Abstract
Aim:
To investigate the effects of vitamin C on the expression of the genes related to apoptosis in extravillous trophoblasts (EVTs) in the first trimester.
Methods:
Extravillous trophoblasts were cultured under 2% O2 followed by 2% O2 or 8% O2 with or without vitamin C. The level of reactive oxygen species (ROS) in the cultured medium was estimated using electron spin resonance spectroscopy. The expression levels of the genes TP53, BCL2, and BAX were quantified using real-time quantitative polymerase chain reaction.
Results:
Reactive oxygen species were found to be decreased after adding vitamin C under increasing oxygen concentrations. In addition, the ratio of BAX/BCL2 also increased after adding vitamin C under conditions of 2% O2, while the gene expression level of BCL2 increased after adding vitamin C under increasing oxygen concentrations. In contrast, the gene expression level of TP53 and the ratio of BAX/BCL2 both decreased.
Conclusion:
We have revealed that vitamin C reduces ROS and may promote the apoptosis of EVTs under conditions of 2% O2 while paradoxically preventing apoptosis under increasing oxygen concentrations.
Keywords: vitamin C, apoptosis, extravillous trophoblasts, RT-PCR, ESR
Introduction
Although the pathogenesis of preeclampsia remains largely unclear, many researchers have reported that the insufficient invasion of extravillous trophoblasts (EVTs) into the uterine decidua and myometrium contributes to the development of deficient placental perfusion, which is subsequently associated with pregnancy-related preeclampsia.1 Deficient perfusion is related to oxidative stress, which is considered to play a crucial role in the onset of preeclampsia.2,3 Several authors have hypothesized that excessive oxidative stress inhibits trophoblast invasion with subsequently poor placentation.2–4 Patients with preeclampsia exhibit profound cellular dysfunction with an increased response of unfolded proteins and apoptosis of villous trophoblasts.5 The levels of proapoptotic proteins, p53 and Bax, are increased in trophoblasts in the placenta at term complicated by preeclampsia.6,7 Adequately high levels of reactive oxygen species (ROS) induce structural and functional damage to lipids. A recent systematic review revealed that patients with preeclampsia have high levels of malondialdehyde, an indicator of lipid peroxidation, low levels of erythrocyte superoxide dismutase (SOD), which functions as an antioxidant,1 and low levels of serum vitamins C and E.8 Such findings indicate the potential role of antioxidant vitamins in maintaining the oxidative status of tissues.
Vitamin C is a chain-breaking antioxidant that halts the propagation of peroxidative processes and reacts with membrane-bound oxidized vitamin E, thus reducing it back to its native form.9,10 Vitamin C also plays a role in many enzyme reactions, including those leading to the synthesis of amino acids and peptide hormones. These findings suggest that vitamin C behaves as an ROS scavenger and may be effective in combating oxidative damage under conditions of increasing oxygen concentrations and apoptosis. Several randomized studies of the efficacy of antioxidant vitamins in preventing preeclampsia have shown no beneficial effects of vitamin C and E supplementation during pregnancy with respect to reducing the risk of preeclampsia.11–15 Intriguingly, epidemiological studies have reported a decreasing trend in the incidence of severe preeclampsia among patients with a high dietary intake of vitamin C and increased plasma vitamin C levels.16,17
During the first trimester, the intervillous space of the developing placenta is separated from the uterine circulation by plugs of trophoblasts that occlude the tips of uteroplacental arteries. At the end of the first trimester, these plugs are subsequently dislocated, allowing the maternal blood to flow freely and continuously in the intervillous space.18 The most convincing evidence regarding this issue, obtained by Jauniaux, is that the oxygen concentration in the intervillous spaces increases from 2% O2 before 9 weeks of gestation to 8% O2 at 10 to 12 weeks of gestation.19 Based on these findings, the process of increasing oxygen is clearly an important phase in trophoblast proliferation. Nevertheless, recent clinical studies of vitamins C and E in a number of different settings have proved unsuccessful. In these clinical trials, the antioxidant vitamins were only given during pregnancy (after 9-16 weeks of gestation); however, placental invasion is already established in early gestation. We therefore hypothesized that the administration of vitamin C at a high physiological concentration prevents apoptosis in EVTs from a burst of oxidative stress under increasing oxygen concentrations.
The aim of the present study was to clarify whether vitamin C administration activates neutralizing ROS and inhibits the expression of genes related to apoptosis in EVTs under conditions of hypoxia and increasing oxygen concentrations.
Materials and Methods
Tissue Collection
Extravillous trophoblasts obtained from surgically removed villous tissue specimens obtained from pregnant females requesting artificial abortion before 7 weeks of gestation were analyzed between December 2013 and June 2014. The villous tissue specimens obtained from cases of multiple gestation, self-reported smokers, illicit drug use, and preexisting medical conditions, such as diabetes, chronic hypertension and renal disease were excluded in the present analysis. The gestational age was decided based on last menses period, which confirmed or corrected with the crown-rump length with detection of the fetal heartbeat at Okayama Clinic (Tokyo, Japan) and Kitamura Clinic (Kawasaki, Japan), and villous samples were collected at 7 to 8 weeks. This study was approved by the Ethics Committee of Human Genomic Analysis at Showa University School of Medicine (approval number: 144/2011). Written informed consent was obtained from each patient prior to participation.
Following collection, the villi were immediately suspended in sterile saline and transported to the laboratory at the Department of Obstetrics and Gynecology at Showa University School of Medicine (Tokyo, Japan) within 3 hours, at which time the villous tissues were washed 3 times in sterile phosphate-buffered saline (PBS) to remove excess blood.
Isolation, Purification, and Treatment of EVTs
Extravillous trophoblasts were isolated from the villous tissues using a previously published method.20–22 Briefly, the villous materials were washed in Hanks balanced salt solution (HBSS; Sigma-Aldrich Co, Missouri). Then, after eliminating the deciduous membrane, the villi tissues were dissected, minced to a size of 0.5 mm3, and digested for 35 minutes at 37°C in 0.25% trypsin (Invitrogen Co, California) and 0.5 mg of DNaseI (Sigma-Aldrich Co) twice. At the end of the warm extraction procedure, the supernatants were collected. Following centrifugation, the cell pellets were resuspended in HBSS and loaded on top of a 5% step-layer Percoll (Sigma-Aldrich Co) gradient ranging from 10% to 70% and centrifuged. Trophoblasts were isolated from the middle layer of 35% to 45% Percoll and cultured within the culture medium (HAM F12 containing 10% fetal bovine serum, 1000 U/mL of penicillin, 1 mg/mL of streptomycin, and 1.5 mg/mL of amphotericin B obtained from Sigma-Aldrich Co)
Extravillous trophoblasts (5 × 105 cells/500 μL per well) were plated in a 24-well plate coated with growth factor-reduced Matrigel (Becton Dickson, East Rutherford, New Jersey) and incubated for 24 hours at 37°C, 5% CO2, and 2% O2 to promote the invasion of EVTs into the Matrigel. The EVTs were subsequently cultured under 4 conditions: 2% O2 with vehicle, 2% O2 with 200 μmol/L of ascorbic acid, 8% O2 with vehicle, and 8% O2 with 200 μmol/L of ascorbic acid, as described in Figure 1. The plasma vitamin C in pharmacokinetic model reported that vitamin C plasma concentrations were tightly controlled when the vitamin is ingested orally and the peak values did not exceed 220 μmol/L after the high oral administration, even at 3 g given orally every 4 hours.23 We performed this study in the experiments under 200 μmol/L of ascorbic acid for the considerable high physiological concentration.
Figure 1.
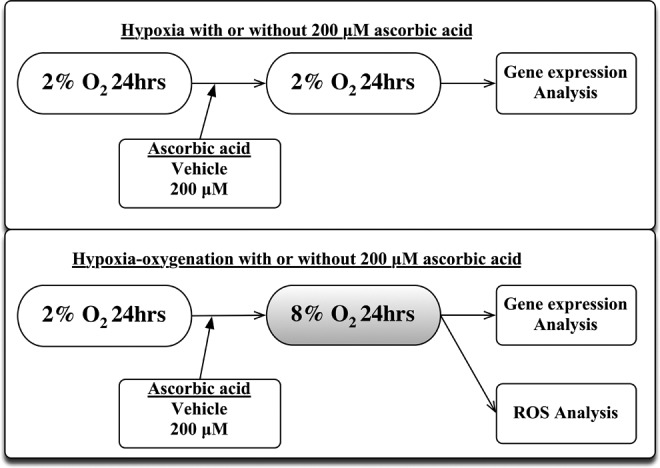
Experimental design of the EVTs culture. EVTs were cultured under conditions of 2% O2 for 24 hours to promote attachment to the Matrigel. The cells were then cultured for an additional 24 hours under conditions of 2% O2 with or without 200 μmol/L of vitamin C or 8% O2 with or without 200 μmol/L of vitamin C. The expression levels of genes related to mitochondria-dependent apoptosis and ROS were subsequently analyzed. EVT indicates extravillous trophoblast.
At the end of the incubation period, the cells were removed from the Matrigel after spreading cell recovery solution (BD Biosciences, Bedford, Massachusetts). The layers of cells and gel were then scraped with 2 mL of cell recovery solution per well, and the samples were left on ice for 1 hour until the Matrigel was completely dissolved, after which the cells were recovered via centrifugation (300g for 5 minutes at 4°C) and washed twice in sterile PBS.
Detection and Quantitative Analysis of ROS
The formation of ROS was observed by electron spin resonance (ESR). The ESR spin trapping technique involves an addition reaction of a short-lived radical to a diamagnetic compound to make a more stable free radical product, spin adduct. The intensity of the spin adduct signal corresponds to the amount of short-lived radicals trapped. In this study, 5,5-dimetyl-1-pyrroline-N-oxide (DMPO; Dojin Chemicals, Kumamoto, Japan) was used to trap free radicals, and the ESR spectra were recorded with a JES RE1X (X-band 100 kHz; JEOL, Tokyo, Japan). In order to analyze free radicals in the medium, 100 μL of the supernatant of the culture medium was immediately mixed with 100 μL of DMPO by vortexing for 5 seconds and immediately stored in liquid nitrogen. Within 10 minutes of thawing, the intensity of free radicals was measured using an ESR spectrometer. The conditions for ESR were applied at room temperature (23°C) with the following spectrometer settings: microwave power = 16 mW, magnetic field = 335.4 ± 5 mT, modulation amplitude = 0.1 mT, time constant = 0.1 seconds, and scanning time = 2 minutes. The intensity of free radicals was defined as the ratio of the signal intensity of the first peak of the free radical spin adduct to the internal ESR signal derived from Mn2+.24,25
RNA Extraction and Reverse Transcription
Total RNA was extracted from the cell pellets using the RNeasy Mini Kit (Qiagen, Valencia) according to the manufacturer’s instructions. The purity of the RNA was determined using a NanoDrop ND-1000 spectrophotometer (Thermo Scientific Inc. Wilmington) by measuring the absorbance at 260 and 280 nm. An OD 260/280 ratio greater than 1.90 was considered to indicate that the sample was acceptable for further processing. All RNA samples met this purity requirement. The extracted total RNA (2 μg) was immediately reverse transcribed into complementary DNA using the PrimeScript RT Master Mix (Takara Bio Inc, Shiga, Japan) according to the manufacturer’s instructions. The process was performed in a Veriti Thermal Cycler (Applied Biosystems, Foster City, California) under the following thermal conditions: 15 minutes at 37°C, followed by 5 minutes at 85°C.
Real-Time Quantitative Polymerase Chain Reaction
The real-time quantitative polymerase chain reaction (RT-qPCR) analysis was performed using the StepOnePlus Real-Time PCR System (Applied Biosystems). Assay-on-Demand TaqMan primers and primers obtained from Applied Biosystems were used to quantify the levels of TP53 (TaqMan Gene Expression Assay ID Hs01034249_m1), BAX (Hs00180269_m1), and BCL2 (Hs00608023_m1). ACTB (Hs01060665_g1) was used as a reference gene. The selected genes were briefly expressed as follows. TP53 encodes a tumor suppressor protein, which induces apoptosis mediated by the stimulation of BAX (a proapoptotic regulator) and repression of BCL2 (an antiapoptotic regulator). The thermal cycling conditions were as follows: 95°C for 30 seconds, followed by 40 cycles at 95°C for 5 seconds and 60°C for 30 seconds. All samples were analyzed in duplicate, and multiple negative water blanks were included in each analysis. The transcript numbers were determined based on the linear regression model of the standard curves. The gene expression levels were normalized to the level of ACTB as a ratio (absolute expression of the target gene/absolute expression of ACTB).
Data Analysis
The data are expressed as the fold change relative to equivalent control and presented as the mean ± standard error of the mean. Statistically significant differences were assessed using the Wilcoxon rank-sum test. All analyses were carried out using the JMP version 11.0.0 software program (SAS Institute, Cary, North Carolina). A P value of <.05 was considered to be statistically significant.
Results
Extravillous trophoblastsobtained from 12 pregnant females without any exclusion criteria. The ESR spin trapping analysis demonstrated the typical ESR spectra for the formation of the alkoxyl radical (RO·) DMPO spin adduct (Figure 2A). Under an increasing oxygen concentration in the presence of 200 μmol/L of vitamin C, significant decreases were observed in the height of the first peak of the spectrum (0.81 ± 0.03 fold lower, P = .013, n = 4), which represented the relative amount of DMPO-RO· adduct, compared to that obtained without the presence of vitamin C in the 2% O2 culture (Figure 2B).
Figure 2.
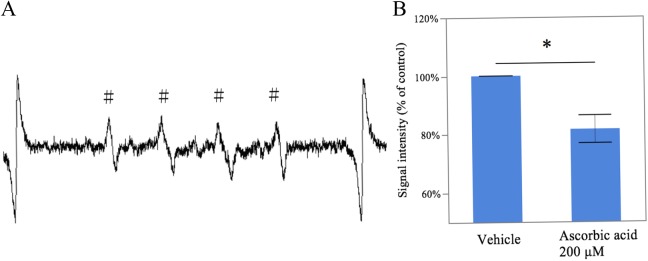
Medium free radical generation under conditions of increasing oxygen concentrations with vitamin C. The medium was mixed with DMPO and subjected to an ESR spin-trapping analysis. The ESR spectrum of the medium without vitamin C is shown, which revealed the presence of RO· (A). The signal intensity of RO· under conditions of an increasing oxygen concentration in the presence of 200 μmol/L of vitamin C was decreased (n = 4; B). # indicates RO· spin adduct. *, P < .05, between the samples cultured with and without vitamin C; DMPO, 5,5-dimetyl-1-pyrroline-N-oxide; ESR, electron spin resonance; RO·, alkoxyl radical.
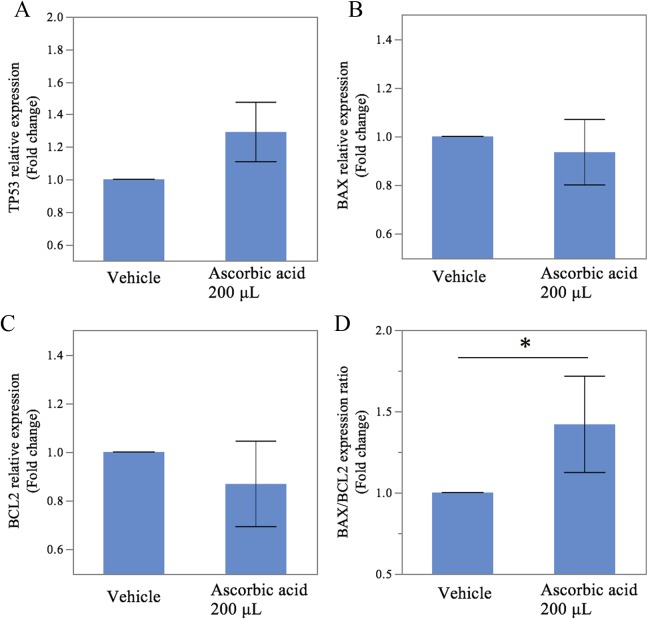
Figure 3 shows the changes in the messenger RNA (mRNA) expression levels of pro- and antiapoptotic genes under the conditions of hypoxia with and without vitamin C at a concentration of 200 μmol/L. The BAX/BCL2 gene expression ratio, an index of apoptosis, was 1.43 ± 0.20-fold higher in the culture with vitamin C (P = .015, Figure 3D). However, there were no significant differences in the expression levels of TP53, BAX, or BCL2 mRNA between the samples cultured with and without vitamin C (1.29 ± 0.12-fold higher; P = .106, Figure 3A, 1.11 ± 0.09-fold higher; P = .247, Figure 3B; and 0.87 ± 0.12-fold lower; P = .699, Figure 3C).
Figure 3.
Expression of apoptosis-related genes induced by the generation of oxidative stress under conditions of hypoxia with or without vitamin C. Using real-time quantitative PCR, a significantly increased ratio of BAX/BCL2 mRNA was observed (D). ACTB mRNA was used to normalize the gene expression. The results are expressed as the mean ± SEM. * indicates P < .05, between the samples cultured with and without vitamin C; PCR, polymerase chain reaction; mRNA, messenger RNA; SEM, standard error of the mean.
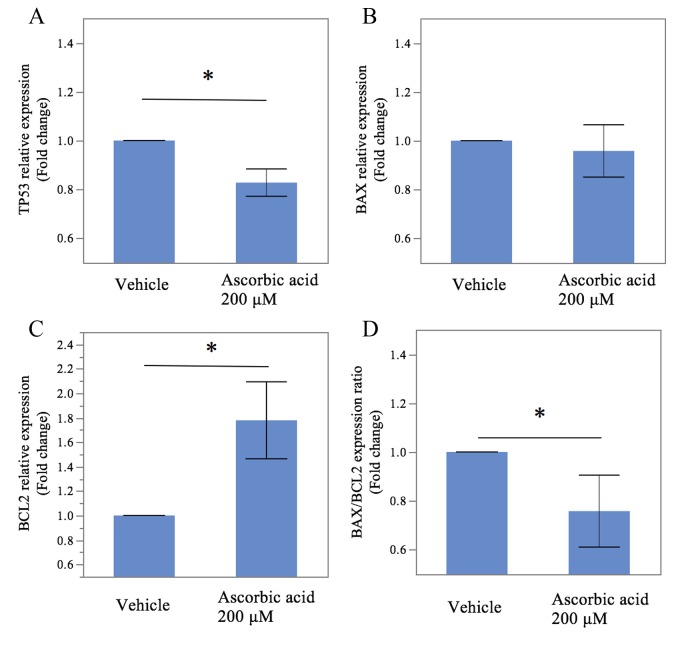
Figure 4 shows the results for the 8% O2 culture. The mRNA level of TP53 in the culture with vitamin C was 0.82 ± 0.03-fold lower (P = .015, Figure 4A) than that obtained without vitamin C. In addition, the BAX/BCL2 gene expression ratio was decreased by the addition of vitamin C (fold change: 0.75 ± 0.10, P = .006, Figure 4D) as was the gene expression level of BCL2, an antiapoptotic regulator (fold change: 1.77 ± 0.22, P = .006, Figure 4C). However, we found no significant differences in the gene expression levels of BAX between the samples treated with and without vitamin C (0.95 ± 0.07; P = .247, Figure 4B).
Figure 4.
Expression of apoptosis-related genes induced by the generation of oxidative stress under conditions of increasing oxygen concentrations with or without vitamin C. Using RT-qPCR, a significantly decreased expression of TP53 mRNA was observed (A) in the samples treated with vitamin C, compared to an increased expression of BCL2 mRNA (C). Vitamin C decreased the ratio of BAX/BCL2 mRNA (D). ACTB mRNA was used to normalize the gene expression. The results are expressed as the mean ± SEM. * indicates P < .05, between the samples cultured with and without vitamin C; RT-qPCR, real-time quantitative polymerase chain reaction; mRNA, messenger RNA; SEM, standard error of the mean.
Discussion
This study demonstrated that vitamin C removes ROS induced by oxidative stress under increasing oxygen concentrations. Specifically, these findings demonstrated that vitamin C increased the gene expression ratio of BAX/BCL2, an index of apoptosis, under conditions of hypoxia. In addition, under increasing oxygen levels, vitamin C increased the gene expression of BCL2, an antiapoptotic regulator, and decreased the gene expression of TP53, a proapoptotic factor, and the ratio of BAX/BCL2 mRNA, an apoptotic index. These findings suggest that vitamin C exhibits a suppressive effect against the apoptosis of EVTs induced by an increasing oxygen concentration, whereas an opposite effect is observed under conditions of 2% O2.
Prior to around 10 weeks of gestation, the oxygen concentration in the intervillous space is approximately 1% to 2%. Following the increase in the maternal blood flow to the placenta at 10 to 12 weeks of gestation, the oxygen concentration in the intervillous space sharply rises to approximately 6% to 8%.26,27 The placenta subsequently adapts to the increment in the oxygen concentration, which supports a normal placental function, by increasing cellular antioxidant defenses.28,29 The EVTs replace the maternal endothelium as far as the inner third of the myometrium in this sequence. The placental bed of patients with preeclampsia is characterized by a decreased number of spiral arteries with transformation of the myometrial segment.30,31 Premature perfusion of the intervillous space at 10 weeks of gestation increases the risk of pregnancy loss.27 Such premature perfusion is associated with an increased level of oxidative stress, enhanced apoptosis, and decreased cell proliferation.32 Gene expression studies of chorionic villous sampling in females who subsequently develop preeclampsia have demonstrated a decreasing expression of SOD.33 These findings indicate that an increased ROS level and consequent uncontrollable state of ROS production result in the onset of preeclampsia and initiation of pregnancy loss.
An ESR spin trapping analysis was employed to directly measure the extent of ROS production in EVTs under increasing oxygen concentrations. The results indicated that ROS production was suppressed by the presence of EVTs with vitamin C, further confirming that vitamin C acts as an effective ROS scavenger, thus mediating the formation of bifunctional electrophiles due to the production of the alkoxy radical.34 The intracellular ROS reduction induced by vitamin C modulates the expression of genes involved in signal transduction pathways leading to cell cycle progression, cell differentiation, and apoptosis.35 The rate of production of ROS and outflow of electrons from electron transport chains are limited by hypoxia and increase sharply if the oxygen level rises.35 Excessive ROS production is an apoptotic signal that leads to cell death via the actions of mitochondria. The mitochondrial pathway is regulated by members of the Bcl-2 family of proteins under the control of p53 proteins. TP53, which produces p53 proteins, is a negative regulator of BCL2 and antiapoptotic gene and acts as a transcriptional activator of BAX, a proapoptotic gene.36 Increased apoptosis among trophoblasts may result in an impaired placental function, and p53 may play a pivotal and complex role in regulating trophoblast cell turnover.37 The present findings are concordant with previous reports in which intracellular vitamin C has been reported to prevent human umbilical vein endothelial cells from progressing to hypoxia–reoxygenation-induced apoptosis.38 Various researchers have also reported that vitamin C decreases the rate of apoptosis induced by hypoxia–reoxygenation in term placental explants.39,40 In addition to a previous study, the present analysis is the first to reveal the antiapoptotic effects of vitamin C alone in EVTs derived from first trimester tissue under conditions of increasing oxygen concentrations.
Paradoxically, vitamin C increased the BAX/BCL gene expression ratio under conditions of hypoxia in the present study. Cells treated with vitamin C exhibit an increased expression of proapoptotic genes induced by UV irradiation and DNA damage35 as well as an increased BAX/BCL2 ratio in the cytosol, which leads to apoptosis.41 Moreover, Hung et al observed enhanced apoptosis following the concomitant supplementation of vitamin C and E in term placental explants under hypoxia-standard culture conditions.42 Some researchers reported that the potential mechanism underlying vitamin C-associated apoptosis is through the regulation of p38 MAPK.43–45 The sustained activation of the p38 MAPK pathway leads to the transcription of the apoptosis-related genes as well as the p53-regulated BAX gene.46
These findings provide information regarding the effects of vitamin C in enhancing susceptibility to apoptosis and protecting mechanisms of apoptosis against increased oxidative stress as an ROS scavenger. It may be suggested that vitamin C shows the apoptotic process in hypoxic EVT of the 8 to 10 weeks of gestation and antiapoptotic process to prevent excessive ROS generation, which leads to p53-induced apoptosis, in EVT of the 10 to 12 weeks of gestation under increasing oxygen concentration. Our study is associated with some limitation. First, the sample size was small. Second, the present study documented only the gene expression. Third, we did not conduct an ESR analysis in the hypoxia medium, as the aim of our study was to assess the effects of antioxidants under increasing oxygen concentrations. Further studies are thus required to clarify the effects of high or low concentrations of vitamins C on the function and apoptosis of trophoblasts.
In conclusion, the findings of this study revealed the effects of vitamin C in neutralizing ROS and promoting the expression of genes related to apoptosis in EVTs obtained from first-trimester tissue under conditions of hypoxia. In addition, our findings discovered that vitamin C paradoxically inhibited the expression of gene-related apoptosis in EVTs under increasing oxygen concentrations. Our results suggest that vitamin C plays a role as an antioxidant and may thereby promote the apoptosis of EVTs under conditions of hypoxia, with a suppressive effect against the apoptosis of EVTs induced by an increasing oxygen concentration. These findings may support the protective use of vitamin C against mitochondria-dependent apoptosis arising from bursts of oxidative stress under conditions of steeply increasing oxygen concentrations. These data indicate that more attention should be focused on the antioxidant status of pregnant females in the early first trimester, namely, from 7 to 8 weeks of gestation.
Acknowledgements
We thank Dr. Katsuhiko Naruse for his valuable advices in the separation and culture system of extravillous trophoblasts.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sport and Culture of Japan (grant number 26462501); Smoking Research Foundation and Ogyaa Foundation.
References
- 1. Lash GE, Otun HA, Innes BA, et al. Regulation of extravillous trophoblast invasion by uterine natural killer cells is dependent on gestational age. Hum Reprod. 2010;25 (5):1137–1145. [DOI] [PubMed] [Google Scholar]
- 2. Hubel CA. Oxidative stress in the pathogenesis of preeclampsia. Proc Soc Exp Biol Med. Society for experimental biology and medicine 1999;222 (3):222–235. [DOI] [PubMed] [Google Scholar]
- 3. Roberts JM, Hubel CA. Is oxidative stress the link in the two-stage model of pre-eclampsia? Lancet. 1999;354 (9181):788–789. [DOI] [PubMed] [Google Scholar]
- 4. Pavan L, Tsatsaris V, Hermouet A, Therond P, Evain-Brion D, Fournier T. Oxidized low-density lipoproteins inhibit trophoblastic cell invasion. J Clin Endocrinol Metabol. 2004;89 (4):1969–1972. [DOI] [PubMed] [Google Scholar]
- 5. Leung DN, Smith SC, To KF, Sahota DS, Baker PN. Increased placental apoptosis in pregnancies complicated by preeclampsia. Am J Obstet Gynecol. 2001;184 (6):1249–1250. [DOI] [PubMed] [Google Scholar]
- 6. Sharp AN, Heazell AE, Baczyk D, et al. Preeclampsia is associated with alterations in the p53-pathway in villous trophoblast. PloS One. 2014;9 (1):e87621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Levy R, Smith SD, Yusuf K, et al. Trophoblast apoptosis from pregnancies complicated by fetal growth restriction is associated with enhanced p53 expression. Am J Obstet Gynecol. 2002;186 (5):1056–1061. [DOI] [PubMed] [Google Scholar]
- 8. Gupta S, Aziz N, Sekhon L, et al. Lipid peroxidation and antioxidant status in preeclampsia: a systematic review. Obstet Gynecol Surv. 2009;64 (11):750–759. [DOI] [PubMed] [Google Scholar]
- 9. Chan AC. Partners in defense, vitamin E and vitamin C. Can J Physiol Pharmacol. 1993;71 (9):725–731. [DOI] [PubMed] [Google Scholar]
- 10. Nagaoka S, Kakiuchi T, Ohara K, Mukai K. Kinetics of the reaction by which natural vitamin E is regenerated by vitamin C. Chem Phys Lipids. 2007;146 (1):26–32. [DOI] [PubMed] [Google Scholar]
- 11. Roberts JM, Myatt L, Spong CY, et al. Vitamins C and E to prevent complications of pregnancy-associated hypertension. N Engl J Med. 2010;362 (14):1282–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xu H, Perez-Cuevas R, Xiong X, et al. An international trial of antioxidants in the prevention of preeclampsia (INTAPP). Am J Obstet Gynecol. 2010;202(3):239. e231–239 e210. [DOI] [PubMed] [Google Scholar]
- 13. Basaran A, Basaran M, Topatan B. Combined vitamin C and E supplementation for the prevention of preeclampsia: a systematic review and meta-analysis. Obstet Gynecol Surv. 2010;65 (10):653–667. [DOI] [PubMed] [Google Scholar]
- 14. Villar J, Purwar M, Merialdi M, et al. World Health Organisation multicentre randomised trial of supplementation with vitamins C and E among pregnant women at high risk for pre-eclampsia in populations of low nutritional status from developing countries. BJOG. 2009;116 (6):780–788. [DOI] [PubMed] [Google Scholar]
- 15. Spinnato JA, II, Freire S, Pinto ESJL, et al. Antioxidant therapy to prevent preeclampsia: a randomized controlled trial. Obstet Gynecol. 2007;110 (6):1311–1318. [DOI] [PubMed] [Google Scholar]
- 16. Klemmensen A, Tabor A, Osterdal ML, et al. Intake of vitamin C and E in pregnancy and risk of pre-eclampsia: prospective study among 57 346 women. BJOG. 2009;116 (7):964–974. [DOI] [PubMed] [Google Scholar]
- 17. Zhang C, Williams MA, King IB, et al. Vitamin C and the risk of preeclampsia--results from dietary questionnaire and plasma assay. Epidemiology. 2002;13 (4):409–416. [DOI] [PubMed] [Google Scholar]
- 18. Hustin J, Schaaps JP. Echographic [corrected] and anatomic studies of the maternotrophoblastic border during the first trimester of pregnancy. Am J Obstet Gynecol. 1987;157 (1):162–168. [DOI] [PubMed] [Google Scholar]
- 19. Jauniaux E, Watson A, Burton G. Evaluation of respiratory gases and acid-base gradients in human fetal fluids and uteroplacental tissue between 7 and 16 weeks' gestation. Am J Obstet Gynecol. 2001;184 (5):998–1003. [DOI] [PubMed] [Google Scholar]
- 20. Lash GE, Naruse K, Innes BA, Robson SC, Searle RF, Bulmer JN. Secretion of angiogenic growth factors by villous cytotrophoblast and extravillous trophoblast in early human pregnancy. Placenta. 2010;31 (6):545–548. [DOI] [PubMed] [Google Scholar]
- 21. Naruse K, Innes BA, Bulmer JN, Robson SC, Searle RF, Lash GE. Secretion of cytokines by villous cytotrophoblast and extravillous trophoblast in the first trimester of human pregnancy. J Reprod Immunol. 2010;86 (2):148–150. [DOI] [PubMed] [Google Scholar]
- 22. Onogi A, Naruse K, Sado T, et al. Hypoxia inhibits invasion of extravillous trophoblast cells through reduction of matrix metalloproteinase (MMP)-2 activation in the early first trimester of human pregnancy. Placenta. 2011;32 (9):665–670. [DOI] [PubMed] [Google Scholar]
- 23. Padayatty SJ, Sun H, Wang Y, et al. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med. 2004;140 (7):533–537. [DOI] [PubMed] [Google Scholar]
- 24. Dohi K, Satoh K, Mihara Y, et al. Alkoxyl radical-scavenging activity of edaravone in patients with traumatic brain injury. J Neurotrauma. 2006;23 (11):1591–1599. [DOI] [PubMed] [Google Scholar]
- 25. Dohi K, Satoh K, Nakamachi T, et al. Novel free radical monitoring in patients with neurological emergency diseases. Acta neurochirur Suppl. 2010;106:315–319. [DOI] [PubMed] [Google Scholar]
- 26. Rodesch F, Simon P, Donner C, Jauniaux E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet Gynecol. 1992;80 (2):283–285. [PubMed] [Google Scholar]
- 27. Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN, Burton GJ. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am J Pathol. 2000;157 (6):2111–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carter AM. Placental oxygen consumption. Part I: in vivo studies--a review. Placenta. 2000;21 (suppl A):S31–S37. [DOI] [PubMed] [Google Scholar]
- 29. Jauniaux E, Cindrova-Davies T, Johns J, et al. Distribution and transfer pathways of antioxidant molecules inside the first trimester human gestational sac. J Clin Endocrinol Metab. 2004;89 (3):1452–1458. [DOI] [PubMed] [Google Scholar]
- 30. Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204 (3):193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gerretsen G, Huisjes HJ, Elema JD. Morphological changes of the spiral arteries in the placental bed in relation to pre-eclampsia and fetal growth retardation. Br J Obstet Gynaecol. 1981;88 (9):876–881. [DOI] [PubMed] [Google Scholar]
- 32. Hempstock J, Jauniaux E, Greenwold N, Burton GJ. The contribution of placental oxidative stress to early pregnancy failure. Hum Pathol. 2003;34 (12):1265–1275. [DOI] [PubMed] [Google Scholar]
- 33. Farina A, Sekizawa A, De Sanctis P, et al. Gene expression in chorionic villous samples at 11 weeks' gestation from women destined to develop preeclampsia. Prenat Diagn. 2008;28 (10):956–961. [DOI] [PubMed] [Google Scholar]
- 34. Lee SH, Oe T, Blair IA. Vitamin C-induced decomposition of lipid hydroperoxides to endogenous genotoxins. Science. 2001;292 (5524):2083–2086. [DOI] [PubMed] [Google Scholar]
- 35. Catani MV, Rossi A, Costanzo A, et al. Induction of gene expression via activator protein-1 in the ascorbate protection against UV-induced damage. The Biochemical journal, May 15 2001;356 (Pt 1):77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bursch W, Karwan A, Mayer M, et al. Cell death and autophagy: cytokines, drugs, and nutritional factors. Toxicology. 2008;254 (3):147–157. [DOI] [PubMed] [Google Scholar]
- 37. Hung TH, Chen SF, Lo LM, Li MJ, Yeh YL, Hsieh TT. Increased autophagy in placentas of intrauterine growth-restricted pregnancies. PloS one. 2012;7 (7):e40957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dhar-Mascareno M, Carcamo JM, Golde DW. Hypoxia-reoxygenation-induced mitochondrial damage and apoptosis in human endothelial cells are inhibited by vitamin C. Free Rad Biol Med. 2005;38 (10):1311–1322. [DOI] [PubMed] [Google Scholar]
- 39. Tjoa ML, Cindrova-Davies T, Spasic-Boskovic O, Bianchi DW, Burton GJ. Trophoblastic oxidative stress and the release of cell-free feto-placental DNA. Am J Pathol. 2006;169 (2):400–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cindrova-Davies T, Spasic-Boskovic O, Jauniaux E, Charnock-Jones DS, Burton GJ. Nuclear factor-kappa B, p38, and stress-activated protein kinase mitogen-activated protein kinase signaling pathways regulate proinflammatory cytokines and apoptosis in human placental explants in response to oxidative stress: effects of antioxidant vitamins. Am J Pathol. 2007;170 (5):1511–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Park S, Han SS, Park CH, et al. L-Ascorbic acid induces apoptosis in acute myeloid leukemia cells via hydrogen peroxide-mediated mechanisms. Int J Biochem Cell Biol. 2004;36 (11):2180–2195. [DOI] [PubMed] [Google Scholar]
- 42. Hung TH, Chen SF, Li MJ, Yeh YL, Hsieh TT. Differential effects of concomitant use of vitamins C and E on trophoblast apoptosis and autophagy between normoxia and hypoxia-reoxygenation. PloS One. 2010;5 (8):e12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lee SK, Kang JS, Jung da J, et al. Vitamin C suppresses proliferation of the human melanoma cell SK-MEL-2 through the inhibition of cyclooxygenase-2 (COX-2) expression and the modulation of insulin-like growth factor II (IGF-II) production. J Cell Physiol. 2008;216 (1):180–188. [DOI] [PubMed] [Google Scholar]
- 44. Lee SA, Son YO, Kook SH, Choi KC, Lee JC. Ascorbic acid increases the activity and synthesis of tyrosinase in B16F10 cells through activation of p38 mitogen-activated protein kinase. Arch Dermatol Res. 2011;303 (9):669–678. [DOI] [PubMed] [Google Scholar]
- 45. Wang Y, Tang C, Wu M, et al. Dehydroascorbic acid taken up by glucose transporters stimulates estradiol production through inhibition of JNK/c-Jun/AP1 signaling in JAR cells. Mol Hum Reprod. 2014;20 (8):799–809. [DOI] [PubMed] [Google Scholar]
- 46. Lin FL, Hsu JL, Chou CH, Wu WJ, Chang CI, Liu HJ. Activation of p38 MAPK by damnacanthal mediates apoptosis in SKHep 1 cells through the DR5/TRAIL and TNFR1/TNF-alpha and p53 pathways. Eur J Pharmacol. 2011;650 (1):120–129. [DOI] [PubMed] [Google Scholar]