Abstract
The prototyping of tissue-engineered bone scaffold (calcined goat spongy bone-biphasic ceramic composite/PVA gel) by 3D printing was performed, and the biocompatibility of the fabricated bone scaffold was studied. Pre-designed STL file was imported into the GXYZ303010-XYLE 3D printing system, and the tissue-engineered bone scaffold was fabricated by 3D printing using gel extrusion. Rabbit bone marrow stromal cells (BMSCs) were cultured in vitro and then inoculated to the sterilized bone scaffold obtained by 3D printing. The growth of rabbit BMSCs on the bone scaffold was observed under the scanning electron microscope (SEM). The effect of the tissue-engineered bone scaffold on the proliferation and differentiation of rabbit BMSCs using MTT assay. Universal testing machine was adopted to test the tensile strength of the bone scaffold. The leachate of the bone scaffold was prepared and injected into the New Zealand rabbits. Cytotoxicity test, acute toxicity test, pyrogenic test and intracutaneous stimulation test were performed to assess the biocompatibility of the bone scaffold. Bone scaffold manufactured by 3D printing had uniform pore size with the porosity of about 68.3%. The pores were well interconnected, and the bone scaffold showed excellent mechanical property. Rabbit BMSCs grew and proliferated on the surface of the bone scaffold after adherence. MTT assay indicated that the proliferation and differentiation of rabbit BMSCs on the bone scaffold did not differ significantly from that of the cells in the control. In vivo experiments proved that the bone scaffold fabricated by 3D printing had no acute toxicity, pyrogenic reaction or stimulation. Bone scaffold manufactured by 3D printing allows the rabbit BMSCs to adhere, grow and proliferate and exhibits excellent biomechanical property and high biocompatibility. 3D printing has a good application prospect in the prototyping of tissue-engineered bone scaffold.
Keywords: 3D printing, tissue-engineered bone, bone scaffold, biocompatibility
Introduction
Dental caries, periodontal diseases, trauma and tumors can cause dentition defect, leading to resorption of residual ridge and the subsequent decrease of mandibular bone mass. This not only results in poor dental repair effect, but also restricts the prosthetic options in dentistry. Allograft and autograft implantation is usually adopted for bone augmentation technique. The overall failure rate of bone augmentation varies from 16% to 50%, and autograft implantation has a lower failure rate [1]. However, both two methods have defects. Allograft implantation causes secondary injury, while autograft may disseminate diseases. The 3D printing provides an ideal solution to these problems. Being one of the most promising rapid prototyping techniques in biomedical engineering, 3D printing was first proposed by Sachs et al. [2] from Massachusetts Institute of Technology. Now this technique has found wide applications in biomedical engineering. Although many studies are devoted to the manufacturing of tissue-engineered bone scaffold using 3D printing, very few domestic researchers deal with the spatial structure and material biocompatibility of bone scaffold made by this technique. Therefore, the research over the manufacturing of tissue-engineered bone scaffold with a reasonable spatial structure and good mechanical performance and biocompatibility using 3D printing has a high practical significance.
Materials and methods
Materials and equipments
Fresh goat vertebrae were purchased from market. Thirty healthy male adult SPF New Zealand white rabbits were provided by Experimental Animal Center of the First Affiliated Hospital of Xinjiang Medical University, weighing 2.0-2.5 KG.
Experimental method
Rapid prototyping of tissue-engineered bone scaffold by 3D printing
The bone scaffold was manufactured by extrusion-based rapid prototyping technique using gels, the principle of which was similar to fused deposition modeling (FDM). The gel extrusion and deposition rapid prototyping system developed by Xinjiang University was used. The pre-designed STL file [3] was imported into the upper computer software Delphi developed by Xinjiang University to convert STL 3D model into G code. The data of cross-sectional contour and the optimal path of movement along Z axis were determined. The workbench made resultant movement along X-Y axis, while the extruder moved along Z axis using the nozzle movement control system. The 15% PVA/calcined goat spongy bone was mixed with biphasic ceramic powder at the volume ratio of 1:2 (the bone powder was compacted before mixing). The mixed powder was loaded to the workbench of extrusion propeller. The nozzle was elevated by a distance equal to the thickness of one cross-section to print the cross-sectional contour by deposition method. This procedure was repeated until the prototyping of the whole scaffold was finished. The gas pressure of the extrusion system was 110-130PSI, and 0.25 mm disposable nozzle was used. The movement speed of the nozzle was 400 mm/min. After the printing of each layer, the extruder was elevated by 0.25 mm along Z axis. Extrusion speed was controlled by precision extrusion control system. The printed scaffold was dried overnight at 40°C in a dryer. Cross-linking reaction proceeded for 30 min at 150°C in muffle furnace [4].
Culture and passage of rabbit BMSCs
Three male adult New Zealand white rabbits were selected for the extraction of 5 ml bone marrow from the ilium under sterile conditions using 1 ml syringe pre-filled with heparin. Primary cell culture was performed by whole bone marrow adherence method [5]. The harvested bone marrow was passed through 100-mesh stainless steel screen and added with DMEM. The supernatant was discarded after centrifugation at 1000 r/min for 5 min. The remaining suspension was mixed with DMEM (containing 10% FBS) at the proportion of 1:3 and then inoculated to 50 ml culture flask. Primary culture cell was conducted at 37°C in a humidified 5% CO2 incubator. The medium was replaced for the first time after 24 h. The cells were washed by PBS buffer twice and further cultured by adding 5 ml DMEM (containing 10% FBS). The medium was replaced every other day. When the cells reached 80% convergence at the bottom of the culture flask, digestion with 0.25% was performed, followed by 1:2 cell passage and amplification.
Preparation of leachate of bone scaffold and MTT proliferation assay for rabbit BMSCs
DMEM/F12 containing 10% FBS was used as the leaching medium at the dose of 1 mL per 0.1 g of bone scaffold. After leaching at 37°C for 72 h, the bone scaffold was taken out. The leachate was centrifuged, and the supernatant was collected. The third generation cells with good growth status were harvested and detected for CD44 by flow cytometry. The CD44-positive cells were made into cell suspension with the concentration of 1*104/ml. Two 96-well plates were used for the experimental group and the control group, 40 wells for each group and 80 wells in total. The cells were inoculated to 96-well plates at 2×103 cells per well and then cultured at 37°C in a 5% CO2 incubator for 24 h. When the cells adhered to the wall under the inverted phase contrast microscope, the medium in the plate was discarded. Into the experimental wells 200 μL leachate of bone scaffold was added, and for the control wells 200 μL DMEM/F12 was added. The medium was replaced once every 2 d for 8 d. At 1 d-8 d of cell culture, 5 wells were selected and added with 40 μl MTT solution (5 mg/ml). Cells were further cultured for 4 h under standard conditions (37°C, 5% CO2, saturation humidity). After the culture was terminated, the medium was removed and 400 μl DMSO was added into each well. The cells were loaded into multimode microplate reader and oscillated at 100 times/min for 10 min, so as to fully dissolve the crystals on the surface of bone scaffold. The absorbance (A) was measured at 490 nm in each well [6]. The stem cells were directly inoculated to the bottom of the wells as a control. The data were analyzed statistically.
SEM observation of cell adherence to the bone scaffold
Three pieces of sterilized scaffold (10 mm×4 mm×2 mm) was completely soaked in low-sugar DMEM for 2 d. Excess water was removed from the bone scaffold with sterile filter paper, and the bone scaffold was placed in 3 mL Petri dish before use. Third generation rabbit BMSCs with good growth status were digested by 0.25% trypsin, with cell concentration adjusted to 1×105/ml. Cell suspension was slowly dropped onto the surface of bone scaffold, 200 μl for each scaffold. After standing for 3 h, the bone scaffold was immersed in culture medium. Medium replacement was performed once every 2 d. At 1 d, 3 d and 6 d of cell culture, 1 piece of bone scaffold-cells composite was collected respectively and fixed in 3% glutaraldehyde. Gradient ethanol dehydration was performed using ethyl acetate as replacement. The bone scaffold was subject to critical point drying and metal spraying and observed under SEM.
Acute toxicity test, pyrogenic test and intracutaneous stimulation test of the bone scaffold
The sterilized scaffold was loaded to 50 mL centrifuge tube containing normal saline and placed in 37°C water bath for 72 h (1 ml leaching medium per 0.1 g of bone scaffold). Bacteria were removed by using 0.22 μ filter. The tube sealed in sterile container was stored at 4°C in the fridge.
Acute toxicity test
Twelve New Zealand white rabbits were selected and randomly divided into 2 groups with 6 rabbits in each group. Body weight was measured for each individual. Leachate of bone scaffold (10 mg/KG) preheated to 38.5°C was injected via the marginal ear vein for the experimental rabbits. Normal saline (10 mg/KG) preheated to 38.5°C was injected for the controls. At 24 h, 48 h, 72 h and 7 d after injection, the conditions, manifestations of toxicity and death were observed in the two groups, respectively. Changes of body weight were recorded. Liver, spleen and kidney were harvested from 3 individuals in each of the two groups at 7 d for HE staining.
Pyrogenic test
Nine New Zealand White rabbits were measured for body temperature since 7 d. The rabbits were fasted from food and water for 2 h before the measurement of rectal temperature. After immobilization, thermometer was inserted into the anus with an insertion depth of 5-6 cm. The temperature was read after 5 min. Readings were made once every hour for a total of 4 times. The normal temperature range was set as 38.7-39.5°C, and the difference between the highest and the lowest temperature should not exceed 0.4°C. Three rabbits that satisfied the body temperature requirement were selected and numbered. At 7 d, pyrogenic test was performed under the same room temperature and humidity. The rabbits were immobilized and measured for rectal temperature once very 1 h for 2 times in total. After 15 min, the leachate of bone scaffold preheated to 38.5°C was injected via the marginal ear vein at the dose of 10 ml/KG. Rectal temperature was measured at 1 h, 2 h and 3 h, respectively. The rise of body temperature was calculated as highest reading minus normal body temperature. Scaffold was considered non-pyrogenic if the rise of body temperature was below 0.6°C and the rise of rectal temperature was below 1.4°C for each rabbit.
Intracutaneous stimulation test
Six New Zealand white rabbits were selected, and the hairs within 5 cm×20 cm on the left of the spine were removed 24 h before test. Injection was performed at 1.5 cm from the spine. The spacing between two adjacent points of injection was 2 cm. Three points on the left side of the head were chosen as the negative controls with the injection of 0.2 ml normal saline; three points near the tail were positive controls with the injection of 0.2 ml 20% ethanol in normal saline; three points in the middle part were injected with 0.2 ml leachate of bone scaffold. Local skin and tissues were observed for signs of irritation at 6 h, 24 h, 48 h and 72 h, respectively. The reactions such as red spots and edema at each point of injection were scored through primary irritation index and average primary irrigation index (APII).
Results
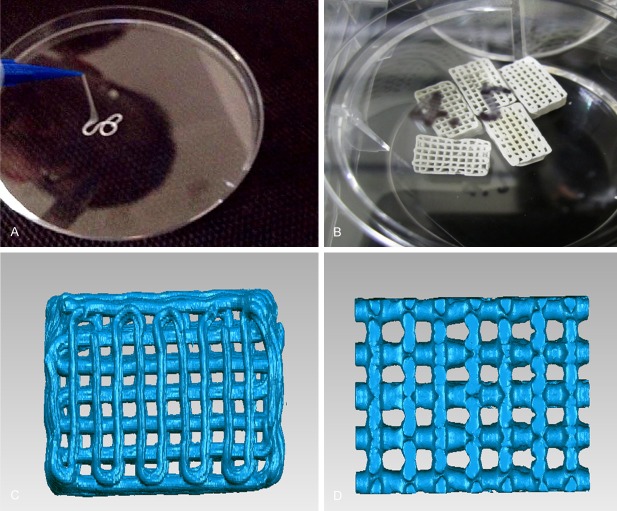
Prototyping of tissue-engineered bone scaffold by 3D printing (Figure 1A and 1B)
Figure 1.
A. Gel extrusion. B. 10×5×5 mm biphasic ceramic powder/PVA bone scaffold prepared by 3D printing. C. Plain view of bone scaffold by MICRO CT analysis. D. Pores over the vertical section of bone scaffold by MICRO CT analysis.
Figure 1C and 1D show the internal structure of bone scaffold using GEOMAGIC reverse engineering software combined with MICRO CT analysis. The bone scaffold had a good permeability, and the pore size was about 380-450 μm, which was suitable for the growth, differentiation and fusion of the cells. The porosity was about 68.3% with good pore interconnection. The bone scaffold exhibited the desired mechanical properties.
Results of biomechanical detection of bone scaffold
According to the measurement results using the universal testing machine, the force-deformation curves were plotted at the feed speed of 1 mm/min as shown in Figure 2A. The average compressive strength was 1513 N. It can be seen from the stress-strain curve that the yield strength of the bone scaffold was about 12.3 MP (Figure 2B).
Figure 2.
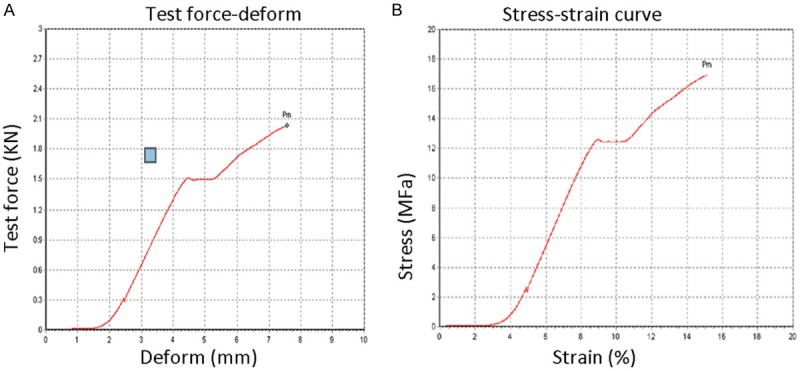
A. Bone scaffold. B. Mechanical properties of calcined goat spongy bone-biphasic ceramic powder composite.
Morphological observation of rabbit BMSCs and the passaged cells
The passaged BMSCs proliferated rapidly and entered the rapid growth phase on day 3. Proliferation peak was reached on day 5, and the stable phase began on day 6. The passaged BMSCs were mostly long spindle-shaped, and cell arrangement had an obvious directionality (Figure 3).
Figure 3.
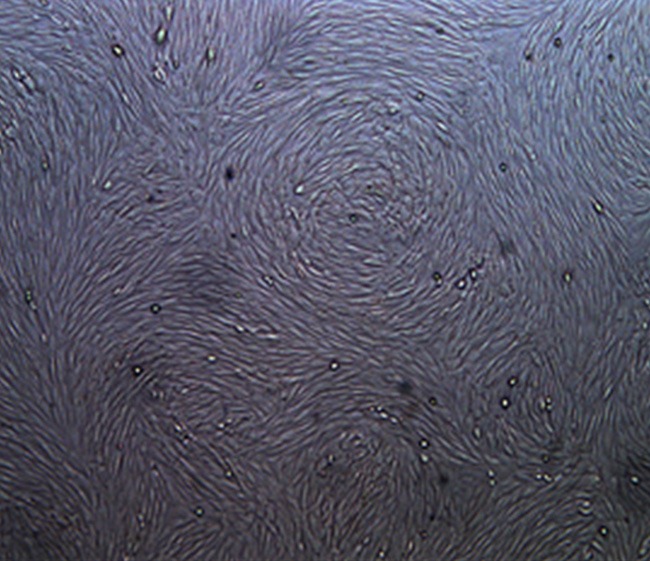
Image of third generation rabbit BMSCs in stable phase (×50).
Effect of scaffold material on proliferability of rabbit BMSCs
As shown in Figure 4, the proliferation curves of the two groups overlapped on 1 d-2 d. The proliferability of the two groups increased on 2-4 d, and the proliferation curve of the control group was slightly above that of the experimental group. On 5 d, the two proliferation curves were nearly parallel, and the absorbance values of the two groups were close. The absorbance of the experimental group was higher than that of the control on 6 d. After entering the stable phase on 7-8 d, the absorbance stabilized at a certain level. On 1-8 d, the absorbance values of the two groups were alternately higher and lower than each other.
Figure 4.
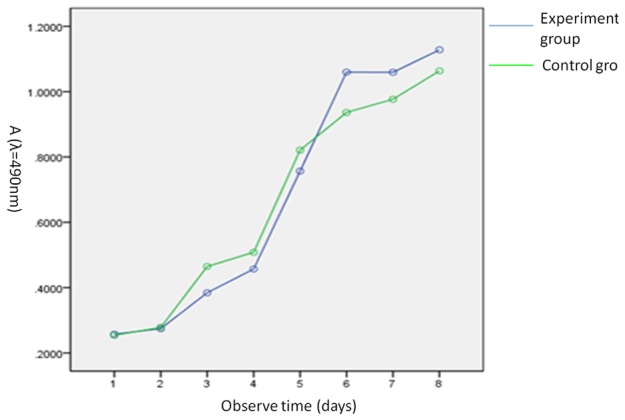
Effect of leachate of bone scaffold on proliferability of rabbit BMSCs in experimental group and control group using MTT proliferation assay.
SPSS19.0 software was used for statistical analyses. Analysis of variance was performed for repeated measurements of MTT assay. There were no significant differences in absorbance between the two groups at 8 d after incubation of rabbit BMSCs with leachate of bone scaffold (P>0.05). The relative proliferation rates of two groups were all higher than 90%. Thus the cytotoxicity of leachate of bone scaffold on rabbit BMSCs belonged to class 0-1, i.e., no obvious cytotoxicity. See Table 1.
Table 1.
Results of MTT assay of rabbit BMSCs cultured in bone scaffold
| Group | 1 d | 2 d | 3 d | 4 d | 5 d | 6 d | 7 d | 8 d |
|---|---|---|---|---|---|---|---|---|
| Experimental | 0.257 ± 0.038 | 0.274 ± 0.012 | 0.384 ± 0.018 | 0.457 ± 0.044 | 0.756 ± 0.041 | 1.059 ± 0.129 | 1.059 ± 0.133 | 1.128 ± 0.195 |
| Control | 0.254 ± 0.025 | 0.278 ± 0.028 | 0.464 ± 0.019 | 0.508 ± 0.040 | 0.821 ± 0.073 | 0.936 ± 0.141 | 0.976 ± 0.044 | 1.063 ± 0.126 |
Note: P>0.05 indicates no statistically significant difference between the experimental group and the control group.
SEM observation of cell adherence and growth on bone scaffold (Figure 5A)
Figure 5.
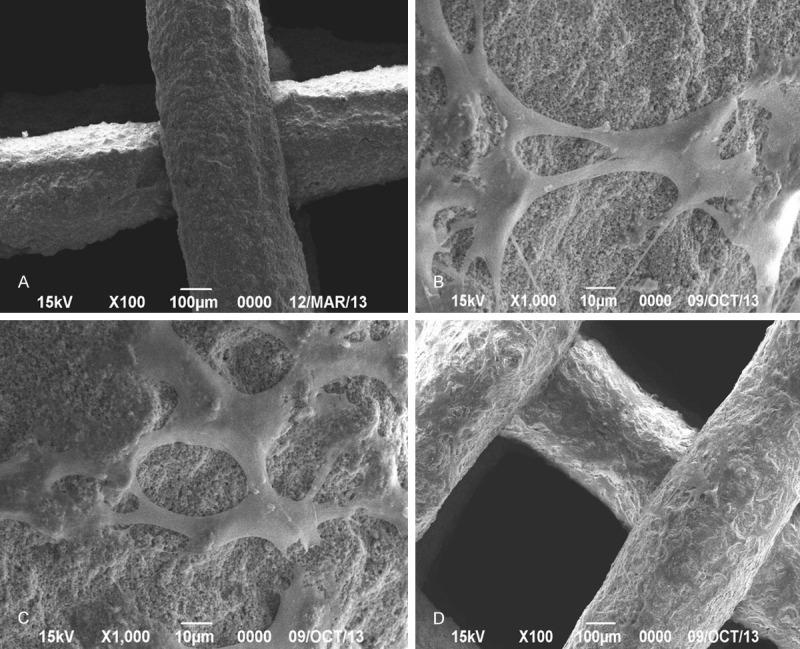
A. SEM image of bone scaffold. B. Cell adherence at 1 d of co-culture. C. Cell adherence at 3 d of co-culture. D. Cell adherence at 6 d of co-culture.
Cell adherence on 1 d of cell culture is shown in Figure 5B. BMSCs tightly adhered to the bone scaffold, being long spindle-shaped or flat and polygonal with filaments. The protrusions were obvious showing an ingrowth into the bone scaffold. On 3 d of cell culture (Figure 5C), a large number of long spindle-shaped or polygonal BMSCs adhered to and grew on the bone scaffold. Part of the scaffold surface was densely covered by interconnected cells, which grew in stacked layers. On 6 d, the majority of the bone scaffold was covered by cells growing in stacked layers (Figure 5D).
Results of acute toxicity test, pyrogenic test and intracutaneous stimulation test of bone scaffold
Results of acute toxicity test of bone scaffold
On 7 d after injection of leachate of bone scaffold, all rabbits showed normal eating and movement and good mental state. There were no signs of toxicity such as paralysis, respiratory inhibition or reduced activities. Body weight increased in the experimental rabbits at different time points. The rabbits were sacrificed after 7 d, and the liver, spleen and kidney were harvested and made into sections. HE staining indicated no abnormalities (Figure 6A and 6B). Thus the bone scaffold had no acute toxicity.
Figure 6.
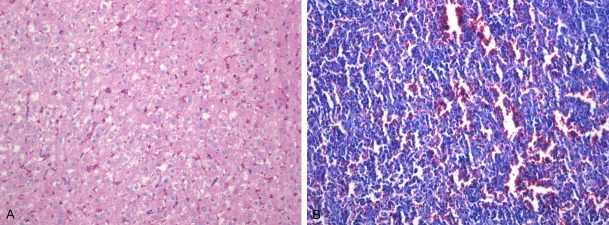
A. HE staining of liver slices of New Zealand white rabbits. B. HE staining of spleen slices of New Zealand white rabbits.
Results of pyrogenic test of bone scaffold
At 1 h, 2 h and 3 h after injection of leachate of bone scaffold, the body temperature rise in all 3 experimental rabbits was lower than 0.6°C, respectively. The total temperature rise was 0.56°C, which satisfied the national standard that the rise should be lower than 1.4°C. One-way ANOVA showed that there were no significant differences in body temperature before and after experiment (P>0.05). For the rabbits receiving chemical treatment, there was an abnormal rise of body temperature. This indicated the material used for physical and chemical treatment did not induce pyrogenic reaction. ANOVA for completely randomized design showed that there were no significant changes of body temperature before and after pyrogenic test (P>0.05, Table 2).
Table 2.
Changes of body temperature of New Zealand white rabbits in pyrogenic test using leachate of bone scaffold (X ± S, n=3, °C)
| Group | Normal body temperature (°C) | Body temperature after experiment | Difference (°C) | P | ||
|---|---|---|---|---|---|---|
|
|
||||||
| 1 h | 2 h | 3 h | ||||
| Experimental | 39.02 ± 0.28 | 39.04 ± 0.18 | 39.14 ± 0.02 | 39.09 ± 0.22 | 0.18 ± 0.08 | >0.05 |
| Control | 39.12 ± 0.11 | 39.87 ± 0.30 | 39.54 ± 0.16 | 39.69 ± 0.10 | 0.82 ± 0.07 | >0.05 |
P>0.05 indicates no statistically significant difference between the experimental and the control group.
Results of intracutaneous stimulation test of bone scaffold
Local skin was observed for red spots, edema and necrosis in the experimental group and the control group immediately after injection and at 6 h, 24 h, 48 h and 72 h, respectively. APII was 0, indicating extremely mild irritation. Obvious red spots were seen at 6 h and 24 h in the positive control, standing above the skin by about 2.5-3 mm. The diameter of the red spot was about 1.5 cm, with edema in the middle. The texture was hard by palpation, and the edema was not mobile. The red spots were deepened after 48 h, and necrosis appeared in the middle at 72 h. A small amount of purulent secretions with scabs were observed near the point of injection. APII was 5.28, indicating strong irritation.
Discussion
3D printing combined with tissue engineering for the manufacturing of tissue-engineered bone scaffold has become a hot research topic in bone tissue engineering. After the rapid prototyping of bone scaffold using 3D printing with high precision, the appropriate seed cells are immobilized onto the surface of the bone scaffold to prepare the tissue-engineered bone suitable for implantation. The key issue is the precision prototyping of bone scaffold that satisfies the requirement of bone tissue engineering. We used calcined goat spongy bone-biphasic ceramic composite/PVA gel in the manufacturing of tissue-engineered bone scaffold. The detection of biocompatibility indicators confirmed the biocompatibility of the material. Thus new method and experimental basis are provided for the manufacturing of tissue-engineered bone scaffold using 3D printing.
STL file after stratification was imported into the 3D printing system. The tissue-engineered bone scaffold in gel state was extruded layer by layer to produce the scaffold body, which was followed by drying and low-temperature crosslinking reaction. SEM and reverse software were used to for multi-angle detection of the bone scaffold. The pore size of the bone scaffold was 380-450 microns, the porosity about 68.3%, and the interconnection rate 84%. The feed rate was 1 mm/min in universal testing machine, and the force-deformation curve was obtained as shown in Figure 2A. It can be seen that the yield strength of the bone scaffold was about 12.3 MP. The manufactured scaffold met the mechanical requirement in bone tissue engineering.
The seed cells to be inoculated are supposed to have high proliferation and differentiation ability, colonization ability in vivo, strong adaptation to the recipient area, easy availability and no immunologic rejection. Besides multi-differentiation potential, BMSCs are multipotential seed cells featured by high proliferability and differentiability and low immunosuppression and immunogenicity. Under different induction conditions, they can be differentiated into osteoblasts, fibroblasts and chondrocytes [7]. Moreover, BMSCs also have the advantages of easy availability and easy separation and culture. BMSCs can maintain the osteogenic potential after many generations of passage. Many studies have been done over the use of rabbit BMSCs as seed cells in allogeneic implantation. In this paper, we combined BMSCs with scaffold fabricated by 3D printing and demonstrated the feasibility of this technique.
Rabbit BMSCs were inoculated to the calcined goat spongy bone-biphasic ceramic composite/PVA gel, and the adherence of the cells to the bone scaffold was observed. Biocompatibility of the bone scaffold was assessed based on the growth and proliferation of the cells on the bone scaffold. At 1 d of cell culture, SEM showed that BMSCs closely adhered to the surface of the bone scaffold. The cells were long spindle-shaped with obvious protuberances or flat and polygonal with filaments, showing the trend of in growth into the bone scaffold. At 3 d of cell culture, a large number of rabbit BMSCs adhered to the surface. The cells were long spindle-shaped or polygonal. In some area, the cells were interconnected and stacked. The majority of the surface of the bone scaffold was covered by stacked layers of cells at 6 d of cell culture. The results indicated that the tissue-engineered bone scaffold manufactured by 3D printing can be used as the carrier for the adherence, growth and proliferation of rabbit BMSCs.
Scaffold is the basis for the manufacturing of tissue-engineered bone. As the seed cells grow and proliferate, the biomaterial is degraded and gradually replaced by the secreted extracellular matrix. The seed cells will finally develop into normal tissues to repair the bone defect.
MTT cytotoxicity assay was performed on the leachate of bone scaffold. From 1 d to 2 d of cell culture, the proliferation curves of the cells in the experimental group and the control group overlapped. From 2 d to 4 d, the proliferation ability of the cells in the two groups increased, with the cell proliferation curve of the control group slightly above that of the experimental group. At 5 d of culture, two cell proliferation curves were almost in parallel, and the two groups had close absorbance values. The absorbance value of the experimental group was higher than that of the control at 6 d. The period from 7 d to 8 d was the plateau phase, where the absorbance stabilized on a certain level. From 1 d to 8 d, the absorbance values of the two groups were alternately higher and lower than each other. Analysis of variance of repeated data was carried out using SPSS19.0 software. The results showed that the absorbance values (A) were not significantly different in the two groups (P>0.05), and the relative proliferation rates were both higher than 90%. The cytotoxicity type of leachate of bone scaffold on rabbit BMSCs was class 0-1, indicating no obvious cytotoxicity. The safety of the bone scaffold was evaluated according to the International Standard ISO-10993, “Biological Evaluation of Medical Devices Part 1”. Biocompatibility of the material was assessed by acute toxicity test, pyrogenic test and intracutaneous stimulation test. To ensure that the bone scaffold had no toxicity and caused no injury to the organism, the leachate of the bone scaffold was injected intracutaneously into New Zealand rabbits. The injection was performed via the ear margin veins. The results showed that the bone scaffold caused no acute toxicity, pyrogenic reaction or stimulation.
Conclusion
Scaffold that met the requirement in bone tissue engineering was manufactured by 3D printing combined with computer modeling. The calcined goat spongy bone-biphasic ceramic composite/PVA gel was used as the scaffold material. The fabricated scaffold had the desired 3D internal structure, excellent biocompatibility and mechanical performance. The 3D printing technique has bright clinical application prospect for manufacturing composite tissues with seed cells. The findings of the present research lay the foundation for the use of 3D printing in making tissue-engineered bone.
Acknowledgements
This work was supported by the national natural science fund project--The study of construct 3 d printing tissue-engineered alveolar bone scaffold. The Item no. (81060088); The xinjiang uygur autonomous region science and technology project--3 d printing build composite signal induced of the tissue engineering tooth. The Item no. (201291173); The science fund project of xinjiang uygur autonomous region--The Item no. (2014211C037).
Disclosure of conflict of interest
None.
References
- 1.Ma PX, Zhang R, Xiao G, Franceschi R. Engineering new bone tissue in vitro on highly porous poly (alpha-hydroxyl acids)/hydroxyapatite composite scaffolds. J Biomed Mater Res. 2001;54:284–293. doi: 10.1002/1097-4636(200102)54:2<284::aid-jbm16>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 2.Somerville MA, Lincoln MA, Lexington MA, Concord MA, inventors. Three-dimensional printing techniques. 5340656 United States Patent.
- 3.Zhang JY, Liu Q, He HY. Three-dimensional printing bone tissue engineering scaffold technology computer-aided modeling and rapid prototyping. J Oral Sci Res. 2013;29:1178–1182. [Google Scholar]
- 4.Wu C, Zhang Y, Zhu Y, Friis T, Xiao Y. Structure-property relationships of silkmodified mesoporous bioglass scaffolds. Biomaterials. 2010;31:3429–38. doi: 10.1016/j.biomaterials.2010.01.061. [DOI] [PubMed] [Google Scholar]
- 5.Arinzeh TL, Peter SJ, Archambault MP, van den Bos C, Gordon S, Kraus K, Smith A, Kadiyala S. Allogeneic mesenchymal stem cells regenerate bone in a critical-sized canine segmental defect. J Bone Joint Surg Am. 2003;85-A:1927–1935. doi: 10.2106/00004623-200310000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Tang Q, Chen JH, Huang NN, Liang HY. A study of tissue-engineered compound of dog bone marrow stromal cells and the novel porous scaffold in vitro. Chinese Journal of Oral Science Research. 2011;5:367–373. [Google Scholar]
- 7.Ouyang HW, Cao T, Zou XH, Heng BC, Wang LL, Song XH, Huang HF. Mesenchymal stem cell sheets revitalize nonviable dense grafts: implications for repair of large-bone and tendon defects. Transplant. 2006;82:170–174. doi: 10.1097/01.tp.0000226232.79106.72. [DOI] [PubMed] [Google Scholar]