procedure. Such biomarker panels will also be useful for monitoring early CVD cases among high-risk population (such as the ones with metabolic syndrome).
Generally speaking, our research disclosed miR-23 suppresses EPC activities in CAD patients via targeting VEGF. With these findings, our research will lead to the development of new diagnosis and/or prognosis approaches for cardiovascular disorders as well as other EPC-related diseases such as diabetes and stroke.
Disclosure of conflict of interest
None.
References
- 1.Wang HW, Huang TS, Lo HH, Huang PH, Lin CC, Chang SJ, Liao KH, Tsai CH, Chan CH, Tsai CF, Cheng YC, Chiu YL, Tsai TN, Cheng CC, Cheng SM. Deficiency of the microRNA-31-microRNA-720 pathway in the plasma and endothelial progenitor cells from patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2014;34:857–69. doi: 10.1161/ATVBAHA.113.303001. [DOI] [PubMed] [Google Scholar]
- 2.Bollati V, Angelici L, Rizzo G, Pergoli L, Rota F, Hoxha M, Nordio F, Bonzini M, Tarantini L, Cantone L, Pesatori AC, Apostoli P, Baccarelli AA, Bertazzi PA. Microvesicle-associated microRNA expression is altered upon particulate matter exposure in healthy workers and in A549 cells. J Appl Toxicol. 2015;35:59–67. doi: 10.1002/jat.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiong XD, Cho M, Cai XP, Cheng J, Jing X, Cen JM, Liu X, Yang XL, Suh Y. A common variant in pre-miR-146 is associated with coronary artery disease risk and its mature miRNA expression. Mutat Res Fundam Mol Mech Mutagen. 2014;761:15–20. doi: 10.1016/j.mrfmmm.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Slagsvold KH, Rognmo O, Høydal M, Wisløff U, Wahba A. Remote ischemic preconditioning preserves mitochondrial function and influences myocardial microRNA expression in atrial myocardium during coronary bypass surgery. Circ Res. 2014;114:851–9. doi: 10.1161/CIRCRESAHA.114.302751. [DOI] [PubMed] [Google Scholar]
- 5.Stratz C, Nührenberg T, Fiebich BL, Amann M, Kumar A, Binder H, Hoffmann I, Valina C, Hochholzer W, Trenk D, Neumann FJ. Controlled type II diabetes mellitus has no major influence on platelet micro-RNA expression. Results from micro-array profiling in a cohort of 60 patients. Thromb Haemost. 2014;111:902–11. doi: 10.1160/TH13-06-0476. [DOI] [PubMed] [Google Scholar]
- 6.D’Alessandra Y, Carena MC, Spazzafumo L, Martinelli F, Bassetti B, Devanna P, Rubino M, Marenzi G, Colombo GI, Achilli F, Maggiolini S, Capogrossi MC, Pompilio G. Diagnostic potential of plasmatic MicroRNA signatures in stable and unstable angina. PLoS One. 2013;8:e80345. doi: 10.1371/journal.pone.0080345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nabiałek E, Wańha W, Kula D, Jadczyk T, Krajewska M, Kowalówka A, Dworowy S, Hrycek E, Włudarczyk W, Parma Z, Michalewska-Włudarczyk A, Pawłowski T, Ochała B, Jarząb B, Tendera M, Wojakowski W. Circulating microRNAs (miR-423-5p, miR-208a and miR-1) in acute myocardial infarction and stable coronary heart disease. Minerva Cardioangiol. 2013;61:627–37. [PubMed] [Google Scholar]
- 8.Vickers KC, Moore KJ. Small RNA overcomes the challenges of therapeutic targeting of microsomal triglyceride transfer protein. Circ Res. 2013;113:1189–91. doi: 10.1161/CIRCRESAHA.113.302732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun X, He S, Wara AK, Icli B, Shvartz E, Tesmenitsky Y, Belkin N, Li D, Blackwell TS, Sukhova GK, Croce K, Feinberg MW. Systemic delivery of microRNA-181b inhibits nuclear factor-κB activation, vascular inflammation, and atherosclerosis in apolipoprotein E-deficient mice. Circ Res. 2014;114:32–40. doi: 10.1161/CIRCRESAHA.113.302089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang F, Long G, Zhao C, Li H, Chaugai S, Wang Y, Chen C, Wang DW. Plasma microRNA-133a is a new marker for both acute myocardial infarction and underlying coronary artery stenosis. J Transl Med. 2013;11:222. doi: 10.1186/1479-5876-11-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Wang R, Du W, Wang S, Yang L, Pan Z, Li X, Xiong X, He H, Shi Y, Liu X, Yu S, Bi Z, Lu Y, Shan H. Downregulation of miR-151-5p contributes to increased susceptibility to arrhythmogenesis during myocardial infarction with estrogen deprivation. PLoS One. 2013;8:e72985. doi: 10.1371/journal.pone.0072985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jansen F, Yang X, Hoelscher M, Cattelan A, Schmitz T, Proebsting S, Wenzel D, Vosen S, Franklin BS, Fleischmann BK, Nickenig G, Werner N. Endothelial microparticle-mediated transfer of MicroRNA-126 promotes vascular endothelial cell repair via SPRED1 and is abrogated in glucose-damaged endothelial microparticles. Circulation. 2013;128:2026–38. doi: 10.1161/CIRCULATIONAHA.113.001720. [DOI] [PubMed] [Google Scholar]
- 13.Tousoulis D, Papageorgiou N, Androulakis E, Siasos G, Latsios G, Tentolouris K, Stefanadis C. Diabetes mellitus-associated vascular impairment: novel circulating biomarkers and therapeutic approaches. J Am Coll Cardiol. 2013;62:667–76. doi: 10.1016/j.jacc.2013.03.089. [DOI] [PubMed] [Google Scholar]
- 14.Liao XB, Zhang ZY, Yuan K, Liu Y, Feng X, Cui RR, Hu YR, Yuan ZS, Gu L, Li SJ, Mao DA, Lu Q, Zhou XM, de Jesus Perez VA, Yuan LQ. MiR-133a modulates osteogenic differentiation of vascular smooth muscle cells. Endocrinology. 2013;154:3344–52. doi: 10.1210/en.2012-2236. [DOI] [PubMed] [Google Scholar]
- 15.Danowski N, Manthey I, Jakob HG, Siffert W, Peters J, Frey UH. Decreased expression of miR-133a but not of miR-1 is associated with signs of heart failure in patients undergoing coronary bypass surgery. Cardiology. 2013;125:125–30. doi: 10.1159/000348563. [DOI] [PubMed] [Google Scholar]
- 16.Coleman CB, Lightell DJ Jr, Moss SC, Bates M, Parrino PE, Woods TC. Elevation of miR-221 and -222 in the internal mammary arteries of diabetic subjects and normalization with metformin. Mol Cell Endocrinol. 2013;374:125–9. doi: 10.1016/j.mce.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang F, Li ML, Fang ZF, Hu XQ, Liu QM, Liu ZJ, Tang L, Zhao YS, Zhou SH. Overexpression of MicroRNA-1 improves the efficacy of mesenchymal stem cell transplantation after myocardial infarction. Cardiology. 2013;125:18–30. doi: 10.1159/000347081. [DOI] [PubMed] [Google Scholar]
- 18.Lu HQ, Liang C, He ZQ, Fan M, Wu ZG. Circulating miR-23 is associated with the severity of coronary artery disease. J Geriatr Cardiol. 2013;10:34–8. doi: 10.3969/j.issn.1671-5411.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagner J, Riwanto M, Besler C, Knau A, Fichtlscherer S, Röxe T, Zeiher AM, Landmesser U, Dimmeler S. Characterization of levels and cellular transfer of circulating lipoprotein-bound microRNAs. Arterioscler Thromb Vasc Biol. 2013;33:1392–400. doi: 10.1161/ATVBAHA.112.300741. [DOI] [PubMed] [Google Scholar]
- 20.Tomé-Carneiro J, Larrosa M, Yáñez-Gascón MJ, Dávalos A, Gil-Zamorano J, Gonzálvez M, García-Almagro FJ, Ruiz Ros JA, Tomás-Barberán FA, Espín JC, García-Conesa MT. One-year supplementation with a grape extract containing resveratrol modulates inflammatory-related microRNAs and cytokines expression in peripheral blood mononuclear cells of type 2 diabetes and hypertensive patients with coronary artery disease. Pharmacol Res. 2013;72:69–82. doi: 10.1016/j.phrs.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Yan CH, Li Y, Xu K, Tian XX, Peng CF, Tao J, Sun MY, Han YL. MicroRNA-31 controls phenotypic modulation of human vascular smooth muscle cells by regulating its target gene cellular repressor of E1A-stimulated genes. Exp Cell Res. 2013;319:1165–75. doi: 10.1016/j.yexcr.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Hutcheson R, Terry R, Chaplin J, Smith E, Musiyenko A, Russell JC, Lincoln T, Rocic P. MicroRNA-145 restores contractile vascular smooth muscle phenotype and coronary collateral growth in the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2013;33:727–36. doi: 10.1161/ATVBAHA.112.301116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Mao H, Chen JY, Wen S, Li D, Ye M, Lv Z. Increased expression of microRNA-221 inhibits PAK1 in endothelial progenitor cells and impairs its function via c-Raf/MEK/ERK pathway. Biochem Biophys Res Commun. 2013;431:404–8. doi: 10.1016/j.bbrc.2012.12.157. [DOI] [PubMed] [Google Scholar]
- 24.Balderman JA, Lee HY, Mahoney CE, Handy DE, White K, Annis S, Lebeche D, Hajjar RJ, Loscalzo J, Leopold JA. Bone morphogenetic protein-2 decreases microRNA-30b and microRNA-30c to promote vascular smooth muscle cell calcification. J Am Heart Assoc. 2012;1:e003905. doi: 10.1161/JAHA.112.003905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kin K, Miyagawa S, Fukushima S, Shirakawa Y, Torikai K, Shimamura K, Daimon T, Kawahara Y, Kuratani T, Sawa Y. Tissue- and plasma-specific MicroRNA signatures for atherosclerotic abdominal aortic aneurysm. J Am Heart Assoc. 2012;1:e000745. doi: 10.1161/JAHA.112.000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu J, Chen T, Yang L, Li Z, Wong MM, Zheng X, Pan X, Zhang L, Yan H. Regulation of microRNA-155 in atherosclerotic inflammatory responses by targeting MAP3K10. PLoS One. 2012;7:e46551. doi: 10.1371/journal.pone.0046551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barjaktarovic Z, Anastasov N, Azimzadeh O, Sriharshan A, Sarioglu H, Ueffing M, Tammio H, Hakanen A, Leszczynski D, Atkinson MJ, Tapio S. Integrative proteomic and microRNA analysis of primary human coronary artery endothelial cells exposed to low-dose gamma radiation. Radiat Environ Biophys. 2013;52:87–98. doi: 10.1007/s00411-012-0439-4. [DOI] [PubMed] [Google Scholar]
- 28.Greliche N, Zeller T, Wild PS, Rotival M, Schillert A, Ziegler A, Deloukas P, Erdmann J, Hengstenberg C, Ouwehand WH, Samani NJ, Schunkert H, Munzel T, Lackner KJ, Cambien F, Goodall AH, Tiret L, Blankenberg S, Trégouët DA Cardiogenics Consortium. Comprehensive exploration of the effects of miRNA SNPs on monocyte gene expression. PLoS One. 2012;7:e45863. doi: 10.1371/journal.pone.0045863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun X, Zhang M, Sanagawa A, Mori C, Ito S, Iwaki S, Satoh H, Fujii S. Circulating microRNA-126 in patients with coronary artery disease: correlation with LDL cholesterol. Thromb J. 2012;10:16. doi: 10.1186/1477-9560-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cardin S, Guasch E, Luo X, Naud P, Le Quang K, Shi Y, Tardif JC, Comtois P, Nattel S. Role for MicroRNA-21 in atrial profibrillatory fibrotic remodeling associated with experimental postinfarction heart failure. Circ Arrhythm Electrophysiol. 2012;5:1027–35. doi: 10.1161/CIRCEP.112.973214. [DOI] [PubMed] [Google Scholar]
- 31.Pan Z, Sun X, Shan H, Wang N, Wang J, Ren J, Feng S, Xie L, Lu C, Yuan Y, Zhang Y, Wang Y, Lu Y, Yang B. MicroRNA-101 inhibited postinfarct cardiac fibrosis and improved left ventricular compliance via the FBJ osteosarcoma oncogene/transforming growth factor-β1 pathway. Circulation. 2012;126:840–50. doi: 10.1161/CIRCULATIONAHA.112.094524. [DOI] [PubMed] [Google Scholar]
- 32.Gao W, He HW, Wang ZM, Zhao H, Lian XQ, Wang YS, Zhu J, Yan JJ, Zhang DG, Yang ZJ, Wang LS. Plasma levels of lipometabolism-related miR-122 and miR-370 are increased in patients with hyperlipidemia and associated with coronary artery disease. Lipids Health Dis. 2012;11:55. doi: 10.1186/1476-511X-11-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hulsmans M, Sinnaeve P, Van der Schueren B, Mathieu C, Janssens S, Holvoet P. Decreased miR-181a expression in monocytes of obese patients is associated with the occurrence of metabolic syndrome and coronary artery disease. J Clin Endocrinol Metab. 2012;97:E1213–8. doi: 10.1210/jc.2012-1008. [DOI] [PubMed] [Google Scholar]
- 34.Tabuchi T, Satoh M, Itoh T, Nakamura M. MicroRNA-34a regulates the longevity-associated protein SIRT1 in coronary artery disease: effect of statins on SIRT1 and microRNA-34a expression. Clin Sci (Lond) 2012;123:161–71. doi: 10.1042/CS20110563. [DOI] [PubMed] [Google Scholar]
- 35.Vavuranakis M, Kariori M, Vrachatis D, Aznaouridis K, Siasos G, Kokkou E, Mazaris S, Moldovan C, Kalogeras K, Tousoulis D, Stefanadis C. MicroRNAs in aortic disease. Curr Top Med Chem. 2013;13:1559–72. doi: 10.2174/15680266113139990105. [DOI] [PubMed] [Google Scholar]
- 36.Eskildsen TV, Jeppesen PL, Schneider M, Nossent AY, Sandberg MB, Hansen PB, Jensen CH, Hansen ML, Marcussen N, Rasmussen LM, Bie P, Andersen DC, Sheikh SP. Angiotensin II Regulates microRNA-132/-212 in Hypertensive Rats and Humans. Int J Mol Sci. 2013;14:11190–207. doi: 10.3390/ijms140611190. [DOI] [PMC free article] [PubMed] [Google Scholar]



