Abstract
Moringa oleifera, an important multipurpose crop, is rich in various phytochemicals: flavonoids, antioxidants, vitamins, minerals and carotenes. The purpose of this study was to profile the groups of metabolites in leaf and stem tissues of M. oleifera. Various sugars, amino acids, and organic acid derivatives were found in all of the M. oleifera tissues with different profiles/peak intensities depending on the tissue. 1D proton nuclear magnetic resonance (NMR) was applied for collecting metabolite spectra. Approximately 30 metabolites with 2 unknown peaks were identified with Chenomx and verified with MMCD databases using carbon data. Among these metabolites, 22 metabolites were identified as common in both leaf and stem tissues. Of the remaining 8 metabolites, 4-aminobutyrate, adenosine, guanosine, tyrosine, and p-cresol were found only in leaf tissues; however, glutamate, glutamine, and tryptophan were found only in stem tissues. Biochemical pathway analysis revealed that 28 identified metabolites were interconnected with 36 different pathways as well as related to different fatty acids and secondary metabolites synthesis biochemical pathways. It is well known that different tissues of M. oleifera have nutritional, medicinal, and therapeutic values; therefore, our main objective is to provide a publicly available M. oliefera tissue specific metabolite database.
Keywords: Moringa oleifera, Metabolomics profile, Nuclear Magnetic Resonance, Spectrum, Biochemical pathway analysis
INTRODUCTION
Moringa oleifera has an impressive range of medicinal uses with high nutritional value. It has long been valued as both a food and medicinal tree. The therapeutic benefits, for which different tree parts such as root, bark, gum, leaf, stem, fruit (pods), flowers, seed and seed oil are used, include analgesic (Sutar et al. 2008), antipyretic (Oliveira et al. 1999), hypocholesterolemic (Mehta et al. 2003), hypoglycemic (Makonnen et al. 1997), anti-inflammatory and hepatoprotective (Kurma and Mishra 1998), anti-hypertensive (Faizi et al. 1995), anti-spasmodic (Caceres et al. 1992), anti-ulcer (Pal et al. 1995), antioxidant (Wangcharoen, Gomolmanee 2011), anticonvulsant (Amrutia et al. 2011), antimicrobial (Caceres et al. 1991), and antitumor (Murakami et al. 1998) activities.
Moringa leaves have been reported to be a rich source of β-carotene, protein, vitamin C, calcium and potassium and act as a good source of natural antioxidant compounds such as ascorbic acid, flavonoids, phenolics and carotenoids (Dillard and German 2000; Siddhuraju and Becker 2003). The high concentrations of ascorbic acid, oestrogenic substances and β-sitosterol, iron, calcium, phosphorus, copper, vitamins A, B and C, α-tocopherol, riboflavin, nicotinic acid, folic acid, pyridoxine, β-carotene, protein, and in particular essential amino acids such as methionine, cystine, tryptophan and lysine present in Moringa leaves and pods make it an ideal dietary supplement (Makkar and Becker 1996). The stem bark has been reported to contain two alkaloids, namely moringine and moringinine (Kerharo 1969). Vanillin, β-sitosterol, β-sitostenone, 4-hydroxymellin and octacosanoic acid have been isolated from the stem of M. oleifera (Faizi et al. 1994a). M. oleifera leaves and stem are the key sources of those secondary metabolites.
Although, Moringa has versatile utility for medicine and nutrition sources, various active ingredients in the extract of leaves and stem are still unknown (Bose 1980). Tissue culture technology has been widely used to isolate cell lines from callus cultures that produce typical levels of secondary metabolites in several plant species (Mansell, Mcintosh 1981). Protocols for the production of secondary metabolites from callus cultures of Moringa have not yet been developed.
Analysis of tissue specific metabolic profiles could shed some light on this gap. With the use of NMR, research may contribute to bridge the information gap in this area. In principle, 1H NMR provides a reliable profile of each sample, and quantities are reflected in the integrals of the individual signals of the spectrum. This makes 1H NMR a unique technique, which enables the quantification of compounds in relation to any other compound in the spectrum (Dagnino, Schripsema 2005). Proton (1H) NMR spectroscopy is particularly a good choice in plant metabolomics studies given the universal occurrence of protons in organic metabolites (Kim et al. 2007; Mahmud et al. 2014a; Mahmud et al. 2014b; Palama et al. 2010). 13C NMR was used to characterize triacylglycerols of M. oleifera seed oil to identify oleic-vaccenic acid (Vlahov et al. 2002) and the same technique is also used for profiling of glucosinolates and phenolics in vegetative and reproductive tissues of M. oleifera L. and M. stenopetala L (Bennett et al. 2003).
The present study deals with the extraction and identification of metabolites, then builds a metabolite list of various plant parts, and ultimately to provide a publicly available M. oliefera tissue specific metabolite database which can be utilized to interpret the broad range of issues related with plant metabolomics.
MATERIALS AND METHODS
Plant material
About 300 mg of fresh leaves and stems of M. oleifera were collected in separate tubes from Claflin University’s greenhouse, in June 2013. For NMR data verification respective tissue samples were collected in six replications. Samples were collected and immediately submerged into liquid nitrogen to quench all metabolic processes and to prevent any stress responses from the cutting process.
Metabolite Extraction
Methanol-chloroform-water extractions of leaf and stem tissues were performed as described by Kim et al. (2010). Briefly, 20 mg of dried sample was used for each sample/replicate and the solvent volumes were calculated using the dry mass to achieve a constant ratio of 2:2:1.8 of chloroform:methanol:water. The dried and homogenized sample material was rehydrated with the appropriate ice-cold methanol:water solution. After vortex-mixing, the mixture was transferred into the ice-cold chloroform:water solution in glass tubes. The sample was incubated on ice for 10 min and centrifuged for 10 min at 2000×g at 4 °C in order to form two liquid layers separated by a solid protein layer. The top liquid layer, i.e. the hydrophilic extract, and the bottom liquid layer, i.e. the hydrophobic extract, were separated. The hydrophilic extracts were dried using a Centrivap centrifuge for ~15 hr and stored at −20 °C until further preparation for NMR analysis. The hydrophobic extracts were stored at −80 °C for future analysis.
NMR Sample Preparation
The dried hydrophilic tissue extracts were resuspended in 620 µL of NMR buffer containing 0.1 M sodium phosphate (pH 7.3), 1 mM 3-(Trimethylsilyl)propionic-2,2,3,3-d4 acid sodium salt (TMSP), 0.1% NaN3 in 100% D2O. Following resuspension, 600 µL of each sample was transferred into standard 5 mm NMR tubes (Norell).
NMR Spectroscopy
1H NOESY (1-D): All NMR spectra were recorded on a Bruker spectrometer operating at 700 MHz. The experiments were conducted at 298 K using 5 mm NMR tubes. Standard 1H 1-D pre-saturation (zgpr) and first increment of 1-D NOESY (noesygppr1d) experiments were recorded and processed for all samples. All data were collected using a spectral width of 16 ppm and 64 K points resulting in an acquisition time of 2.9 s; on-resonance pre-saturation was used for solvent suppression during a 2.0 s recycle delay. The zgpr experiment was used to screen samples and check shimming. For all of the samples, the first increment of 1D NOESY spectra were collected with 120 scans, 4 dummy scans, 50 ms mixing time, and pre-saturation at the residual water frequency. The 90° pulse widths, measured for each sample using the automatic pulse calculation experiment (pulsecal) in TopSpin2.1 (BrukerBioSpin), were between 9.62 and 10.54 µs.
1H-13C-HSQC (2-D): Two dimensional edited heteronuclear single quantum correlation (HSQC) spectra with adiabatic 13C decoupling (hsqcedetgpsisp2.2) were collected for one of the control samples to aid in metabolite identification. A relaxation delay equal to 1.5 s was used between acquisitions and a refocusing delay of 3.60 ms was implemented. The 2048 data points with 256 scans per increment were acquired with spectral widths of 11 ppm in F2 and 180 ppm in F1 (13C). The FIDs were weighted using a shifted sine squared function in both dimensions. Manual two-dimensional phasing was applied.
Identification of Metabolites
The spectral peaks from each type of tissue spectrum were analyzed using the ChenomX NMR software Suite package (ChenomX Inc.). ChenomX allows users to generate a list of tentatively assigned metabolites via targeted analysis, thus providing us with a spectral metabolic profile. Upon assigning metabolites, these compounds were cross-examined with a 2D 1H-13C-HSQC experiment for further validation.
Pathway Analysis
Metabolic pathway analysis was performed on hydrophilic cell extract results with MetPA (MetaboAnalyst 2.0) which combines pathway enrichment analysis with pathway topology analysis to help identify the most relevant pathways involved in the callus conditions under study. Lists of identified metabolites were input and the following parameters were selected for analysis: Arabidopsis thaliana as the reference metabolome, hypogeometric tests for the over representation analysis and, relative-betweeness centrality in the pathway topology analysis. This served as a measure of the number of shortest paths running through the nodes within a metabolic network. Of the resulting pathways, significance was determined based on an impact factor threshold value greater than zero and a p-value<0.05.
Results and Discussion
This research presents the use of NMR to study the identification of tissue specific metabolic profiles and then to use this spectral information for interpretation of biological significance using biochemical pathway analysis.
1D Proton (1H) NMR spectral comparisons
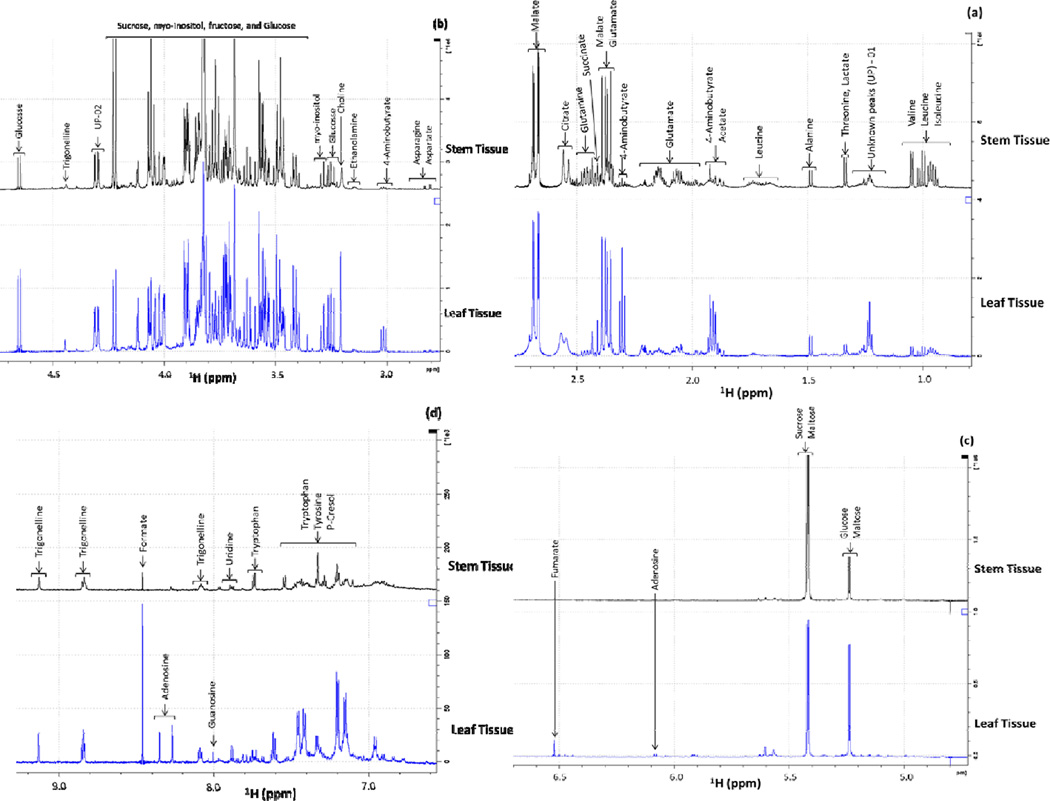
Stacked 1D 1H-NMR spectra (0.85–9.20 ppm) of leaf and stem tissues of M. oliefera were analyzed with expansions of the lower intensity aliphatic region from 0.85–3.19 ppm (Figure 1a & 1b), sugar region 3.34–5.40 ppm (Figure 1b& 1c), and aromatic region from 5.9–9.2 ppm (Figure 1d). Using Chenomx and online based MMCD metabolite database, 30 different metabolites with 2 unknown peaks were identified from 1D 1H-NMR spectra. Among 30 metabolites, 22 different metabolites including amino acids such as valine, leucine, isoleucine, threonine, alanine, aspartate, and asparagine, sugar compounds such as glucose, fructose, sucrose, and maltose, organic acid derivatives such as lactate, acetate, malate, succinate, citrate, ethanolamine, choline, formate, fumarate, and trigonelline were identified as common metabolites in both leaf and stem tissues. Qualitatively different metabolites 4-aminobutyrate, adenosine, guanosine, tyrosine, and p-Cresol were identified only in leaf tissues, and glutamate, glutamine, and tryptophan were identified only in stem tissues of M. oleifera.
Figure 1.
Stacked 1D 1H-NMR spectra of both leaf and stem tissues, with enlargements of the aliphatic (0.85–3.19 ppm), sugar (3.20–5.40ppm) and aromatic (5.5–9.2ppm) regions. a) Spectral profile showing aliphatic region, b) aliphatic and sugar region, c) sugar region, and d) aromatic region. UP 01–02 = unknown peak regions.
In this study, 1D 1H-NMR-based metabolic profile analysis was conducted to build the metabolite database of tissue specific M. oliefera. Currently, NMR-based 1D 1H spectral comparisons are widely used in plant science and biotechnology field to decipher clues related with economically and commercially significant crops. 1D 1H-NMR spectroscopy was used to compare the inner and outer cells of catharanthus roseus calli (Yang et al. 2009), healthy and infected C. roseus leaves (Choi et al. 2004), to distinguish the metabolic profile differences between embryogenic and non-embryogenic callus tissues of sugarcane (Mahmud et al. 2014b), to identify the broad range of compounds such as amino acids, carbohydrates, organic acids and phenolic compounds from callus tissue (Palama et al. 2010) and to distinguish between metabolic profiles of powdery mildew resistant and susceptible watermelon (Mahmud et al. 2014a). Information in this section suggests that the results presented here using 1D 1H NMR for identifying metabolites from both tissues of M. oliefera can be useful for building an NMR-based metabolite database.
In this study, using 1D 1H-NMR spectroscopy, we identified various essential amino acids, sugar compounds, organic acid compounds, and aromatic compounds (listed in table 1). The high concentrations of various organic acids such as ascorbic acid, riboflavin, nicotinic acid, and folic acid, protein content in particular essential amino acids such as methionine, cystine, tryptophan and lysine from Moringa leaves and pods were identified (Makkar and Becker 1996). Different simple sugars and their derivatives such as rhamnose (Fahey et al. 2001), L-arabinose, L-galactose, L-glucuronic acid, L-rhamnose, L-mannose, and L-xylose (Bhattacharya et al. 1982) were identified from Moringaoleifera. In this study, trigonelline, one of the important metabolically active pyridine alkaloids, was identified from both leaf and stem tissues of M. oleifera. Trigonelline has been isolated from various plant parts and callus cultures of M. oleifera Lam., Moringaceae, and was identified using TLC, GLC, GC-MS, which was comparable to that of the standard trigonelline. The trigonelline recovery was found to be maximumin the pods and minimum in flowers (Mathur and Kamal 2012).
Table 1. The list of identified metabolites and unidentified peaks.
Metabolites serial 1–17 were identified from the aliphatic region, 18–22 were from sugar regions, and 23–30 were from aromatic regions of 1D 1H NMR spectra of both leaf and stem tissues. Unknown peaks (UP) – 01 were identified from the aliphatic region and UP – 02 were identified from the sugar region of the same spectral comparisons.
| List of identified metabolites | Leaf Tissues | Stem tissues | 1H chemical shifts (ppm) | |
|---|---|---|---|---|
| 1 | Valine | √ | √ | 0.98, 1.03 |
| 2 | Leucine | √ | √ | 0.94, 0.95 |
| 3 | Isoleucine | √ | √ | 1.00 |
| 4 | Threonine | √ | √ | 1.32 |
| 5 | Lactate | √ | √ | 1.32 |
| 6 | Alanine | √ | √ | 1.47 |
| 7 | 4-Aminobutyrate | √ | - | 1.89, 2.28, 3.00 |
| 8 | Acetate | √ | √ | 1.90 |
| 9 | Glutamate | - | √ | 2.05 |
| 10 | Malate | √ | √ | 2.35, 2.66 |
| 11 | Succinate | √ | √ | 2.40 |
| 12 | Glutamine | - | √ | 2.42 |
| 13 | Citrate | √ | √ | 2.53 |
| 14 | Aspartate | √ | √ | 2.80 |
| 15 | Asparagine | √ | √ | 2.85 |
| 16 | Ethanolamine | √ | √ | 3.13 |
| 17 | Choline | √ | √ | 3.19 |
| 18 | Myo-inositol | √ | √ | 3.27, 3.61 |
| 19 | Glucose | √ | √ | 5.22, 4.63, 3.24 |
| 20 | Fructose | √ | √ | 4.01, 3.69, 3.58 |
| 21 | Sucrose | √ | √ | 5.40, 4.20, 3.46 |
| 22 | Maltose | √ | √ | 5.22, 5.40 |
| 23 | Adenosine | √ | - | 6.07, 8.25 |
| 24 | Trigonelline | √ | √ | 4.43, 8.07, 8.82, 9.12 |
| 25 | Fumarate | √ | √ | 6.51 |
| 26 | Guanosine | √ | - | 7.99 |
| 27 | Tryotophan | - | √ | 7.27, 7.31, 7.53 |
| 28 | Tyrosine | √ | - | 7.18 |
| 29 | p-Cresol | √ | - | 7.14 |
| 30 | Formate | √ | √ | 8.44 |
| 34 | Unknown peaks (UP) - 01 | √ | √ | 1.16 – 1.27 |
| 35 | UP – 02 | - | √ | 4.27–4.32 |
Metabolic Pathways Analysis
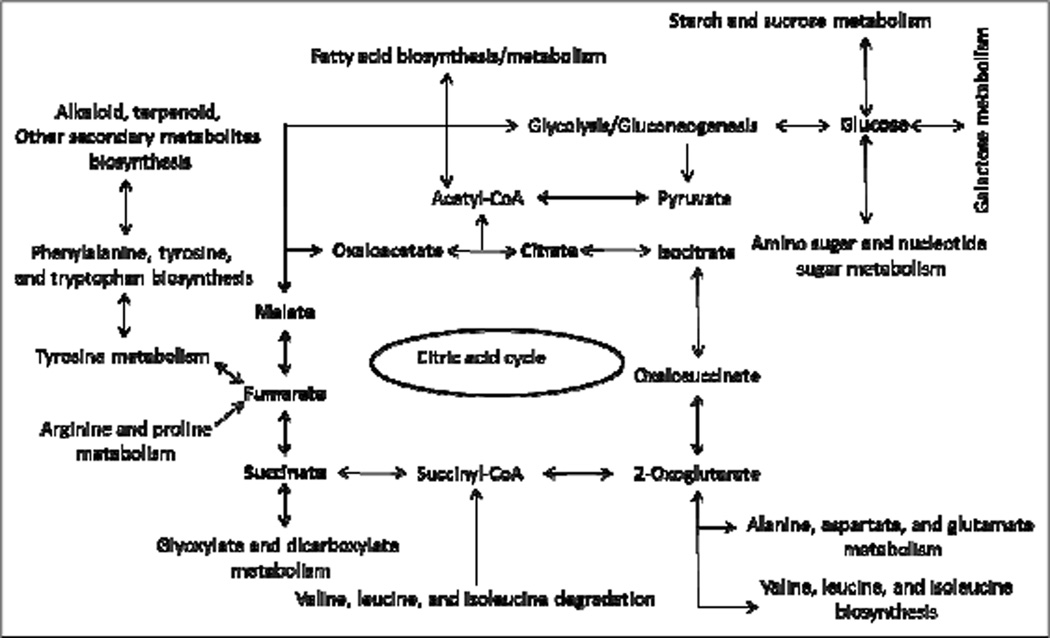
Metabolic pathway analysis was performed using the 30 different identified metabolites. The pathway analysis suggested that these metabolites have involvement with 36 different metabolic pathways based on p-values < 0.05 and an impact factor threshold greater than zero. Metabolic profile analysis of leaf and stem tissues revealed that biochemical relationships between these two types of tissues regulate an array of metabolites involved in the pathways (figure 2).
Figure 2.
Metabolic pathways leading to synthesis of metabolites. KEGG database and MetaboAnalyst 2.0 were used to elucidate metabolic networks. The metabolites that accumulated, and were identified by NMR, are shown in bold.
Based on the biochemical pathway analysis, different aromatic amino acids such as tyrosine, tryptophan, and phenylalanine could be precursors of synthesizing various secondary metabolites as well as could be regulators of the citric acid cycle for producing biological currency. Different organic acid derivatives such as succinate, malate, fumarate, and citrate are involved in the citric acid cycle and can enter into glycolysis or gluconeogenesis to synthesize the different sugar compounds as well as may regulate the various amino acid metabolism/biosynthesis/degradation pathways. Acetyl-CoA, an important metabolite of TCA cycle, and the central compound of metabolism, could be entering into fatty acid biosynthesis/metabolism process (Figure 2). The results explained in this section are in agreement with the relationship among various biochemical pathways in M. oleifera leaf and stem tissues that can be applied to identify the bioactive compounds such as various secondary metabolites.
Acknowledgements
IM is supported by Biology Department, Claflin University and AB is supported by SC-INBRE (2 P20 GM103499).
References
- Amrutia JN, Minaxi L, Srinivasa U, Shabaraya AR, Samuel MR. Anticonvulsant activity of Moringa oleifera leaf. Int Res J Pharm. 2011;2:160–162. [Google Scholar]
- Bennett RN, Mellon FA, Foidl N, Pratt JH, Dupont MS, Perkins L, Kroon PA. Profiling Glucosinolates and Phenolics in Vegetative and Reproductive Tissues of the Multi-Purpose Trees Moringa oleifera L. (Horseradish Tree) and Moringa stenopetala L. J Agric Food Chem. 2003;51(12):3546–3553. doi: 10.1021/jf0211480. [DOI] [PubMed] [Google Scholar]
- Bhattacharya SB, Das AK, Banerji N. Chemical investigations on the gum exudates from Sonja (Moringa oleifera) Carbohydr Res. 1982;102:253–262. [Google Scholar]
- Bose B. Enhancement of nodulation of Vigna mungo by ethanolic extracts of Moringa leaves – a new report. Nat Acad Sci Lett. 1980;3:103–104. [Google Scholar]
- Caceres A, Cabrera O, Morales O, Mollinedo P, Mendia P. Pharmacological properties of Moringa oleifera. 1: Preliminary screening for antimicrobial activity. J Ethnopharmacol. 1991;33:213–216. doi: 10.1016/0378-8741(91)90078-r. [DOI] [PubMed] [Google Scholar]
- Caceres A, Saravia A, Rizzo S, Zabala L, Leon ED, Nave F. Pharmacologic properties of Moringa oleifera. 2: Screening for antispasmodic, anti-infl ammatory and diuretic activity. J Ethnopharmacol. 1992;36:233–237. doi: 10.1016/0378-8741(92)90049-w. [DOI] [PubMed] [Google Scholar]
- Choi YH, Tapias EC, Kim HK, Lefeber AWM, Erkelens C, Verhoeven JTJ, Brzin J, Zel J, Verpoorte R. Metabolic Discrimination of Catharanthus roseus Leaves Infected by Phytoplasma Using 1H-NMR Spectroscopy and Multivariate Data Analysis. Plant Physiology. 2004;135:2398–2410. doi: 10.1104/pp.104.041012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagnino D, Schripsema J. 1H NMR quantification in very dilute toxin solutions: application to anatoxin-a analysis. Toxicon. 2005;46:236–240. doi: 10.1016/j.toxicon.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Dillard CJ, German JB. Phytochemicals: nutraceuticals and human health: A review. J Sci Food Agric. 2000;80:1744–1756. [Google Scholar]
- Fahey JW, Zalcmann AT, Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 2001;56:5–51. doi: 10.1016/s0031-9422(00)00316-2. [DOI] [PubMed] [Google Scholar]
- Faizi S, Siddiqui B, Saleem R, Saddiqui S, Aftab K. Isolation and structure elucidation of new nitrile and mustard oil glycosides from Moringa oleifera and their effect on blood pressure. J Nat Prod. 1994a;57:1256–1261. doi: 10.1021/np50111a011. [DOI] [PubMed] [Google Scholar]
- Faizi S, Siddiqui BS, Saleem R, Siddiqui S, Aftab K, Gilani AH. Fully acetylated carbonate and hypotensive thiocarbamate glycosides from Moringa oleifera. Phytochemistry. 1995;38:957–963. doi: 10.1016/0031-9422(94)00729-d. [DOI] [PubMed] [Google Scholar]
- Kerharo PJ. Un remede populaire Sengalais: Le ‘Nebreday’ (Moringa oleifera lann.) employs therapeutiques en milieu Africain chimie et pharmacologie. Plantes Med Phytother. 1969;3:14–219. [Google Scholar]
- Kim HK, Choi YH, Verpoorte R. NMR-based metabolomic analysis of plants. Nature Protocols. 2010;5:536–549. doi: 10.1038/nprot.2009.237. [DOI] [PubMed] [Google Scholar]
- Kim SW, Ban SH, Jeong SC. Genetic Discrimination between Catharanthus roseus Cultivars by Metabolic Fingerprinting Using 1H NMR Spectra of Aromatic Compounds. Biotechnology and Bioprocess Engineering. 2007;12:646–652. [Google Scholar]
- Kurma SR, Mishra SH. Antiinflammatory and hepatoprotective activities of fruits of Moringa. Ind J Nat Prod. 1998;14:3–10. [Google Scholar]
- Mahmud I, Kousik C, Hassell R, Chowdhury K, Boroujerdi A. Identification and Translocation of Metabolites from Powdery Mildew Resistant Rootstocks to Susceptible Watermelon Scions using NMR. Metabolomics. 2014a doi: 10.1021/acs.jafc.5b02108. (Submitted) [DOI] [PubMed] [Google Scholar]
- Mahmud I, Shrestha B, Thapaliya M, Boroujerdi A, Chowdhury K. NMR-based Metabolomics Profile Comparisons to Distinguish BetweenEmbryogenic and Non-embryogenic Callus Tissue of Sugarcane at the Biochemical Level. In Vitro Cell and Dev Biol-Plant. 2014b (Submitted) [Google Scholar]
- Makkar HPS, Becker K. Nutritional value and antinutritional components of whole and ethanol extracted Moringa oleifera leaves. Anim Feed Sci Technol. 1996;63:211–228. [Google Scholar]
- Makonnen E, Hunde A, Damecha G. Hypoglycemic effect of Moringa Stenopetala aqueous extracts in Rabbits. Phytother Res. 1997;11:147–148. [Google Scholar]
- Mansell RL, Mcintosh CA. In-vitro culture and production of Naringin and Limonin. In: Bajaj YPS, editor. Biotechnology in Agriculture and Forestry. 5. Medicinal and Aromatic Plants III. Berlin, Heidelberg: Springer-Verlag; 1981. p. 198. [Google Scholar]
- Mathur M, Kamal R. Studies on trigonelline from Moringa oleifera and its in vitro regulation by feeding precursor in cell cultures. Brazilian Journal of Pharmacognosy. 2012;22(5):994–1001. [Google Scholar]
- Mehta LK, Balaraman R, Amin AH, Bafna PA, Gulati OD. Effect of fruits of Moringa oleifera on the lipid profile of normal and hypercholesterolemic rabbits. Ethnopharmacol. 2003;86:191–195. doi: 10.1016/s0378-8741(03)00075-8. [DOI] [PubMed] [Google Scholar]
- Murakami A, Kitazono Y, Jiwajinda S, Koshimizu K, Ohigashi H. Niaziminin, a thiocarbamate from the leaves of Moringa oleifera, holds a strict structural requirement for inhibition of tumor-promoter-induced Epstein-Barr virus activation. Planta Med. 1998;64:319–323. doi: 10.1055/s-2006-957442. [DOI] [PubMed] [Google Scholar]
- Oliveira JTA, Silveira SB, Vasconcelos IM, Cavada BS, Moreira RA. Compositional and nutritional attributes of seeds from the multipurpose tree Moringa oleifera Lamarck. J Sci Food Agric. 1999;79:815–520. [Google Scholar]
- Pal SK, Mukherjee PK, Saha BP. Studies on the antiulcer activity of Moringa oleifera leaf extract on gastric ulcer models in rats. Phytother Res. 1995;9:463–465. [Google Scholar]
- Palama TL, Menard P, Fock I, Choi YH, Bourdon E, Soulange JG, Bahut M, Payet B, Verpoorte R, Kodja H. Shoot differentiation from protocorm callus cultures of Vanilla planifolia (Orchidaceae): proteomic and metabolic responses at early stage. BMC Plant Biology. 2010;10:82. doi: 10.1186/1471-2229-10-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddhuraju P, Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agro-climatic origins of drumstick tree (Moringa oleifera Lam.) J Agric Food Chem. 2003;15:2144–2155. doi: 10.1021/jf020444+. [DOI] [PubMed] [Google Scholar]
- Sutar NG, Bonde CG, Patil VV, Narkhede SB, Patil AP, Kakade RT. Analgesic activity of seeds of Moringa oleifera Lam. Int J Green Pharm. 2008;2:108–110. [Google Scholar]
- Vlahov G, Chepkwony PK, Ndalut PK. 13C NMR Characterization of Triacylglycerols of Moringa oleifera Seed Oil: An “Oleic-Vaccenic Acid” Oil. J Agric Food Chem. 2002;50(5):970–975. doi: 10.1021/jf011054a. [DOI] [PubMed] [Google Scholar]
- Wangcharoen W, Gomolmanee S. Antioxidant capacity and total phenolic content of Moringa oleifera grown in Chiang Mai, Thailand. Thai J Agric Sci. 2011;44:118–124. [Google Scholar]
- Yang SO, Kim SH, Kim Y, Kim HS, Chun YJ, Choi HK. Metabolic descrimination of Catharanthus roseus calli according to their relative locations usinf 1H NMR and principal component analysis. Biosci Biotechnol Biochem. 2009;73(9):2032–2036. doi: 10.1271/bbb.90240. [DOI] [PubMed] [Google Scholar]