Genetic diversity assessment plays an important role in plant improvement. It becomes more significant when evaluation is done at different ploidy and geographical origin levels. The present study provides a better understanding of the genetic association of Indian and Turkish hexaploid and tetraploid wheat. The Turkish hexaploid population demonstrated its close association with Indian hexaploid and tetraploid varieties. This confirmed their relatedness within the diverse gene pool. The results revealed in this study can be effectively used by breeders and evolutionary biologists for the development of genetically diverse, promising and healthier wheat varieties.
Keywords: Genetic diversity, molecular markers, ploidy level, population structure, wheat
Abstract
Genetic diversity among plant species offers prospects for improving the plant characteristics. Its assessment is necessary to help tackle the threats of environmental fluctuations and for the effective exploitation of genetic resources in breeding programmes. Although wheat is one of the most thoroughly studied crops in terms of genetic polymorphism studies, phylogenetic affinities of Indian and Turkish Triticum species have not been assessed to date. In this study, genetic association of 95 tetraploid and hexaploid wheat genotypes originating from India and Turkey was determined for the first time. Combined analysis of random amplified polymorphic DNA and inter-simple sequence repeat markers disclosed 177 polymorphic bands, and both the dendrogram and two-dimensional scatterplot showed similar groupings of the wheat genotypes. Turkish hexaploid varieties were basically divided into two clusters, one group showed its close association with Indian hexaploid varieties and the other with Indian tetraploid varieties. Analysis of molecular variance revealed high (77 %) genetic variation within Indian and Turkish populations. Population structure analysis elucidated distinct clustering of wheat genotypes on the basis of both geographical origin and ploidy. The results revealed in this study will support worldwide wheat breeding programmes and assist in achieving the target of sustainable wheat production.
Introduction
Genetic variability in natural plant populations holds the potential to deal with multiple biotic and abiotic stresses. The potential to select a superior line increases with genetic diversity, the discovery of which becomes an important tool in plant breeding. On depletion of genetic variability, plants are unable to cope with unfavourable environmental conditions or pathogens and pests. Diversity studies also facilitate the conservation and management aims of a particular plant species. For the effective use of genetic diversity in plant breeding, knowledge of its extent and distribution plays a crucial role. Considering its significance, a large number of studies have been performed to estimate genetic diversity employing various methodologies in multiple plant species. Assessment of genetic variability employing molecular markers has proved to be a keystone to understanding the genomic constitution, categorizing the genes responsible for important traits, the classification and conservation of genetic variation in plant germplasm and developing selective proliferation approaches for plant propagation.
Wheat production in developing countries moved from defective to surplus (Pingali 2012) during the Green Revolution (Gollin et al. 2005). Being a good source of carbohydrate, protein, sugar, fat, fibre and minerals, it provides half of the energy requirements of the human population (Simmonds 1989; Shewry 2007; Topping 2007). A constantly rising population demands an increment in wheat production (Ehrlich 1975; Evans 1998; Tilman et al. 2011; Ray et al. 2013). As the crop already covers a wide agricultural area, there is a negligible possibility of area expansion (Young 1999; Bruinsma 2003; Cassman et al. 2003). Wheat faces multiple demands including its growth under warmer conditions (Vermeulen et al. 2012; Wheeler 2012), fighting various diseases (Summers and Brown 2013), reduced energy input for sustainable growth (Ziaei et al. 2015) and high nutritional quality (Shewry 2007, 2009). Considering this situation, Lynch (2007) has suggested a need for a ‘second green revolution’. This second green revolution must place emphasis on the utilization of inherent resources and the thorough understanding of genetic diversity.
During the course of evolution, wheat gained sufficient genetic diversity along the road from einkorn to bread wheat. Today, however, its diversity is weakening due to repeated cultivation of landraces for specific characters, narrow adaptation, farmers' varietal selection and the requirement of uniform varieties in industrial seed grain processing (Bellon 1996; Smale 1997; Heal et al. 2004). Implementation of high-yielding commercial varieties played an important part in loss of genetic variation. This depletion has now encouraged the use of genetic resources in wheat breeding programmes.
Genetic diversity is crucial for adaptability and survival of wheat species against the threat of disease attack/onset (Fu and Somers 2009). If all the individuals of a population are identical, they will behave similarly to a stress condition and potentially be equally unable to cope with the situation. Hence, it is beneficial to assess the genetic diversity at a particular level that may facilitate the efficient exploitation of the germplasm. Furthermore, in addition to the fact that genetic diversity plays a part in the development of high-yielding bread wheat varieties, issues like the spread of coeliac disease necessitate the development of new genetic variants of tetraploid wheat (van Herpen et al. 2006; van den Broeck et al. 2010).
Polyploidy and genome evolution of wheat are also partially responsible for maintaining its genetic diversity. In a review, Wendel (2000) shed light on several aspects of the genome duplication and divergence leading to the development of evolutionary genetic diversity. Polyploidy resulting from hybridization leads to gene duplication across the entire genome and thus underlies the emergence of genetic variation. The agriculturally important phenomenon of hybrid vigour in polyploids is a consequence of genetic variability. As wheat is a polyploid species, it is beneficial to include tetraploid and hexaploid varieties in genetic variability assessment programmes. Such assessment programmes are imperative for managing populations by identifying the breeding genotypes. For a long time, depiction of diversity was dependent on morphological characterization (Tesfaye et al. 1991; Marić et al. 2004; Takumi et al. 2009). But due to the influence of environmental conditions and changes during developmental stages, morphological traits are considered unreliable for diversity estimation, mainly for closely associated populations.
Momentous progress in molecular genetics benefitted our understanding of the wheat genome and provided approaches for breeding. With the expansion of novel technologies like molecular markers, researchers utilized a range of Triticeae species for genotypic identification (Khan et al. 2014). Molecular marker techniques vary from each other in data generation efficiency and the genome area covered in the study. Selection of the type of marker tool for a study depends on the target crop and the issue. For example, random amplified polymorphic DNA (RAPD) markers are known for their simplicity, cost efficiency, fast polymorphism assessment, no prior information of DNA sequences being required and extensive coverage of the intact genome being possible. However, due to low reproducibility of the RAPD system, expense of the amplified fragment length polymorphism (AFLP) marker system and requirement of prior information about DNA sequences in SSR analysis, another dominant marker system, inter-simple sequence repeats (ISSRs), was included in the study. Due to high annealing temperature and extended sequence in comparison to RAPD markers, ISSR primers can produce more reproducible and reliable band patterns. Inter-simple sequence repeat markers are employed for distinguishing DNA on the basis of single base variation or insertions and deletions, and are equivalent to the SSR system in reproducibility. These markers are widely implemented for DNA fingerprinting, identification of species association, genetic variability studies and for recognizing the geographic origin of different plant species along with their ploidy status (Vierling and Nguyen 1992; Joshi and Nguyen 1993a, b; Autrique et al. 1996; Nagaoka and Ogihara 1997; Sun et al. 1998; Fahima et al. 1999; Pasqualone et al. 2000; Pecetti et al. 2001; Bered et al. 2002; Mukhtar et al. 2002; Pujar et al. 2002; Mandoulakani et al. 2003; Marić et al. 2004; Bhutta et al. 2005; Motawei et al. 2007; Carvalho et al. 2009; Najaphy et al. 2011; Saleh 2012; Izzatullayeva et al. 2014). Hence, in the present study, RAPD and ISSR were chosen among the various marker systems to yield the benefits of both the techniques, diminishing their drawbacks and increasing the credibility of our results.
India and Turkey play crucial roles in supporting food security through wheat production. India holds first and second place in wheat growing area and production, respectively. It has become a priority to replace the uniform high-yielding varieties spread during the Green Revolution with diverse high-quality varieties. Turkey is found to be the place of origin of both tetraploid and hexaploid wheat domestication (Heun et al. 1997; Nesbitt and Samuel 1998; Dubcovsky and Dvorak 2007) and India is known to be the centre of origin of some promising varieties. An assessment of genetic variability and association of tetraploid and hexaploid wheat varieties from the two developing countries would be of immense benefit to wheat improvement programmes.
Association and contrast among the wheat cultivars from different countries can provide a useful overview on the evolutionary record of the genotypes and, hence, can facilitate the reach of breeding improvement. Although a number of genetic similarity studies were conducted on diverse wheat germplasm using ISSR and RAPD marker systems, phylogenetic association of Indian and Turkish Triticum species has not been documented to date. The present study represents the first effort in this direction, its objectives being to gain a better understanding of the genetic association and population structure of Indian and Turkish wheat on the basis of both geographical origin and ploidy. The share of the genetic variations within and among populations was also revealed so that the information provided can be effectively used by scientists for the development of genetically diverse, promising and healthier wheat varieties.
Methods
Study materials
The object of the present diversity study was a collection of 95 Indian and Turkish wheat genotypes including tetraploid (Triticum turgidum ssp. durum) and hexaploid (Triticum aestivum L.) wheat cultivars (Table 1). Well-known varieties were chosen for the experiment to facilitate the use of results in future breeding programmes.
Table 1.
Name and ploidy of 95 Indian and Turkish wheat genotypes used in the study.
| Sl. no. | Name of genotype | Genotype number | Ploidy | Origin |
|---|---|---|---|---|
| 1 | 30_KR-8 | G1 | 6X | India |
| 2 | AAI_2 | G2 | 6X | India |
| 3 | AKAW_4006 | G3 | 6X | India |
| 4 | AKDW_2997 | G4 | 4X | India |
| 5 | DBW_14 | G5 | 6X | India |
| 6 | DBW_39 | G6 | 6X | India |
| 7 | DDK_1025 | G7 | 6X | India |
| 8 | DT_132 | G8 | 4X | India |
| 9 | GW_03-12 | G9 | 6X | India |
| 10 | GW_03-2 | G10 | 6X | India |
| 11 | GW_03-3 | G11 | 6X | India |
| 12 | GW_03-4 | G12 | 6X | India |
| 13 | GW_03-9 | G13 | 6X | India |
| 14 | HD_2177 | G14 | 6X | India |
| 15 | HD_2236 | G15 | 6X | India |
| 16 | HD_2270 | G16 | 6X | India |
| 17 | HD_2307 | G17 | 6X | India |
| 18 | HD_2329 | G18 | 6X | India |
| 19 | HD_2380 | G19 | 6X | India |
| 20 | HD_2402 | G20 | 6X | India |
| 21 | HD_2501 | G21 | 6X | India |
| 22 | HD_2643 | G22 | 6X | India |
| 23 | HD_2881 | G23 | 6X | India |
| 24 | HUW_12 | G24 | 6X | India |
| 25 | HUW_251 | G25 | 6X | India |
| 26 | HUW_37 | G26 | 6X | India |
| 27 | HUW_468 | G27 | 6X | India |
| 28 | HUW_533 | G28 | 6X | India |
| 29 | HUW_55 | G29 | 6X | India |
| 30 | K_01006 | G30 | 6X | India |
| 31 | K_0204 | G31 | 6X | India |
| 32 | K_616 | G32 | 6X | India |
| 33 | K_8020 | G33 | 6X | India |
| 34 | K_86 | G34 | 6X | India |
| 35 | K_88 | G35 | 6X | India |
| 36 | K_911 | G36 | 6X | India |
| 37 | KALYANSONA | G37 | 6X | India |
| 38 | KBD_65 | G38 | 4X | India |
| 39 | KBD_821 | G39 | 4X | India |
| 40 | KBD_921 | G40 | 4X | India |
| 41 | KBD_922 | G41 | 4X | India |
| 42 | KBD_925 | G42 | 4X | India |
| 43 | KBD_9452 | G43 | 4X | India |
| 44 | KBD_9915 | G44 | 4X | India |
| 45 | KD_9851 | G45 | 4X | India |
| 46 | KLP_306 | G46 | 6X | India |
| 47 | KLP_307 | G47 | 6X | India |
| 48 | KLPD_1106 | G48 | 4X | India |
| 49 | NAW_1448 | G49 | 6X | India |
| 50 | NIDW_295 | G50 | 4X | India |
| 51 | NW_1076 | G51 | 6X | India |
| 52 | NW_2036 | G52 | 6X | India |
| 53 | PBW_550 | G53 | 6X | India |
| 54 | RAJ_1482 | G54 | 6X | India |
| 55 | RAJ_1555 | G55 | 4X | India |
| 56 | RAJ_3072 | G56 | 6X | India |
| 57 | RAJ_3077 | G57 | 6X | India |
| 58 | RAJ_3777 | G58 | 6X | India |
| 59 | RAJ_4027 | G59 | 6X | India |
| 60 | RAJ_4037 | G60 | 6X | India |
| 61 | RAJ_4120 | G61 | 6X | India |
| 62 | RAJ_6560 | G62 | 4X | India |
| 63 | RD_1008 | G63 | 4X | India |
| 64 | RD_1063 | G64 | 4X | India |
| 65 | RD_1093 | G65 | 4X | India |
| 66 | RD_1097 | G66 | 4X | India |
| 67 | SAW_327 | G67 | 6X | India |
| 68 | SAW_337 | G68 | 6X | India |
| 69 | SAW_94 | G69 | 6X | India |
| 70 | SONALIKA | G70 | 6X | India |
| 71 | UP_2338 | G71 | 6X | India |
| 72 | UP_2511 | G72 | 6X | India |
| 73 | UP_2525 | G73 | 6X | India |
| 74 | UP_2696 | G74 | 6X | India |
| 75 | VEERI | G75 | 6X | India |
| 76 | VL_832 | G76 | 6X | India |
| 77 | WR_1381 | G77 | 6X | India |
| 78 | WR_1408 | G78 | 6X | India |
| 79 | WR_1421 | G79 | 6X | India |
| 80 | BAYRAKTAR 2000 | G80 | 6X | Turkey |
| 81 | SEVAL | G81 | 6X | Turkey |
| 82 | KENANBEY | G82 | 6X | Turkey |
| 83 | BEZOSTAJA 1 | G83 | 6X | Turkey |
| 84 | GÜN_91 | G84 | 6X | Turkey |
| 85 | KONYA_2002 | G85 | 6X | Turkey |
| 86 | AKBUĞDAY | G86 | 6X | Turkey |
| 87 | GEREK_79 | G87 | 6X | Turkey |
| 88 | KIRAÇ_66 | G88 | 6X | Turkey |
| 89 | ESER | G89 | 6X | Turkey |
| 90 | SÖNMEZ 2001 | G90 | 6X | Turkey |
| 91 | HARMANKAYA 99 | G91 | 6X | Turkey |
| 92 | KINACI 97 | G92 | 6X | Turkey |
| 93 | YÜREĞIR 89 | G93 | 6X | Turkey |
| 94 | ALTAY 2000 | G94 | 6X | Turkey |
| 95 | LÜTFIBEY | G95 | 6X | Turkey |
Plant genomic DNA extraction
Two to three weeks grown seedlings were utilized for total wheat DNA extraction following the cetyltrimethylammonium bromide (CTAB) method (Doyle 1990) with some modifications. Initially, cells were disrupted and purified with 2 % CTAB buffer and 10 μL RNase A, respectively, followed by incubation at 65 °C. This was followed by protein extraction employing phenol : chloroform : isoamyl alcohol and finally the CTAB–DNA complex was precipitated with isopropanol. The DNA pellet was twice washed with 70 % ethanol, dried and ultimately, dissolved in 100 μL DNase–RNase-free water. Purified DNA quantity and quality were verified using spectrophotometry and 1 % agarose gel electrophoresis, respectively. The DNA samples were diluted to a concentration of 50 ng μL−1 as templates for polymerase chain reactions (PCRs).
Inter-simple sequence repeats analysis
Twenty-seven ISSR primers (Metabion) were examined for distinguishing the polymorphism patterns, and among those 10 primers showed positive outcomes (Table 2) against chosen wheat varieties. Every PCR mixture of 25 μL contained 2.5 μL of 10× Taq buffer containing ammonium sulfate (except ISSR F3 where KCl was used), 3 μL of 25 mM MgCl2, 0.4 μL of 25 mM dNTP, 0.5 μL of 10 μM primer, 1.5 units of Taq DNA Polymerase and 100 ng of template DNA. The two-step ISSR–PCR reactions were performed in a Eppendorf Master Cycler. The physical reaction conditions and the number of initial and final PCR cycles were optimized for each individual ISSR primer.
Table 2.
Characteristics and polymorphism revealed by ISSR primers for 95 wheat genotypes used in the study.
| ISSR primer | Sequence | Melting temperature (Tm) | Total number of bands | Polymorphic bands | Per cent polymorphism detected |
|---|---|---|---|---|---|
| ISSR F3 | 5′-(AG)8 CG-3′ | 56.0 | 8 | 7 | 87.5 |
| ISSR F4 | 5′-(AG)8 TG-3′ | 53.7 | 12 | 12 | 100 |
| ISSR F9 | 5′-(GAA)5-3′ | 39.6 | 13 | 12 | 92.3 |
| ISSR M1 | 5′-(AGC)6 G-3′ | 63.1 | 12 | 11 | 91.6 |
| ISSR M2 | 5′-(ACC)6 G-3′ | 63.1 | 14 | 14 | 100 |
| ISSR M3 | 5′-(AGC)6 C-3′ | 63.1 | 17 | 16 | 94.1 |
| ISSR M8 | 5′-(AC)9 G-3′ | 56.7 | 13 | 12 | 92.3 |
| ISSR M9 | 5′-(AC)8 CG-3′ | 56.0 | 13 | 13 | 100 |
| ISSR M12 | 5′-(GACAC)4-3′ | 61.4 | 6 | 6 | 100 |
| ISSR M17 | 5′-CAG (CA)8-3′ | 56.7 | 8 | 7 | 87.5 |
| Total | 116 | 110 | 94.8 |
Random amplified polymorphic DNA analysis
For RAPD reactions, a total of 43 primers (MWG Biotech-AC) were screened for polymorphism using selected genotypes, and 10 primers were selected for the final reactions (Table 3). The PCR mixture contained 1.5 μL of 10× Taq buffer with ammonium sulfate, 2.5 μL of 25 mM MgCl2, 3 μL of 1 mM dNTP, 3 units Taq DNA polymerase (Thermoscientific), 1.5 μL of 5 μM RAPD primer and 50 ng of template DNA in a total volume of 15 μL. Polymerase chain reaction amplifications were carried out utilizing a Eppendorf Master Cycler with initial denaturation at 94 °C for 3 min, followed by repeated cycles of denaturation at 94 °C for 45 s, annealing as per the primer's melting temperature for 1 min and primer extension at 72 °C for 1 min. On completion of the repeated number of cycles, final extension was performed at 72 °C for 10 min.
Table 3.
Characteristics and polymorphism revealed by RAPD primers for 95 wheat genotypes used in the study.
| RAPD primer | Sequence | Melting temperature (Tm) | Total number of bands | Polymorphic bands | Per cent polymorphism detected |
|---|---|---|---|---|---|
| cRAPD1 | 5′-GAA ACG GGT G-3′ | 32 | 6 | 4 | 66.6 |
| cRAPD2 | 5′-GTG ACG TAG G-3′ | 32 | 12 | 11 | 91.6 |
| RAPD B3 | 5′-GTG ACG TAG G-3′ | 34 | 9 | 7 | 77.7 |
| RAPD B4 | 5′-CTC ACC GTC C-3′ | 34 | 6 | 5 | 83.3 |
| RAPD B5 | 5′-GAC GGA TCA G-3′ | 32 | 11 | 10 | 90.9 |
| RAPD B10 | 5′-CTA CTG CGC T-3′ | 32 | 7 | 6 | 85.7 |
| RAPD B13 | 5′-TTC AGG GTG G-3 | 32 | 5 | 3 | 60.0 |
| RAPD L2 | 5′-GTT TCG CTC C-3′ | 32 | 8 | 7 | 87.5 |
| RAPD L4 | 5′- AAG AGC CCG T-3′ | 32 | 11 | 9 | 81.8 |
| RAPD L6 | 5′-CCC GTC AGC A-3′ | 34 | 7 | 5 | 71.4 |
| Total | 82 | 67 | 81.7 |
Data analysis
All the ISSR- and RAPD-based PCRs were repeated three times for the identification of reproducible amplified bands. Amplified fragments were counted from smaller to larger size. A binary data matrix was obtained by scoring the gel as 1 and 0 to show the presence and absence of bands, respectively. Information capacity of the primers and polymorphism content of the genotypes were estimated by calculating the total number of bands and of polymorphic bands. The binary data matrix was used to obtain the similarity matrix depending on simple matching (SM) coefficient by Numerical Taxonomy and Multivariate Analysis System (NTSYS-PC) version 2.02e software (Rohlf 1997). This similarity matrix was utilized in R software for constructing a combined dendrogram of RAPD and ISSR.
On the basis of SM coefficients, the similarity matrix was double centred using the DCENTER module of NTSYS-PC. Then eigen analyses were performed using the EIGEN module of NTSYS-PC to construct two-dimensional scatterplots by the R package. Scatterplots were drawn for the substantiation of the dendrograms and verification of genotypes clustering according to both ploidy and geographical origin.
To explain the population structure of Indian and Turkish wheat genotypes, analysis of molecular variance (AMOVA) was performed using GenAlEx 6.5 software (Peakall and Smouse 2006, 2012) with 1000 permutations. The programme was used for the determination of variance components and estimating the total variation within and among the populations.
Bayesian model-based clustering with assumed K populations was employed for genetically homogenous group estimation in Indian and Turkish wheat germplasm. A parameter of 50 000 burn-in period and 100 000 Markov Chain Monte Carlo replication, along with the admixture model and correlated allele frequencies, was used in STRUCTURE, version 2.3.4 (Pritchard et al. 2000; Falush et al. 2003, 2007; Hubisz et al. 2009). A total of 10 independent runs were performed for each value of K (from 1 to 4 assumed) (Evanno et al. 2005). For the determination of the best possible K value elucidating the genetically distinctive clusters in the data, the Structure Harvester v6.0 (Earl and vonHoldt 2012) programme was used implementing parameters described by Evanno et al. (2005).
Results
Genetic diversity
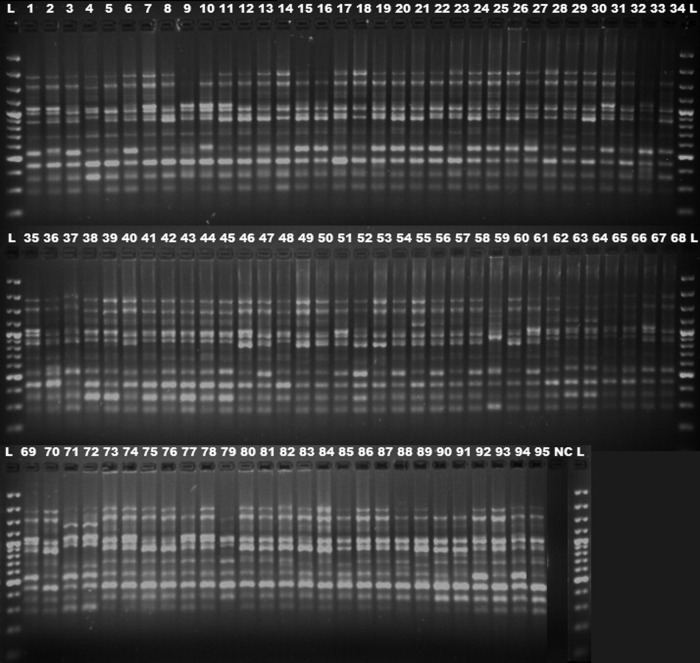
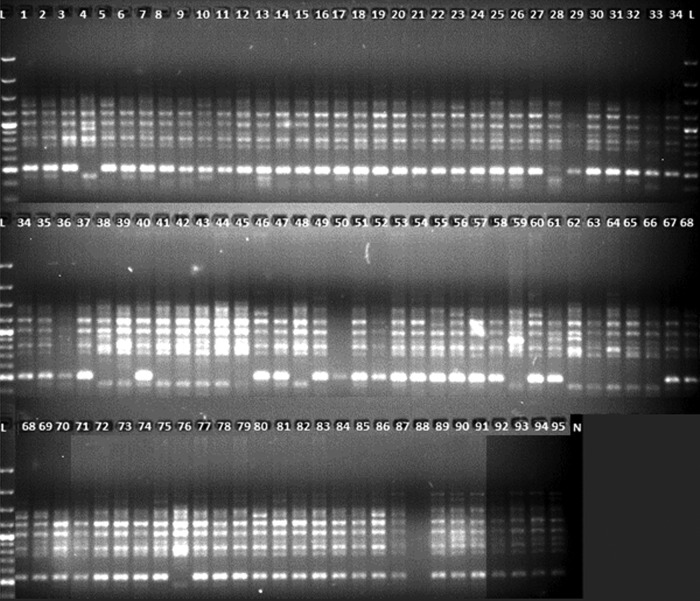
Ninety-five Indian and Turkish wheat varieties were amplified using 43 and 27 ISSR and RAPD markers, respectively. The 10 most polymorphic ISSR and RAPD primers generated 116 and 82 genetic loci, respectively, with a total of 198 loci. Among ISSR primers, ISSR M3 generated the maximum number of polymorphic fragments (16) and cRAPD2 was the most prolific RAPD primer (11). In total, 94.8 and 81.7 % bands were found to be polymorphic among ISSR and RAPD markers. The average number of polymorphic bands per primer was 11.0 and 6.7 for ISSR and RAPD primers, respectively (Tables 2 and 3). For both primer types, the main amplified region was in the range of 300–2000 bp (Figs 1 and 2).
Figure 1.
The inter-simple sequence repeat M3 primer amplification profile of 95 Indian and Turkish wheat genotypes.
Figure 2.
Random amplified polymorphic DNA B5 primer amplification profile of 95 Indian and Turkish wheat genotypes.
Genetic relationships/association
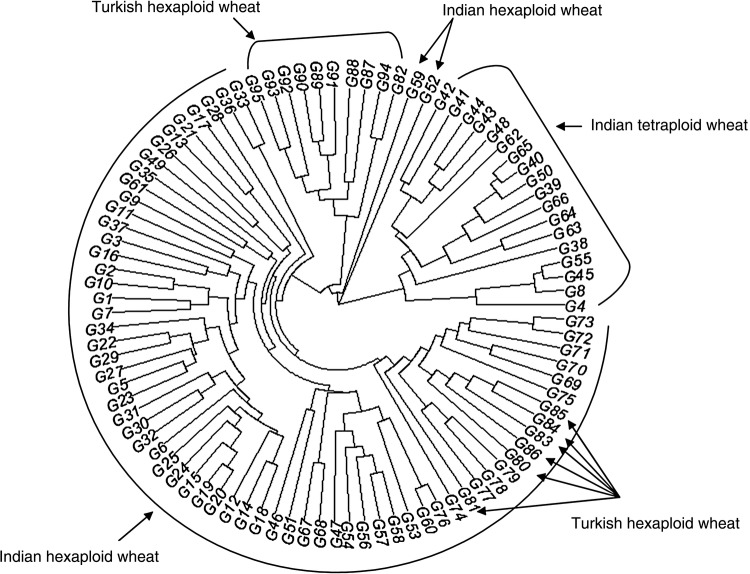
A Fan dendrogram of the combined RAPD and ISSR data showed clear groupings of genotypes on the basis of both ploidy and origin (Fig. 3). On combining both RAPD and ISSR data, individual errors of either marker system are reduced and combined the dendrogram provided a more robust overview of the relatedness of Indian and Turkish populations.
Figure 3.
Simple matching coefficient-based Fan dendrogram using NTSYS-PC and R software package of 95 Indian and Turkish wheat genotypes.
On the basis of ploidy, wheat varieties were divided into three clusters, containing 18 tetraploid and 77 hexaploid varieties. Among hexaploid varieties, two Indian genotypes, NW 2036 and RAJ 4027, were separated as outliers from the rest. However, all the hexaploid genotypes were basically divided into two groups, and six Turkish genotypes were clustered with the Indian Hexaploid group. Similarity coefficients among Indian hexaploid varieties ranged from 0.71 to 0.98 while among Turkish hexaploid varieties ranged from 0.42 to 0.95.
Furthermore, the molecular variance factor in both Indian and Turkish populations was compared as a further measure of genetic diversity. Results from AMOVA for geographical origin indicated 77 % genetic variation within populations, while the variation between the populations was 23 % (PhiPT = 0.232; P = 0.010). On the basis of ploidy, AMOVA detected higher genetic variation within tetraploid and hexaploid populations (92 %); however, the genetic variation between ploidies was only 8 % (PhiPT = 0.078; P = 0.010) (Table 4).
Table 4.
Analysis of molecular variance in Indian and Turkish wheat populations.
| Source of variation | df | Square sum | Variance component | Percentage | Probability |
|---|---|---|---|---|---|
| Geographic origin | |||||
| Among populations | 1 | 133.52 | 4.46 | 23 | <0.001 |
| Within populations | 93 | 1370.776 | 14.74 | 77 | |
| Ploidy | |||||
| Among populations | 1 | 54.21 | 1.32 | 8 | <0.001 |
| Within populations | 93 | 1450.09 | 15.59 | 92 | |
Population structure
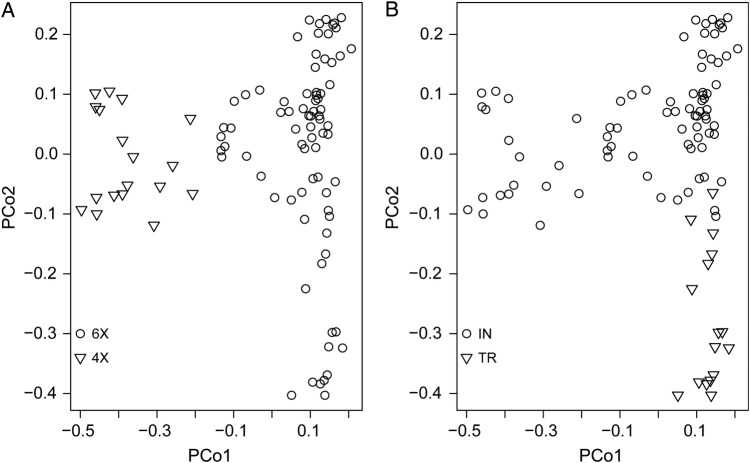
Principal coordinate analysis (PCoA) serves as a platform to provide a spatial illustration of the comparative genetic distances between the individuals. It also assesses the robustness of the differentiation among the groups classified by the dendrogram (Liu et al. 2013). In our scatterplots, the first two principal components explained 17.6 and 10.7 % of the total variation, respectively. In accordance with the dendrogram, hexaploid individuals were clearly separated from tetraploid varieties by the first principal coordinate (Fig. 4A). Similarly, the second principal coordinate (10.71 % of total variation) divided the Turkish populations from the Indian ones (Fig. 4B).
Figure 4.
Principal coordinate analysis of 95 Indian and Turkish wheat genotypes based on (A) ploidy level of the genotypes and (B) geographic origin of the genotypes.
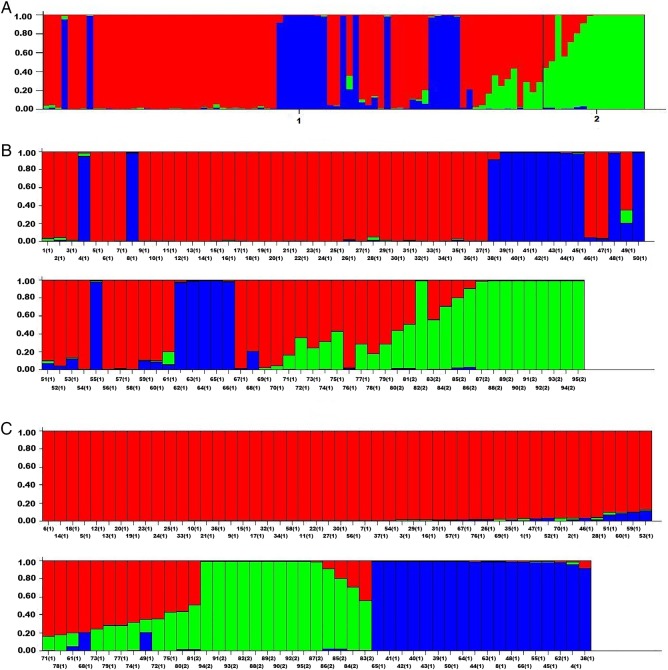
For population genetic structure analysis, Bayesian clustering modelling was executed in the STRUCTURE software using genotyping data generated by 177 RAPD and ISSR loci. As the clustering model presumes the underlying existence of K clusters, an Evano test was performed and yielded K = 3 as the highest log-likelihood. This means that 3 was the optimum number of subpopulations, indicating that the two major population groups actually represent three distinct clusters.
The analysis of structure according to the geographical origin was performed by setting the range of possible number of subpopulations (K) from 1 to 4. Indian and Turkish populations involved in this procedure showed separation from each other in accordance with clusters obtained in PCoA. At K = 3, wheat genotypes were divided into three clusters with two main populations, 1 and 2 (Fig. 5A) representing Indian and Turkish wheat gene pool, respectively. Red colour bars represent individuals belonging to the Indian wheat gene pool while those in green belong to the Turkish gene pool. The Indian wheat gene pool was again distributed into subclusters with blue representing the tetraploid wheat population (Fig. 5B). The Indian population consisted of 79 accessions, of which 72 % belonged to the first cluster, 4 % to the second cluster and 24 % to the third; whereas the Turkish group consisted of 16 accessions with 13, 86 and 1 % belonging to the first, second and third cluster, respectively (Table 5). Some of the Indian and Turkish hexaploid genotypes, including NW_2036, RAJ_4027, Bayraktar_2000, Seval, Gün_91, Konya_2002, showed admixture clustering (Fig. 5C). Within the first, second and third clusters, expected heterozygosity within individuals was found to be 0.18, 0.15 and 0.16, respectively.
Figure 5.
(A) Three clusters inferred from population STRUCTURE analysis; red zone consists of basically Indian varieties with blue zone representing Indian tetraploid subpopulation and green zone includes basically Turkish varieties. (B) For distinctive clusters, vertical coordinates denote membership coefficients and each vertical line along with the horizontal coordinate denotes individual genotypes. Numbers in brackets denote their main population group, India and Turkey. (C) Collection of genotypes on the basis of Q, which explains the proportion of every individual genome that belongs to two distinct clusters.
Table 5.
Proportion of membership of each pre-defined population in each of the three clusters obtained from STRUCTURE analysis.
| Given population | Inferred clusters |
Number of individuals | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| 1 | 0.720 | 0.039 | 0.241 | 79 |
| 2 | 0.133 | 0.857 | 0.009 | 16 |
Discussion
The complex nature and huge size of the wheat genome pose serious challenges towards genetic means of increasing its production. Hence, furthering our understanding of the wheat genome utilizing a variety of analyses has assisted efforts towards the genetic improvement of modern cultivars. Our examination of the literature to date found no prior genotypic characterization of the Indian and Turkish wheat varieties, simultaneously using RAPD and ISSR markers (Khan et al. 2014). The present study constitutes the first attempt to better understand jointly the origin, evolution and molecular diversity of Indian and Turkish wheat varieties at different ploidy levels.
Evaluating genetic diversity in Indian and Turkish wheat
Since their introduction, ISSR and RAPD markers have been broadly utilized for variability estimation of wheat genotypes. Several RAPD- and ISSR-based diversity studies including diploid, tetraploid and hexaploid wheat have been published (Castagna et al. 1997; Nagaoka and Ogihara 1997; Pujar et al. 1999, 2002; Barcaccia et al. 2002; Teshale et al. 2003; Mantzavinou et al. 2005; Thomas et al. 2006; Aliyev et al. 2007; Grewal et al. 2007; Anand et al. 2008; Cenkci et al. 2008; Sawalha et al. 2008; Tahir 2008; Carvalho et al. 2009; Pandey et al. 2012). Due to the possession of diverse (A, B, D) genomes of wheat, tetraploid and hexaploid varieties were involved in the study.
Although Indian and Turkish wheat germplasms were not simultaneously used earlier, the average RAPD- and ISSR primer-based polymorphism, 81.7 and 94.8 %, respectively, revealed in this study, were comparable with several prior diversity studies. The very first attempt made by other researchers among Indian tetraploid wheat varieties revealed high genetic variability in durum released cultivars (50.6 %) in comparison to landraces (44.8 %) (Pujar et al. 1999). Teshale et al. (2003) found 79.6 % polymorphism among 27 tetra- and hexaploid Indian genotypes using RAPD markers. A detailed study on 96 commercial Indian wheat genotypes, including tetraploids, triticale and hexaploids, indicating 78.8 % polymorphism, revealed a narrow genetic base of tetraploid cultivars in comparison to hexaploids (Thomas et al. 2006).
The similarity coefficient values range among Indian hexaploid varieties observed in our study (0.71–0.98) was found to be higher than that in a previous study by Grewal et al. (2007) (0.52–0.82) using RAPD markers. In the present work, the average count of polymorphic bands per primer was higher in the case of ISSR (11) compared with that in RAPD (6.7). These results were consistent with a previous study by Pujar et al. (2002) on Indian tetraploid wheat varieties. Although limited studies have been performed on diversity assessment of Turkish wheat, Akar and Ozgen (2007) assessed the genetic variability of 100 durum wheat varieties using RAPD markers and observed higher genetic diversity in landraces than in cultivars. Cifci and Yagdi (2012) distinguished 16 Turkish bread wheat varieties using RAPD markers with product size in the range of 300–2800 bp, which was similar to our results.
Analysis of genetic relationships among wheat genotypes
The dendrogram obtained in this study clearly clustered the genotypes according to their ploidy level, consistently with the evolution of wheat (Alamerew et al. 2004). Furthermore, Indian and Turkish varieties were grouped separately. The information revealed by the dendrogram highlighted the parentage association of the varieties. Varieties HD_2177 and HD_2329 grouped together in the dendrogram with 95 % similarity share three common parents, HD_1962, E_4870, K_65. HD_2402 also grouped with its parent variety HD_2236 and showed 96 % similarity. Varieties Raj_1482, Raj_3072 and Raj_3077 were clustered together. Within this cluster, Raj_1482 is the parent of Raj_3077, with 93 % similarity. HD_2307 and HD_2501, which were grouped separately from other ‘HD’ varieties, share as a common parent HD_2160 and consistently exhibited 93 % similarity. Not only hexaploids, but also some of the tetraploid varieties like AKDW_2997 were also allocated in the same subcluster with its parent Raj_1555 and showed 88 % similarity (Fig. 3).
Analysis of molecular variance results disclosed in the study were in agreement with the UPGMA clustering and supported a high level of diversity within-country samples. Although the variation between Indian and Turkish populations was lower in comparison to within-population variation, it was significant according to the partitioning value (P = 0.010) (Table 4). The results suggest that similarity association between the countries was affected by within-country inconsistencies of the varieties. This high variation within groups can be attributed to selective adaptation towards the growth conditions at the time of breeding.
Investigating the Indian and Turkish wheat population structure
Similar separation of Indian and Turkish wheat varieties was observed by PCoA on the basis of ploidy and geographical region. The outcomes of the two methods (cluster analysis and PCoA) were comparable. Both of them classified 95 wheat genotypes mainly into three clusters and offered similar alignment of the genotypes with a few negligible discrepancies. The groups attained were in agreement with the recognized geographical origin as well.
Population structure analyses indicated that wheat accessions can be efficiently categorized on the basis of both geographical origin and ploidy. Using the maximum membership probability in STRUCTURE, Indian and Turkish populations showed similar grouping to the UPGMA and PCoA clustering. The PCoA clustering divided Indian and Turkish populations basically into similar clusters to those produced by the Structure bar plot at K = 3. In PCoA, some of the Indian and Turkish varieties showed close association with each other, and similar varieties demonstrated admixture in Structure analysis confirming their relatedness within the diverse gene pool. In dendrogram also, these varieties showed distinct clustering with the main population groups. Closeness of some of the Turkish hexaploid genotypes with Indian hexaploid genotypes in PCoA was also supported by the population structure as well as the dendrogram. Similar and mutually supportive results from all the statistical analyses demonstrated the capability of RAPD and ISSR markers to distinguish Indian and Turkish wheat varieties efficiently.
Conclusions
Genetic diversity evaluation serves as a crucial platform in plant improvement. The present study provides a detailed understanding of the genetic association of Indian and Turkish hexaploid and tetraploid wheat. The Turkish hexaploid populations showed their closeness to Indian genotypes, confirming their alliance within the diverse gene pool. The present genetic diversity study of wheat material obtained from diverse regions will support breeders in expanding the genetic variation of breeding accessions and utilizing the studied wheat resources more effectively.
Sources of Funding
M.K.K. has been granted ‘2216 Research Fellowship for Foreign Citizens’ by TÜBİTAK (The Scientific and Technological Research Council of Turkey) for performing the present research work. Also, a part of the research was funded by BAP (Turkish Scientific Research Project agency) under Project No. 14401106.
Contributions by the Authors
M.K.K. initiated and obtained the funding for the research work. M.K.K. and A.P. contributed in performing the research work and preparation of the manuscript. M.K.K., A.P., S.A.K. and Y.O. carried out all statistical analyses. All other authors have provided suggestions and guidance for the successful completion of the work. All authors read and approved the final manuscript.
Conflict of Interest Statement
None declared.
Acknowledgements
We thank Enrique Lopez-Juez for language editing and Hall Cushman, Kermit Ritland and the anonymous reviewers for their valuable suggestions that have greatly improved the manuscript.
Literature Cited
- Akar T, Ozgen M. 2007. Genetic diversity in Turkish durum wheat landraces. In: Buck HT, Nisi JE, Salomón N, eds. Wheat production in stressed environments. The Netherlands: Springer, 753–760. [Google Scholar]
- Alamerew S, Chebotar S, Huang X, Röder M, Börner A. 2004. Genetic diversity in Ethiopian hexaploid and tetraploid wheat germplasm assessed by microsatellite markers. Genetic Resources and Crop Evolution 51:559–567. 10.1023/B:GRES.0000024164.80444.f0 [DOI] [Google Scholar]
- Aliyev RT, Abbasov MA, Mammadov AC. 2007. Genetic identification of diploid and tetraploid wheat species with RAPD markers. Turkish Journal of Biology 31:173–180. [Google Scholar]
- Anand A, Gupta AK, Teshale ET, Mishra A, Khanna VK. 2008. Phylogenetic relationship to study the ploidy status and resistance to Karnal Bunt in Indian wheat cultivars using RAPD technique. Biotechnology 7:430–438. 10.3923/biotech.2008.430.438 [DOI] [Google Scholar]
- Autrique E, Nachit M, Monneveux P, Tanksley SD, Sorrells ME. 1996. Genetic diversity in durum wheat based on RFLPs, morphophysiological traits, and coefficient of parentage. Crop Science 36:735–742. 10.2135/cropsci1996.0011183X003600030036x [DOI] [Google Scholar]
- Barcaccia G, Molinari L, Porfiri O, Veronesi F. 2002. Molecular characterization of emmer (Triticum dicoccon Schrank) Italian landraces. Genetic Resources and Crop Evolution 49:417–428. 10.1023/A:1020650804532 [DOI] [Google Scholar]
- Bellon MR. 1996. The dynamics of crop infraspecific diversity: a conceptual framework at the farmer level 1. Economic Botany 50:26–39. 10.1007/BF02862110 [DOI] [Google Scholar]
- Bered F, Barbosa-Neto JF, de Carvalho FIF. 2002. Genetic variability in common wheat germplasm based on coefficients of parentage. Genetics and Molecular Biology 25:211–215. 10.1590/S1415-47572002000200015 [DOI] [Google Scholar]
- Bhutta WM, Shahzad A, Ibrahim M, Akhtar J. 2005. Assessment of genetic divergence among wheat (Triticum aestivum) genotypes using random amplified polymorphic DNA (RAPD) analysis. Biologia 60:671–674. [Google Scholar]
- Bruinsma J. 2003. World agriculture: towards 2015/2030. An FAO perspective. London: Earthscan Publications Ltd. [Google Scholar]
- Carvalho A, Lima-Brito J, Maçãs B, Guedes-Pinto H. 2009. Genetic diversity and variation among botanical varieties of old Portuguese wheat cultivars revealed by ISSR assays. Biochemical Genetics 47:276–294. 10.1007/s10528-009-9227-5 [DOI] [PubMed] [Google Scholar]
- Cassman KG, Dobermann A, Walters DT, Yang H. 2003. Meeting cereal demand while protecting natural resources and improving environmental quality. Annual Review of Environment and Resources 28:315–358. 10.1146/annurev.energy.28.040202.122858 [DOI] [Google Scholar]
- Castagna R, Gnocchi S, Perenzin M, Heun M. 1997. Genetic variability of the wild diploid wheat Triticum urartu revealed by RFLP and RAPD markers. Theoretical and Applied Genetics 94:424–430. 10.1007/s001220050432 [DOI] [Google Scholar]
- Cenkci S, Yildiz M, Konuk M, Eren Y. 2008. RAPD analyses of some wild Triticum L. and Aegilops L. species and wheat cultivars in Turkey. Acta Biologica Cracoviensia Series Botanica 50:35–42. [Google Scholar]
- Cifci EA, Yagdi K. 2012. Study of genetic diversity in wheat (Triticum aestivum) varieties using random amplified polymorphic DNA (RAPD) analysis. Turkish Journal of Field Crops 17:91–95. [Google Scholar]
- Doyle JJ. 1990. Isolation of plant DNA from fresh tissue. Focus 12:13–15. [Google Scholar]
- Dubcovsky J, Dvorak J. 2007. Genome plasticity a key factor in the success of polyploid wheat under domestication. Science 316:1862–1866. 10.1126/science.1143986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl DA, vonHoldt BM. 2012. STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conservation Genetics Resources 4:359–361. 10.1007/s12686-011-9548-7 [DOI] [Google Scholar]
- Ehrlich PR. 1975. The population bomb. Rivercity, MA: Rivercity Press. [Google Scholar]
- Evanno G, Regnaut S, Goudet J. 2005. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Molecular Ecology 14:2611–2620. 10.1111/j.1365-294X.2005.02553.x [DOI] [PubMed] [Google Scholar]
- Evans LT. 1998. Feeding the ten billion: plants and population growth. Cambridge: Cambridge University Press. [Google Scholar]
- Fahima T, Sun GL, Beharav A, Krugman T, Beiles A, Nevo E. 1999. RAPD polymorphism of wild emmer wheat populations, Triticum dicoccoides, in Israel. Theoretical and Applied Genetics 98:434–447. 10.1007/s001220051089 [DOI] [Google Scholar]
- Falush D, Stephens M, Pritchard JK. 2003. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics 164:1567–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falush D, Stephens M, Pritchard JK. 2007. Inference of population structure using multilocus genotype data: dominant markers and null alleles. Molecular Ecology Notes 7:574–578. 10.1111/j.1471-8286.2007.01758.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y-B, Somers DJ. 2009. Genome-wide reduction of genetic diversity in wheat breeding. Crop Science 49:161–168. 10.2135/cropsci2008.03.0125 [DOI] [Google Scholar]
- Gollin D, Morris M, Byerlee D. 2005. Technology adoption in intensive post-green revolution systems. American Journal of Agricultural Economics 87:1310–1316. 10.1111/j.1467-8276.2005.00824.x [DOI] [Google Scholar]
- Grewal S, Kharb P, Malik R, Jain S, Jain R. 2007. Assessment of genetic diversity among some Indian wheat cultivars using random amplified polymorphic DNA (RAPD) markers. Indian Journal of Biotechnology 6:18–23. [Google Scholar]
- Heal G, Walker B, Levin S, Arrow K, Dasgupta P, Daily G, Ehrlich P, Maler K-G, Kautsky N, Lubchenco J, Schneider S, Starrett D. 2004. Genetic diversity and interdependent crop choices in agriculture. Resource and Energy Economics 26:175–184. 10.1016/j.reseneeco.2003.11.006 [DOI] [Google Scholar]
- Heun M, Schäfer-Pregl R, Klawan D, Castagna R, Accerbi M, Borghi B, Salamini F. 1997. Site of Einkorn wheat domestication identified by DNA fingerprinting. Science 278:1312–1314. 10.1126/science.278.5341.1312 [DOI] [Google Scholar]
- Hubisz MJ, Falush D, Stephens M, Pritchard JK. 2009. Inferring weak population structure with the assistance of sample group information. Molecular Ecology Resources 9:1322–1332. 10.1111/j.1755-0998.2009.02591.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzatullayeva V, Akparov Z, Babayeva S, Ojaghi J, Abbasov M. 2014. Efficiency of using RAPD and ISSR markers in evaluation of genetic diversity in sugar beet. Turkish Journal of Biology 38:429–438. 10.3906/biy-1312-35 [DOI] [Google Scholar]
- Joshi CP, Nguyen HT. 1993a. Application of the random amplified polymorphic DNA technique for the detection of polymorphism among wild and cultivated tetraploid wheats. Genome 36:602–609. 10.1139/g93-081 [DOI] [PubMed] [Google Scholar]
- Joshi CP, Nguyen HT. 1993b. RAPD (random amplified polymorphic DNA) analysis based intervarietal genetic relationships among hexaploid wheats. Plant Science 93:95–103. 10.1016/0168-9452(93)90038-2 [DOI] [Google Scholar]
- Khan MK, Pandey A, Choudhary S, Hakki EE, Akkaya MS, Thomas G. 2014. From RFLP to DArT: molecular tools for wheat (Triticum spp.) diversity analysis. Genetic Resources and Crop Evolution 61:1001–1032. 10.1007/s10722-014-0114-5 [DOI] [Google Scholar]
- Liu J, Shi S, Chang E, Yang W, Jiang Z. 2013. Genetic diversity of the critically endangered Thuja sutchuenensis revealed by ISSR markers and the implications for conservation. International Journal of Molecular Sciences 14:14860–14871. 10.3390/ijms140714860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP. 2007. TURNER REVIEW No. 14. Roots of the second green revolution. Australian Journal of Botany 55:493–512. 10.1071/BT06118 [DOI] [Google Scholar]
- Mandoulakani B, Tabatabaei B, Bushehri A, Ghannadha M, Omidi M. 2003. Assessment of genetic diversity among wheat cultivars by RAPD–PCR. Iranian Journal of Agricultural Sciences 34:447–454. [Google Scholar]
- Mantzavinou A, Bebeli PJ, Kaltsikes PJ. 2005. Estimating genetic diversity in Greek durum wheat landraces with RAPD markers. Australian Journal of Agricultural Research 56:1355–1364. 10.1071/AR04245 [DOI] [Google Scholar]
- Marić S, Bolarić S, Martinčić J, Pejić I, Kozumplik V. 2004. Genetic diversity of hexaploid wheat cultivars estimated by RAPD markers, morphological traits and coefficients of parentage. Plant Breeding 123:366–369. 10.1111/j.1439-0523.2004.00956.x [DOI] [Google Scholar]
- Motawei M, Al-Doss A, Moustafa K. 2007. Genetic diversity among selected wheat lines differing in heat tolerance using molecular markers. Journal of Food Agriculture and Environment 5:180–183. [Google Scholar]
- Mukhtar MS, Rahmanw M, Zafar Y. 2002. Assessment of genetic diversity among wheat (Triticum aestivum L.) cultivars from a range of localities across Pakistan using random amplified polymorphic DNA (RAPD) analysis. Euphytica 128:417–425. 10.1023/A:1021261811454 [DOI] [Google Scholar]
- Nagaoka T, Ogihara Y. 1997. Applicability of inter-simple sequence repeat polymorphisms in wheat for use as DNA markers in comparison to RFLP and RAPD markers. Theoretical and Applied Genetics 94:597–602. 10.1007/s001220050456 [DOI] [Google Scholar]
- Najaphy A, Parchin RA, Farshadfar E. 2011. Evaluation of genetic diversity in wheat cultivars and breeding lines using Inter Simple Sequence Repeat markers. Biotechnology & Biotechnological Equipment 25:2634–2638. 10.5504/BBEQ.2011.0093 [DOI] [Google Scholar]
- Nesbitt M, Samuel D. 1998. Wheat domestication: archaeobotanical evidence. Science 279:1431 10.1126/science.279.5356.1431e9508710 [DOI] [Google Scholar]
- Pandey A, Khan MK, Choudhary S, Hakki EE, Akkaya MS, Thomas G. 2012. “RAPD and wheat diversity analysis”—still a centre of interest. Flora and Fauna 18:67–75. [Google Scholar]
- Pasqualone A, Lotti C, Bruno A, De Vita P, Di Fonzo N, Blanco A. 2000. Use of ISSR markers for cultivar identification in durum wheat. CIHEAM—Options Mediterraneennes 40:157–161. [Google Scholar]
- Peakall R, Smouse PE. 2006. Genalex 6: genetic analysis in Excel. Population genetic software for teaching and research. Molecular Ecology Notes 6:288–295. 10.1111/j.1471-8286.2005.01155.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peakall R, Smouse PE. 2012. GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research—an update. Bioinformatics 28:2537–2539. 10.1093/bioinformatics/bts460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecetti L, Doust MA, Calcagno L, Raciti CN, Boggini G. 2001. Variation of morphological and agronomical traits, and protein composition in durum wheat germplasm from easternEurope. Genetic Resources and Crop Evolution 48:609–620. 10.1023/A:1013825821856 [DOI] [Google Scholar]
- Pingali PL. 2012. Green revolution: impacts, limits, and the path ahead. Proceedings of the National Academy of Sciences of the USA 109:12302–12308. 10.1073/pnas.0912953109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. 2000. Inference of population structure using multilocus genotype data. Genetics 155:945–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujar S, Tamhankar SA, Rao VS, Gupta VS, Naik S, Ranjekar PK. 1999. Arbitrarily primed-PCR based diversity assessment reflects hierarchical groupings of Indian tetraploid wheat genotypes. Theoretical and Applied Genetics 99:868–876. 10.1007/s001220051307 [DOI] [Google Scholar]
- Pujar S, Tamhankar SA, Gupta VS, Rao VS, Ranjekar PK. 2002. Note: diversity analysis of Indian tetraploid wheat using intersimple sequence repeat markers reveals their superiority over random amplified polymorphic DNA markers. Biochemical Genetics 40:63–69. 10.1023/A:1014593206886 [DOI] [PubMed] [Google Scholar]
- Ray DK, Mueller ND, West PC, Foley JA. 2013. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 8:e66428 10.1371/journal.pone.0066428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlf FJ. 1997. NTSYS-PC. Numerical taxonomy and multivariate analysis system, version 2.02e. Exeter Software, New York. [Google Scholar]
- Saleh B. 2012. Characterization of some upland cotton varieties using AFLP and NIR techniques. Agriculture 58:85–92. [Google Scholar]
- Sawalha K, Eideh H, Laham S, Hasasneh H, Mezeid B. 2008. Genetic diversity studies on wheat landraces in Palestine using RAPD markers in comparison to phenotypic classification. Journal of Applied Biological Sciences 2:29–34. [Google Scholar]
- Shewry PR. 2007. Improving the protein content and composition of cereal grain. Journal of Cereal Science 46:239–250. 10.1016/j.jcs.2007.06.006 [DOI] [Google Scholar]
- Shewry PR. 2009. Wheat. Journal of Experimental Botany 60:1537–1553. 10.1093/jxb/erp058 [DOI] [PubMed] [Google Scholar]
- Simmonds DH. 1989. Wheat and wheat quality in Australia. Melbourne: CSIRO. [Google Scholar]
- Smale M. 1997. The Green Revolution and wheat genetic diversity: some unfounded assumptions. World Development 25:1257–1269. 10.1016/S0305-750X(97)00038-7 [DOI] [Google Scholar]
- Summers RW, Brown JKM. 2013. Constraints on breeding for disease resistance in commercially competitive wheat cultivars. Plant Pathology 62:115–121. 10.1111/ppa.12165 [DOI] [Google Scholar]
- Sun Q, Ni Z, Liu Z, Gao J, Huang T. 1998. Genetic relationships and diversity among Tibetan wheat, common wheat and European spelt wheat revealed by RAPD markers. Euphytica 99:205–211. 10.1023/A:1018316129246 [DOI] [Google Scholar]
- Tahir NA-R. 2008. Assessment of genetic diversity among wheat varieties in Sulaimanyah using random amplified polymorphic DNA (RAPD) analysis. Jordan Journal of Biological Sciences 1:159–164. [Google Scholar]
- Takumi S, Nishioka E, Morihiro H, Kawahara T, Matsuoka Y. 2009. Natural variation of morphological traits in wild wheat progenitor Aegilops tauschii Coss. Breeding Science 59:579–588. 10.1270/jsbbs.59.579 [DOI] [Google Scholar]
- Tesfaye T, Getachew B, Worede M. 1991. Morphological diversity in tetraploid wheat landrace populations from the central highlands of Ethiopia. Hereditas 114:171–176. 10.1111/j.1601-5223.1991.tb00321.x [DOI] [Google Scholar]
- Teshale ET, Bansal S, Mishra A. 2003. DNA fingerprinting of wheat genotypes by RAPD markers. Wheat Information Service (Japan) 96:23–27. [Google Scholar]
- Thomas G, Mohapatra T, Rao A, Sharma R. 2006. Distinguishing Indian commercial wheat varieties using RAPD based DNA fingerprints. Indian Journal of Biotechnology 5:200–206. [Google Scholar]
- Tilman D, Balzer C, Hill J, Befort BL. 2011. Global food demand and the sustainable intensification of agriculture. Proceedings of the National Academy of Sciences of the USA 108:20260–20264. 10.1073/pnas.1116437108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topping D. 2007. Cereal complex carbohydrates and their contribution to human health. Journal of Cereal Science 46:220–229. 10.1016/j.jcs.2007.06.004 [DOI] [Google Scholar]
- van den Broeck H, Hongbing C, Lacaze X, Dusautoir JC, Gilissen L, Smulders M, van der Meer I. 2010. In search of tetraploid wheat accessions reduced in celiac disease-related gluten epitopes. Molecular BioSystems 6:2206–2213. 10.1039/c0mb00046a [DOI] [PubMed] [Google Scholar]
- van Herpen TWJM, Goryunova SV, van der Schoot J, Mitreva M, Salentijn E, Vorst O, Schenk MF, van Veelen PA, Koning F, van Soest LJM, Vosman B, Bosch D, Hamer RJ, Gilissen LJWJ, Smulders MJM. 2006. Alpha-gliadin genes from the A, B, and D genomes of wheat contain different sets of celiac disease epitopes. BMC Genomics 7:1 10.1186/1471-2164-7-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen SJ, Campbell BM, Ingram JSI. 2012. Climate change and food systems. Annual Review of Environment and Resources 37:195–222. 10.1146/annurev-environ-020411-130608 [DOI] [Google Scholar]
- Vierling RA, Nguyen HT. 1992. Use of RAPD markers to determine the genetic diversity of diploid, wheat genotypes. Theoretical and Applied Genetics 84:835–838. [DOI] [PubMed] [Google Scholar]
- Wendel JF. 2000. Genome evolution in polyploids. Plant Molecular Biology 42:225–249. 10.1023/A:1006392424384 [DOI] [PubMed] [Google Scholar]
- Wheeler T. 2012. Agriculture: wheat crops feel the heat. Nature Climate Change 2:152–153. 10.1038/nclimate1425 [DOI] [Google Scholar]
- Young A. 1999. Is there really spare land? A critique of estimates of available cultivable land in developing countries. Environment, Development and Sustainability 1:3–18. 10.1023/A:1010055012699 [DOI] [Google Scholar]
- Ziaei SM, Mazloumzadeh SM, Jabbary M. 2015. A comparison of energy use and productivity of wheat and barley (case study). Journal of the Saudi Society of Agricultural Sciences 14:19–25. 10.1016/j.jssas.2013.04.002 [DOI] [Google Scholar]