Abstract
Perivascular adipose tissue (PVAT) contributes to vasoregulation. The role of this adipose tissue bed in pregnancy has not been examined. Here, we tested the hypothesis that PVAT in pregnant rats decreases resistance artery tone. Mesenteric arteries from nonpregnant (NP) and late pregnant (LP) rats were exposed to phenylephrine (PHE) or KCl in the presence (+) versus absence (−) of PVAT. The LP PVAT(+) vessels showed a 44% decrease in sensitivity to PHE in the presence of PVAT. There was no attenuation of the contractile response to KCl when PVAT was present. The LP arteries perfused with LP or NP PVAT underwent vasodilation; unexpectedly, NP vessels in the presence of PVAT from LP rats sustained a 48% vasoconstriction. The PVAT attenuates vasoconstriction by a mechanism that involves hyperpolarization. The vasoconstriction observed when nonpregnant vessels were exposed to pregnant PVAT suggests pregnant vessels adapt to the vasoconstricting influence of pregnant PVAT.
Keywords: perivascular adipose tissue, vasoregulation, pregnancy, phenylephrine, hyperpolarization
Introduction
Perivascular adipose tissue (PVAT; Figure 1) is a functionally unique and specialized adipose tissue depot, which surrounds most blood vessels throughout the body, and is particularly evident in the splanchnic circulation. Under normal physiological conditions, PVAT has been found to decrease vascular tone in animals1–4 and humans.5,6
Figure 1.
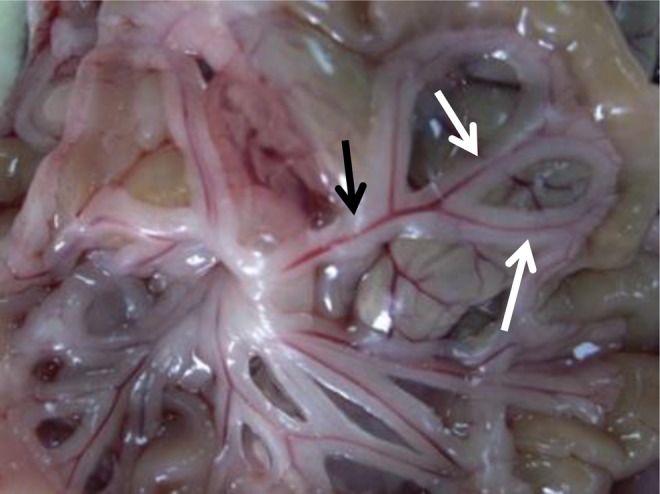
Perivascular adipose tissue (PVAT) in the mesenteric circulation. The superior mesenteric artery (black arrow) and its branches surround by adipose tissue (white arrow).
Modulation of vascular tone by PVAT has been attributed to the release of adipokines that act in a paracrine manner on the underlying vascular smooth muscle and/or endothelium.6 These adipokines promote vasorelaxation in a healthy state or vasoconstriction in pathological states.7–13 Although the exact mechanism of action has yet to be elucidated, it has been hypothesized that there may be a PVAT-derived relaxing factor such as adiponectin, angiotensin1-7, or hydrogen sulfide that when released acts in an endothelium-dependent and/or independent manner (depending on the species and vascular bed) resulting in vasodilation through the activation of vascular smooth muscle K+ channels.4,5,7–9
Alterations in PVAT structure and function have been demonstrated in several vascular disease states including hypertension,10–13 diabetes,14 and atherosclerosis.15,16 However, to date no studies have examined whether or how PVAT affects vascular function in pregnancy, although pregnancy is associated with a significant alteration in lipid metabolism and reduction in systemic vascular resistance and vascular tone. The reduction in vascular tone is most often attributed to an increase in endothelial-derived vasodilators (nitric oxide [NO], prostacyclin, and endothelium-derived hyperpolarization [EDH]), sometimes combined with a reduction in vascular smooth muscle sensitivity to vasoconstrictor agonists such as phenylephrine (PHE) and angiotensin II.17
Here, we hypothesize that PVAT will have a significant vasodilatory effect on mesenteric resistance arteries from pregnant animals and that this influence will be modified relative to PVAT from nonpregnant (NP) animals. We tested this hypothesis with the following objectives: (1) determine whether PVAT has a vasorelaxing effect on mesenteric resistance vessels from pregnant animals; (2) determine whether hyperpolarization is necessary for PVAT’s vasodilatory effect; and (3) evaluate the effect of pregnancy on mesenteric vascular reactivity by examining the effects of fat from NP versus late pregnant (LP) rats on vessels obtained from each type of animal.
Methods
Animals
Female Sprague-Dawley rats at 13 to 14 weeks of age were purchased from Charles River, Canada, in either a nonpregnant or a pregnant state. Pregnant animals were studied during LP, on day 20 of a 22-day gestation. This study conforms with and was approved by the guidelines of University of Vermont Animal Care Committee. Animals were anesthetized on day 20 by isoflourane (3%) and killed by decapitation.
Isolated Mesenteric Artery Preparation
A segment of the gut approximately 5 cm distal to the pylorus was excised from the abdominal cavity and pinned in a Sylgard-coated Petri dish filled with physiological saline solution (PSS; Figures 1 and 2). Third-order branches of the superior mesenteric artery (mean diameter 240 µm) were dissected and quickly transferred to a pressure arteriograph that contained oxygenated PSS. For the PVAT+ arteries, PVAT was carefully removed from 1 side of the vessel (and left intact on the contralateral side) using fine scissors under a dissecting microscope being careful not to damage the underlying adventitial layer.
Figure 2.
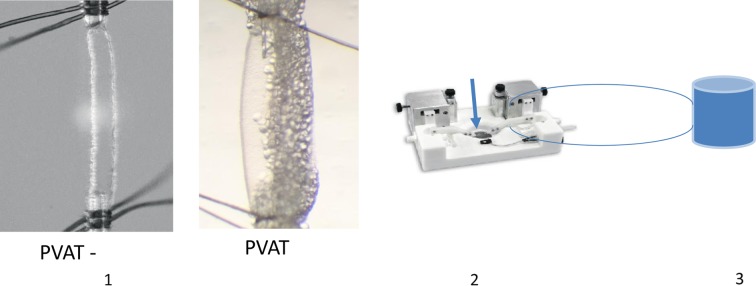
Representative picture demonstrating pressure arteriograph methodology. (1) Appearance of third-order branches of the superior mesenteric artery with (PVAT+) and without PVAT (PVAT−). (2) Arteries were mounted in a pressure arteriograph (37° C, pH 7.4). The blue arrow indicates where the artery is mounted. (3) Depending on the protocol, drugs (phenylephrine [PHE], acetylcholine [Ach], and KCl) were added to the reservoir. For solution transfers studies, PVAT was added to the reservoir. PVAT indicates perivascular adipose tissue.
A 2- to 3-mm segment of artery was cannulated at each end onto a glass micropipette. The proximal end was secured with a nylon ligature and gently flushed of residual blood. The distal end was then secured in a similar fashion and thereafter under no-flow conditions at an intraluminal pressure of 50 mm Hg using a pressure servo system with an in-line pressure transducer (Living Systems Instrumentation, Vermont). A 37°C circulating reservoir of PSS was maintained at pH 7.4 by bubbling with a gas mixture of 10% O2, 85% N2, and 5% CO2. The chamber was set on the stage of an inverted microscope with a video camera attached to the viewing cylinder. Lumen diameter was measured by transilluminating each vessel segment and using a video dimension analyzer system (VDA; Living Systems Instrumentation). Signals from the pressure servo system and VDA were simultaneously collected by a computer data acquisition system (DATAQ; Akron, Ohio) and recorded.
All drugs were added to a buffer reservoir that allowed for continuous recirculation. Vessels were allowed to equilibrate for 30 to 45 minutes prior to the beginning of an experiment. At the end of each experiment, 10−6 mol/L acetylcholine was added to each vessel to confirm the presence of viable endothelium. Vessels that did not dilate to within 10% of their initial baseline were excluded from the analysis.
Functional Assessment of PVAT Effects on Isolated Mesenteric Arteries (Experiments 1 and 2)
For the first series of experiments, vessels with and without PVAT from LP and NP rats were exposed to cumulative concentrations of PHE (10−8 to 10−5 mol/L for 3 minutes at each concentration) or KCl (LP rats only; 5-60 mmol/L) to determine the influence of perivascular PVAT on vasoconstrictor sensitivity. Different vessels were used for PHE and KCl.
In a second series of studies, solution transfer experiments (Figure 2) were performed in the following manner. Prior to starting the experiment, 0.2 g of PVAT from an LP mesenteric artery was placed in a continuously warmed (37°C) beaker containing 5 cm3 PSS and allowed to equilibrate for 1 hour. Vessels were primed with a concentration of PHE that would cause maximal constriction, followed by a wash out with PSS. Vessels were then preconstricted with PHE to obtain a 30% to 50% reduction in artery diameter. Once constriction stabilized (≈10 minutes), 5 cm3 of the total 50 cm3 of circulating PSS was removed and replaced with a 5-cm3 PVAT/PSS solution that contained an equivalent concentration of PHE. Lumen diameter was measured after 30 minutes, as this time was found to be the optimal period for the vessel to develop a stable diameter.
Crossover Study: Vascular Reactivity Effects of Nonpregnant versus Pregnant PVAT (Experiment 3)
For these studies, the solution transfer paradigm described previously was used with the following changes. The PVAT was removed from an LP or NP artery and the artery was mounted and cannulated in an arteriograph as described earlier. After the 10-minute stabilization period, the 5 cm3 PVAT/PSS aliquot from an NP or an LP rat was added to the reservoir with the PVAT(−) mounted vessel from a pregnant or nonpregnant rat (eg, each type of vessel was exposed to both kinds of fat). In order to account for time-dependent changes that occur with PHE, after the 10-minute stabilization period, each vessel was also exposed to PHE only and the lumen diameter was measured after 30 minutes. This value was then subtracted from the 30-minute lumen diameter that was obtained in the presence of PVAT.
Chemicals
The composition of PSS was 115 mmol/L NaCl, 4.6 mmol/L KCl, 1 mmol/L CaCl2, 25 mmol/L NaHCO3, 1.2 mmol/L KH2PO4, 1.2 mmol/L MgS04, 0.01 mmol/L EDTA, and 11 mmol/L glucose (Fisher Scientific, Hampton, New Hampshire). Potassium concentration response curves were generated by the cumulative addition of KCl (0.5 mol/L stock). The PHE and acetylcholine (Sigma, St Louis, Missouri) were administered from prepared stock solutions dissolved in deionized water.
Statistical analysis
All values are presented as mean ± standard error of the mean. Half-maximal effective concentration (EC50) values (concentration of drug required to produce a half-maximal response) were calculated as percentage of the maximum response obtained with each drug, determined by no additional change in diameter following the addition of more drug. The EC50 value was obtained from a plot of the concentration-dependent response (GraphPad Prism version 5.00 for Windows). Individual EC50 values (µmol/L) and maximal constrictor responses (percentage change from baseline at highest concentration) were determined for PHE and KCl to provide measures of sensitivity and efficacy, respectively, and averaged values were compared using a t test. For the crossover studies, the time-related changes in vessel diameter with PHE only were subtracted at 30 minutes and were analyzed with a 2-way analysis of variance and Tukey test for multiple comparisons. P < .05 was considered statistically significant.
Results
Pressure Myography: PVAT Has an Anticontractile Effect on LP Mesenteric Arteries
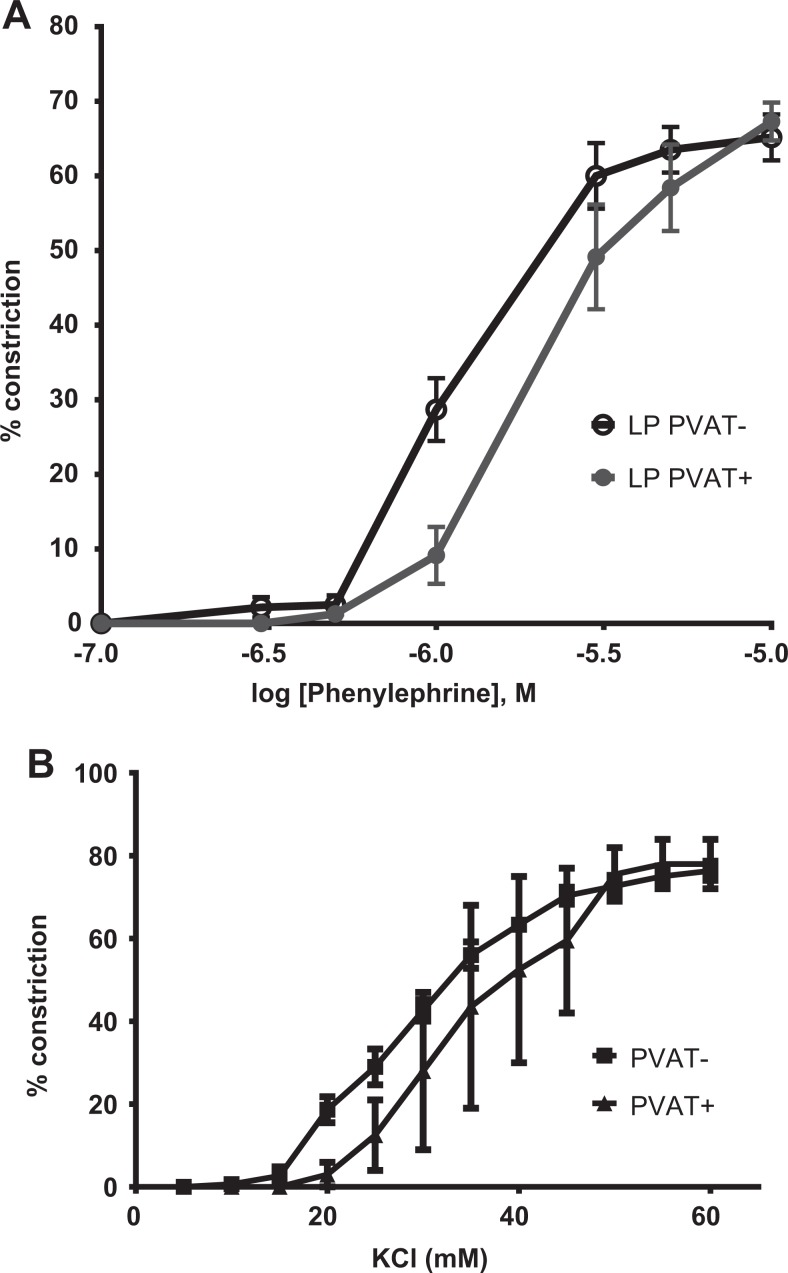
The LP arteries with PVAT (PVAT+) were significantly less sensitive to PHE as revealed by the right shift of the concentration response curve and the reduction in EC50 of vessels with versus without PVAT (4.7 ± 07 vs 2.8 ± 0.4 µmol/L; P < .03; Figure 3A). The presence of PVAT had no effect on efficacy as the mean response was 68% ± 3%. In order to determine whether hyperpolarization was necessary for PVAT-mediated relaxation, we examined the response of arteries in the presence of KCl and found that the contractile responses to increasing concentrations of KCl were no different for PVAT− versus PVAT+ vessels (Figure 3B) as both EC50 (31.3 ± 2.3 vs 30.1 ± 3.9 µmol/L) values and efficacy (68% ± 3%) were similar. The NP arteries with PVAT also had a reduction in sensitivity when exposed to increasing concentrations of PHE (Table 1).
Figure 3.
Constriction of vessel with PVAT (PVAT+) and without PVAT (PVAT−) to increasing concentrations of phenylephrine (A) and KCl (B). A, PVAT+ (n = 14) and PVAT− (n = 11). B, PVAT+ (n = 5) and PVAT− (n = 5). PVAT indicates perivascular adipose tissue.
Table 1.
Half-maximal effective concentration (EC50; μmol/L) Calculated From Concentration Response to Phenylephrine for Mesenteric Arteries With (+) and Without (−) Perivascular Adipose Tissue (PVAT) From Late Pregnant (LP) and Nonpregnant (NP) Rats.
| PVAT− | PVAT+ | |
|---|---|---|
| LP | a2.8 ± 0.4 (n = 11) | 4.7 ± 0.7 (n = 14) |
| NP | b4.3 ± 0.6 (n = 10) | c19.7 ± 6.3(n = 10) |
aLP− versus LP+; P < .03.
bLP− versus NP−; P < .04.
cNP− versus NP+; P < .007.
Solution Transfer Studies Demonstrate That Pregnant PVAT Releases a Vasorelaxing Factor
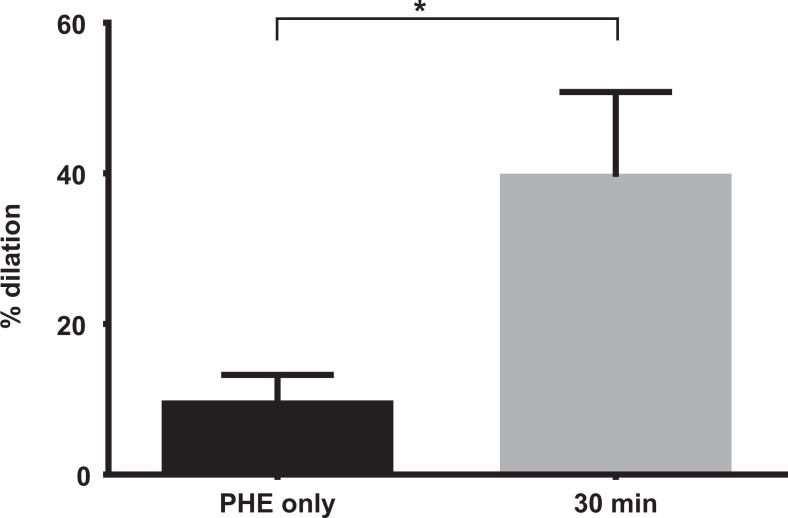
After the addition of the 0.2 g PVAT/PSS solution (containing the same concentration of PHE), PHE-preconstricted arteries underwent significant vasodilation (36% ± 9%, P < .03) in comparison to the baseline derived from time controls (Figure 4), which were exposed to PHE without PVAT.
Figure 4.
Response of preconstricted late pregnant vessel to phenylephrine (30%-50% resting diameter) after addition of 0.2 g PVAT. Phenylephrine (PHE) represents the response of the vessel after 30 minutes with PVAT solution added the circulating bath (n=9). *P < .03. PVAT indicates perivascular adipose tissue.
Perivascular Adipose Tissue From LP Rat Has a Vasoconstrictor Effect on NP Mesenteric Artery
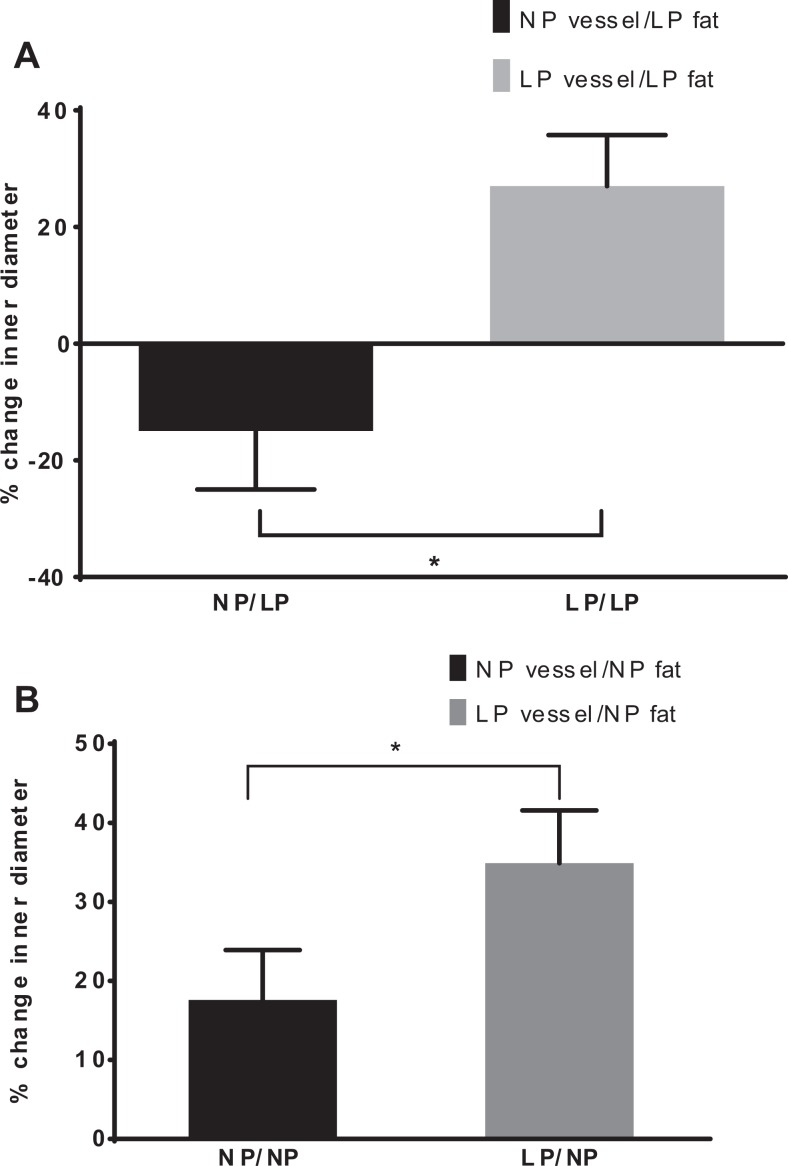
The LP vessels showed a 34% ± 13% increase in lumen diameter in response to the 0.2 g PVAT/PSS solution from NP animals (Figure 5). On the other hand, although LP vessels relaxed to PVAT derived from either NP or pregnant animals, exposure of vessels from NP rats to LP adipose tissue resulted in significant vasoconstriction (−48% ± 9%, P < .03) in comparison to LP vessels perfused with LP fat (Figure 5A and B).
Figure 5.
Effects of NP versus LP adipose tissue on vascular reactivity. Percentage change (from baseline) in vessel diameter by different vessel type when exposed to pregnant fat. A, Effect of late pregnant (LP) adipose tissue on reactivity in mesenteric arteries from nonpregnant (NP) rats (n = 10-14) *P < .001. B, Effect of nonpregnant fat on LP arteries (n = 10-14; P < .05).
Discussion
The present investigation evaluated the effects of pregnancy on PVAT-mediated changes in mesenteric resistance artery reactivity. There were 3 major findings, that is, (1) this study is the first to show that PVAT from pregnant animals has a significant vasodilatory influence on resistance artery diameter; (2) this effect is mediated by a mechanism that involves hyperpolarization, as it was not present in vessels preconstricted with a depolarizing solution of KCl; (3) PVAT from pregnant animals induced a significant opposite effect in vessels from NP animals, that is, vasoconstriction. Each of these points is discussed subsequently.
Influence of PVAT on Resistance Arteries From LP Rats
The mesenteric circulation undergoes changes in reactivity to accommodate the increased cardiac output and vascular volume characteristic of mammalian pregnancy.18 One of the adaptations that facilitates this change is a reduced pressor response of the mesenteric arteries to receptor-mediated vasoconstrictor agonists.19 Here, we demonstrate that PVAT from third-order mesenteric arteries of LP rats can further attenuate the contractile response when these vessels are preconstricted with PHE, presumably via the release of vasodilatory adipokines such as adiponectin or angiotensin1-7.2–7 Specifically, the presence of PVAT was associated with a 50% reduction in sensitivity to PHE. We hypothesize that PVAT’s attenuation of vasoconstriction may facilitate the increased plasma volume that is necessary for a healthy pregnancy and contribute to the reduction in blood pressure observed during pregnancy.
Role of Vascular Smooth Muscle K+ Channels in PVAT-Mediated Vasodilation
In the healthy nonpregnant rat, PVAT’s vasorelaxing effect has been attributed to the action of a PVAT-derived relaxing factor that causes hyperpolarization of the underlying smooth muscle cells by activation of hyperpolarizing K+ channels.2,4,6 The elimination of PVAT’s anticontractile effects in the presence of KCl suggests that the pathway associated with PVAT-mediated vasodilation does not interfere with the vascular contraction induced by the Ca2+ entry through voltage-dependent calcium channels but is more likely due to inactivation of K+ conductance channels. Although our study did not address the mechanism of PVAT-mediated vasorelaxation, we speculate that the loss of PVAT’s anticontractile effect in the presence of high external K+ concentrations can be attributed to K+ channel inactivation.18 Since mesenteric arteries are more hyperpolarized in pregnancy,20 it is possible that the adipose tissue that surrounds these blood vessels may contribute to the hyperpolarized resting membrane potential present in pregnancy. Additional electrophysiological studies will be necessary for direct measurement of the resting membrane potential in intact mesenteric arteries as well pharmacologic studies to investigate the mechanism of PVAT-mediated K+ channel activation (eg, NO and/or EDH factor).
Effect of Pregnant PVAT on Arteries From NP Animals
One of the unexpected findings in this study was how NP mesenteric arteries responded to adipose tissue from LP rats. When NP vessels were exposed to pregnant PVAT, the NP vessel constricted; this constriction is in contrast to the dilation that occurred when a pregnant vessel was perfused with pregnant adipose tissue. This finding suggests that PVAT takes on a more vasoconstrictive phenotype during pregnancy that is attenuated as a result of pregnancy-related adaptations in vascular smooth muscle and/or endothelium.
Although the mechanism is unclear, we speculate that inflammatory changes that normally occur in visceral adipose tissue during pregnancy may also occur in the PVAT. Studies of PVAT in an inflammatory milieu have shown that PVAT will promote vasoconstriction in this environment.21,22 Therefore, it appears that pregnancy produces a vascular adaptation that counteracts the vasoconstricting properties acquired by pregnant adipose tissue such that the mesenteric arteries from LP rats undergo an adaptation that results in the pregnant vessel being more vasorelaxed in a healthy pregnant state. The above-mentioned finding is also consistent with the greater reduction in sensitivity to PHE in nonpregnant vessels than in LP vessels. Clearly, PVAT undergoes changes in pregnancy that attenuates its vasorelaxing effect. Future studies are needed to investigate the mechanism underlying these changes.
The demonstration of PVAT-mediated anticontractility in late gestation rat mesenteric resistance arteries adds to the growing body of literature supporting the critical vasoregulatory role of PVAT. The findings from this study open up a new area of exploration for understanding how vascular contractility is regulated during pregnancy. Future studies are necessary to determine the specific role of PVAT in pregnancy vasoregulation, that is, is PVAT the primary regulator of blood flow distribution and vascular tone or does it play a more passive role in responding to endothelial cell release of vasoregulatory molecules? Additionally, the mechanism of this effect with respect to the interaction between the PVAT and endothelial cells will be important as well as how PVAT contributes to the NO-dependent and -independent components of vasoregulation in pregnancy. Although our focus in this study was the mesenteric circulation, future studies that examine other vascular beds and conduit vessels will be important to enhance our understanding of vasoregulation in pregnancy.
In summary, our results support the hypothesis that adipose tissue around mesenteric resistance vessels contributes to the reduction in vascular tone that occurs in pregnancy. Expanding our understanding of PVAT not only in the mesenteric circulation but also in other vascular beds and conduit vessels will be important to our understanding of vasoregulation in pregnancy. Studies have already shown that PVAT contributes to an increase in vascular tone in hypertension13 and that PVAT-mediated vasodilation is impaired in diabetes and obesity.23–25 Therefore, we hypothesize that alterations in PVAT function may be part of the pathophysiology of pregnancy-related vascular diseases such as preeclampsia and gestational diabetes. Future studies will be needed to understand this important adipose tissue bed.
Perspectives and Clinical Significance
Vascular dysfunction in pregnancy has many etiologies. One area that has yet to be investigated is the role of PVAT, the adipose tissue depot that surrounds most systemic blood vessels. The results from this study suggest that it is not just the endothelium and vascular smooth muscle that impact vascular contractility in pregnancy; the adipose tissue bed surrounding blood vessels is also important. Although it has been established that adipose tissue in pregnancy releases factors that impact insulin resistance and vascular function, this study suggests that the PVAT also impacts vascular contractility in pregnancy. With this information, there is the potential for an exciting new avenue of investigation that will not only enhance our understanding of vasoregulation in pregnancy but also may facilitate our understanding how PVAT changes in conditions such as maternal obesity and how PVAT may play a role in vascular diseases such as preeclampsia and diabetes.
Footnotes
Authors’ Note: Presented at the Society for Maternal Fetal Medicine, February 2014, New Orleans, LA, USA.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: NIH WRHR K12 HD0638082-03.
References
- 1. Soltis EE, Cassis LA. Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin Exp Hypertens A. 1991;13 (2):277–296. [DOI] [PubMed] [Google Scholar]
- 2. Lohn M, Dubrovska G, Lauterbach B, Luft FC, Gollasch M, Sharma AM. Periadventitial fat releases a vascular relaxing factor. FASEB J. 2002;16 (9):1057–1063. [DOI] [PubMed] [Google Scholar]
- 3. Dubrovska G, Verlohren S, Luft FC, Gollasch M. Mechanisms of ADRF release from rat aortic adventitial adipose tissue. Am J Physiol Heart Circ Physiol. 2004;286 (3):H1107–H1113. [DOI] [PubMed] [Google Scholar]
- 4. Gao YJ, Lu C, Su LY, Sharma AM, Lee RM. Modulation of vascular function by perivascular adipose tissue: the role of endothelium and hydrogen peroxide. Br J Pharmacol. 2007;151 (3):323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gao YJ, Zeng ZH, Teoh K, et al. Perivascular adipose tissue modulates vascular function in the human internal thoracic artery. J Thorac Cardiovasc Surg. 2005;130 (4):1130–1136. [DOI] [PubMed] [Google Scholar]
- 6. Verlohren S, Dubrovska G, Tsang SY, et al. Visceral periadventitial adipose tissue regulates arterial tone of mesenteric arteries. Hypertension. 2004;44 (3):271–276. [DOI] [PubMed] [Google Scholar]
- 7. Gollasch M. Vasodilator signals from perivascular adipose tissue. Br J Pharmacol. 2012;165 (3):633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gollasch M, Dubrovska G. Paracrine role for periadventitial adipose tissue in the regulation of arterial tone. Trends Pharmacol Sci. 2004;25 (12):647–653. [DOI] [PubMed] [Google Scholar]
- 9. Lee RM, Lu C, Su LY, Gao YJ. Endothelium-dependent relaxation factor released by perivascular adipose tissue. J Hypertens. 2009;27 (4):782–790. [DOI] [PubMed] [Google Scholar]
- 10. Galvez B, de Castro J, Herold D, et al. Perivascular adipose tissue and mesenteric vascular function in spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol. 2006;26 (6):1297–1302. [DOI] [PubMed] [Google Scholar]
- 11. Lee RM, Ding L, Lu C, Su LY, Gao YJ. Alteration of perivascular adipose tissue function in angiotensin II-induced hypertension. Can J Physiol Pharmacol. 2009;87 (11):944–953. [DOI] [PubMed] [Google Scholar]
- 12. Lee YC, Chang HH, Chiang CL, et al. Role of perivascular adipose tissue-derived methyl palmitate in vascular tone regulation and pathogenesis of hypertension. Circulation. 2011;124 (10):1160–1171. [DOI] [PubMed] [Google Scholar]
- 13. Lu C, Su LY, Lee RM, Gao YJ. Alterations in perivascular adipose tissue structure and function in hypertension. Eur J Pharmacol. 2011;656 (1-3):68–73. [DOI] [PubMed] [Google Scholar]
- 14. Eringa EC, Bakker W, van Hinsbergh VW. Paracrine regulation of vascular tone, inflammation and insulin sensitivity by perivascular adipose tissue. Vascul Pharmacol. 2012;56 (5-6):204–209. [DOI] [PubMed] [Google Scholar]
- 15. Chang L, Milton H, Eitzman DT, Chen YE. Paradoxical roles of perivascular adipose tissue in atherosclerosis and hypertension. Circ J. 2013;77 (1):11–18. [DOI] [PubMed] [Google Scholar]
- 16. Verhagen SN, Visseren FL. Perivascular adipose tissue as a cause of atherosclerosis. Atherosclerosis. 2011;214 (1):3–10. [DOI] [PubMed] [Google Scholar]
- 17. Hermsteiner M, Zoltan DR, Kunzel W. The vasoconstrictor response of uterine and mesenteric resistance arteries is differentially altered in the course of pregnancy. Eur J Obstet Gynecol Reprod Biol. 2001;100 (1):29–35. [DOI] [PubMed] [Google Scholar]
- 18. D'Angelo G, Osol G. Regional variation in resistance artery diameter responses to alpha adrenergic stimulation during pregnancy. Am J Physiol. 1993;264(1 pt 2):H78–H85. [DOI] [PubMed] [Google Scholar]
- 19. Paller MS. Mechanism of decreased pressor responsiveness to ANG II, NE, and vasopressin in pregnant rats. Am J Physiol. 1984;247 (1 pt 2):H100–H108. [DOI] [PubMed] [Google Scholar]
- 20. Meyer MC, Brayden JE, McLaughlin MK. Characteristics of vascular smooth muscle in the maternal resistance circulation during pregnancy in the rat. Am J Obstet Gynecol. 1993;169 (6):1510–1516. [DOI] [PubMed] [Google Scholar]
- 21. Bhattacharya I, Dragert K, Albert V, et al. Rictor in perivascular adipose tissue controls vascular function by regulating inflammatory molecule expression. Arterioscler Thromb Vasc Biol. 2013;33 (9):2105–2111. [DOI] [PubMed] [Google Scholar]
- 22. Guzik TJ, Marvar PJ, Czesnikiewicz-Guzik M, Korbut R. Perivascular adipose tissue as a messenger of the brain-vessel axis: role in vascular inflammation and dysfunction. J Physiol Pharmacol. 2007;58 (4):591–610. [PubMed] [Google Scholar]
- 23. Aghamohammadzadeh R, Heagerty AM. Obesity-related hypertension: epidemiology, pathophysiology, treatments, and the contribution of perivascular adipose tissue. Ann Med. 2012;44 (suppl 1):S74–S84. [DOI] [PubMed] [Google Scholar]
- 24. Eringa EC, Bakker W, Smulders YM, Serne EH, Yudkin JS, Stehouwer CD. Regulation of vascular function and insulin sensitivity by adipose tissue: focus on perivascular adipose tissue. Microcirculation. 2007;14 (4-5):389–402. [DOI] [PubMed] [Google Scholar]
- 25. Meijer RI, Serne EH, Smulders YM, van Hinsbergh VW, Yudkin JS, Eringa EC. Perivascular adipose tissue and its role in type 2 diabetes and cardiovascular disease. Curr Diab Rep. 2011;11 (3):211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]