Abstract
Background:
Preterm prelabor rupture of the fetal membranes (PPROM) is a significant contributor to the morbidity and mortality of preterm birth, particularly in the setting of chorioamnionitis. No sensitive or specific diagnostic or predictive test currently exists for the accurate diagnosis of chorioamnionitis. Our aim was to measure messenger RNA (mRNA) coding cytokines in the maternal blood and examine whether they were increased in association with chorioamnionitis at delivery.
Methods/Results:
We performed a prospective cohort study of women recruited with PPROM at a mean gestational age of 28.9 weeks at risk of developing chorioamnionitis. Blood was sampled from participants, and the expression of mRNA coding for proinflammatory genes was measured in women with and without chorioamnionitis at the time of delivery as well as gestation-matched healthy controls. Expression was measured using quantitative polymerase chain reaction (PCR) and also digital PCR. Interleukin 1β (IL1B) mRNA expression in maternal blood was elevated in women with chorioamnionitis compared to gestation-matched controls. Importantly, among women admitted with PPROM, digital PCR confirmed a significant increase in IL1B expression in maternal blood in women with chorioamnionitis compared to women without chorioamnionitis. Polymerase chain reaction array revealed that CD14, nuclear factor of κ light polypeptide gene enhancer in B-cells 1 (NFKB1), and tumor necrosis factor receptor super family-interacting serine–threonine kinase 1 mRNA were significantly increased in women with chorioamnionitis compared to controls. Digital PCR confirmed that NFKB1 mRNA was significantly increased in patients with chorioamnionitis compared to controls and that CD14 levels increased over time in patients with PPROM having chorioamnionitis.
Conclusion:
Measuring circulating proinflammatory mRNA in women with PPROM may distinguish those with chorioamnionitis from those without, in turn providing better targeted therapies and appropriate timing of delivery.
Keywords: preterm birth, preterm prelabor rupture of the fetal membranes, chorioamnionitis, PCR, digital PCR, mRNA, IL1B, inflammation, intrauterine infection
Introduction
Preterm prelabor rupture of the fetal membranes (PPROM) is a pregnancy complication that is a major contributor burden of prematurity, accounting for 25% of preterm births.1
If the pregnancy is significantly preterm (ie, less than 34 weeks of gestation), clinicians will attempt to delay delivery as long as possible, so the fetus can continue to gain gestation in utero with the aim of minimizing the risks of respiratory distress syndrome, intraventricular hemorrhage, necrotizing enterocolitis in the neonatal period, and cerebral palsy to the fetus in the long term.
However, the approach of delaying delivery is not without risk. Rupture of the fetal membranes means that an important anatomical and physiological barrier to ascending infection is gone, placing the fetus at greatly increased risk of intrauterine infection.2 Although intrauterine infection and chorioamnionitis can occur preceding PPROM or occur with intact membranes, PPROM remains an important risk factor for infection. Intrauterine infection or chorioamnionitis is an independent additional risk factor for major developmental morbidity and cerebral palsy.3–5
Deciding whether chorioamnionitis is either present or imminent is largely based on clinical assessment of symptoms and signs such as maternal fever, tachycardia, uterine tenderness, a change in the color and odor of the amniotic fluid, and fetal tachycardia. Unfortunately by the time these clinical signs are present, often infection is already established and the resulting inflammation in the premature fetus may already have adversely impacted on the fetal brain, lung, and other organs.6
A biomarker that can detect chorioamnionitis early in its evolution or predict the development of intrauterine infection before it occurs could substantially improve outcomes. It would allow clinicians to better time delivery, safely leaving the fetus in utero to gain gestation and also inform the timing of delivery before there is severe fetal infection. Although there have been avid attempts to identify one, no such biomarker exists.
For almost a decade, it has been recognized that messenger RNA (mRNA) circulates in the maternal circulation where it can be sampled and readily quantified.7 The likely sources of these mRNA transcripts are maternal leukocytes, fetus, and placenta. The placenta and the maternal leukocytes possess the molecular machinery of the innate immune system, one component of which is the presence of toll-like receptors. These receptors can sense bacterial antigens and respond by secreting cytokines that then mobilize the immune response.8 Therefore, we hypothesized that for women with PPROM and chorioamnionitis (i) there would be a change in expression of the genes of inflammatory cytokines and other infection-associated genes (in either, or both of, placenta and maternal leukocytes), (ii) these changes would also result in the upregulation and release of mRNA coding cytokines in the maternal circulation, and (iii) measuring mRNA transcripts coding cytokines could be used as a clinical biomarker to identify chorioamnionitis.
We therefore performed a prospective study from a cohort of women with preterm rupture of membranes without labor and obtained serial blood samples.
Methods
Ethics Statement
Ethics approval was obtained from The Mercy Hospital for Women Human Research Ethics Committee (R11/34: Establishment of a Tissue Bank at the Department of Obstetrics and Gynaecology, Mercy Hospital for Women). Written informed consent was obtained from each participant prior to sample collection.
Study Participants and Specimens
Participants were recruited from the Mercy Hospital for Women (Heidelberg, Melbourne). To examine the role of inflammatory cytokines in women with PPROM and chorioamnionitis, women admitted with PPROM, less than 34 weeks of gestation, were recruited and maternal blood samples collected serially (at the time of other routine blood tests, ie, full blood examination and C-reactive protein) from the time of recruitment until delivery.
Inpatient management was determined by the treating clinical team and the decision when to deliver was made on usual obstetric indications, mostly clinical suspicion of chorioamnionitis.
Placental samples were obtained immediately after delivery; placental swabs for microscopy and culture were obtained from the maternal and fetal surfaces and the placenta was sent for histological examination. Microbiological analysis was performed by Gram stain, and bacterial culture was performed on MacConkey, Horse-blood, and Ureaplasma agar.
Chorioamnionitis was defined by the presence of histological chorioamnionitis and 2 or more clinical signs of infection (ie, maternal pyrexia, tachycardia, uterine tenderness, offensive liquor, and fetal tachycardia). No chorioamnionitis was defined as: no histological chorioamnionitis and one or fewer clinical signs of chorioamnionitis. Blood was obtained from the gestation-matched control group who went on to have normal pregnancies without complication.
Sample Collection
Maternal peripheral blood of 2.5 mL was collected in PAXgene whole blood RNA tubes (Preanalytix/BD, Hombrechtikon, Switzerland). The tubes were stored according to the manufacturer’s instructions—at room temperature for 24 hours and thereafter at −80°C until RNA extraction.
Placental samples were obtained following delivery from the maternal surface of the placenta excluding the decidua and fetal membranes. Placental samples were washed in sterile phosphate-buffered saline, snap frozen, or collected into RNAlater (Ambion, Austin, Texas) and frozen at −80°C until processing.
RNA Preparation
Total RNA was extracted from maternal blood samples using the PAXgene RNA (Preanalytix/BD) system according to the manufacturer’s instructions. RNA was extracted from placental samples using the RNeasy (QIAGEN, Venlo, the Netherlands) kit according to the manufacturer’s instructions. Genomic DNA was removed using DNAse treatment. Concentration and purity of RNA were determined with NanoDrop ND100 spectrophotometer (Thermofisher, Waltham, Massachusetts).
Reverse Transcription, Quantitative Polymerase Chain Reaction Analysis, and Digital PCR
Reverse transcription of 100 ng of total RNA was performed using Superscript VILO (Invitrogen, Carlsbad, California). For the first experiment examining inflammatory mRNA expression of 6 genes, quantitative polymerase chain reaction (qPCR) was performed using Taqman (Life Technologies, Carlsbad, California) primers and probes. The PCR array was performed using Roche Realtime Ready Custom PCR Array plates (Roche, Penzberg, Germany); G6PD, HPRT1, and YWHAZ (included on the array) were used as internal housekeeper genes. The comparative cycle threshold (Ct) method was used to determine relative expression. Digital PCR was performed using the Quantstudio 3D digital PCR platform (Life Technologies) using Taqman primers and probes.
Statistical Methods
We used descriptive statistics to describe our clinical data. Analysis of variance and chi-square test was used to compare clinical data between the 3 cohorts (PPROM with Chorio, PPROM without Chorio, and controls). The Mann-Whitney U test was used to systematically compare gene expression. We used the following a priori approach to compare gene expression between the groups: (1) the sample taken at consent was compared with samples taken on the day of delivery within the clinical subgroups (ie, Chorio or No Chorio subgroups), (2) consent versus consent and delivery versus delivery samples between these 2 subgroups, and (3) all samples from the subgroups versus controls (ie, a cohort of 12 women without PPROM who progressed to an uncomplicated term birth). GraphPad Prism Version 6 (GraphPad, San Diego, California) was used for statistical analysis.
Results
Expression of Cytokine mRNA in Maternal Blood in Patients With and Without Chorioamnionitis
We first examined whether mRNA expression of the proinflammatory cytokines, interleukin (IL) 1β (IL1B), IL6, CXCL8 (previously IL8), IL10, tumor necrosis factor (TNF), and colony stimulating factor (CSF) 3 (previously granulocyte CSF) when detected in maternal blood was differentially expressed in women with and without chorioamnionitis. These cytokines were selected on the basis that they have previously been reported to be differentially expressed with PPROM, chorioamnionitis, and preterm birth.9
Our cohort of women with PPROM was classified into 2 groups: presence of chorioamnionitis at delivery (“Chorio,” n = 11) or no chorioamnionitis (“No Chorio,” n = 4). For each group we examined the following: (1) blood taken on the day of admission when consent was obtained (named “CONSENT” samples) and (2) blood taken on the day of delivery (“DELIVERY” samples). Importantly, we also included a control (n = 12) cohort of women with normal pregnancies without PPROM that progressed to term, where blood was taken at a similar gestation to those women with PPROM (“CONTROL” samples). Hence, we examined 5 groups (see Table 1).
Table 1.
Patient Characteristics for PPROM With and Without Chorioamnionitis and Control Cohorts.
| Gestational Controls (n = 12) | PPROM/No chorioamnionitis (n = 8) | PPROM/chorioamnionitis (n = 19) | P Value | |
|---|---|---|---|---|
| Maternal age, yearsa | 32 (7.3) | 29 (1.5) | 32 (5.2) | .59 |
| Nulliparity, % | 42 | 25 | 58 | .27 |
| Gestational age at birth, weeksa | 40.1 (1.4) | 32.0 (2.8) | 28.9 (3.7) | <.0001 |
| Gestational age at sampling, weeksa | 29.6 (1.2) | 32.0 (2.8) | 28.9 (3.7) | .07 |
| Birthweight, ga | 3403 (244) | 1724 (550) | 909 (487) | <.0001 |
| Latency, days | N/A | 6.4 (5-11) | 8.3 (4-28) | .32 |
| Smoking, % | 0 | 13 | 16 | .36 |
| Illicit drug use, % | 0 | 0 | 5 | .58 |
| Gestational diabetes, % | 8 | 0 | 16 | .42 |
| Antenatal corticosteroids, % | 0 | 100 | 100 | <.0001 |
| Antenatal antibiotics, % | 33 | 88 | 100 | .0001 |
| Magnesium sulfate, % | 0 | 50 | 56 | .006 |
| Placental/vaginal microbes (cases) | Group B Streptococcus (4) | Mixed flora (4) and no growth (4) | Group B Streptococcus (1), Escherichia coli (3), Acinetobacter lwoffii (1), Peptoniphilus asaccharolyticus (1), mixed anaerobic flora (3), Ureaplasma urealyticum (3), no growth (6) |
Abbreviations: SD, standard deviation; PPROM, preterm prelabor rupture of the fetal membranes; N/A, not applicable.
aMean (SD).
For the purpose of our study, chorioamnionitis was defined as placental histological chorioamnionitis and the presence of 2 or more clinical signs of infection in the mother. This definition recognizes that chorioamnionitis is also a clinical diagnosis and that the histological findings alone are not sufficient in determining the disease.
Of these 6 genes examined, IL1B was significantly upregulated in the blood of women with chorioamnionitis on the day of delivery (Figure 1A) compared to healthy gestational age-matched controls who progressed to term. The expression of IL1B mRNA measured at delivery in the chorioamnionitis group was also nonsignificantly increased compared to (1) the same cohort where blood was taken on the day of consent and entry to the study and (2) a cohort with PPROM who never subsequently developed chorioamnionitis.
Figure 1.
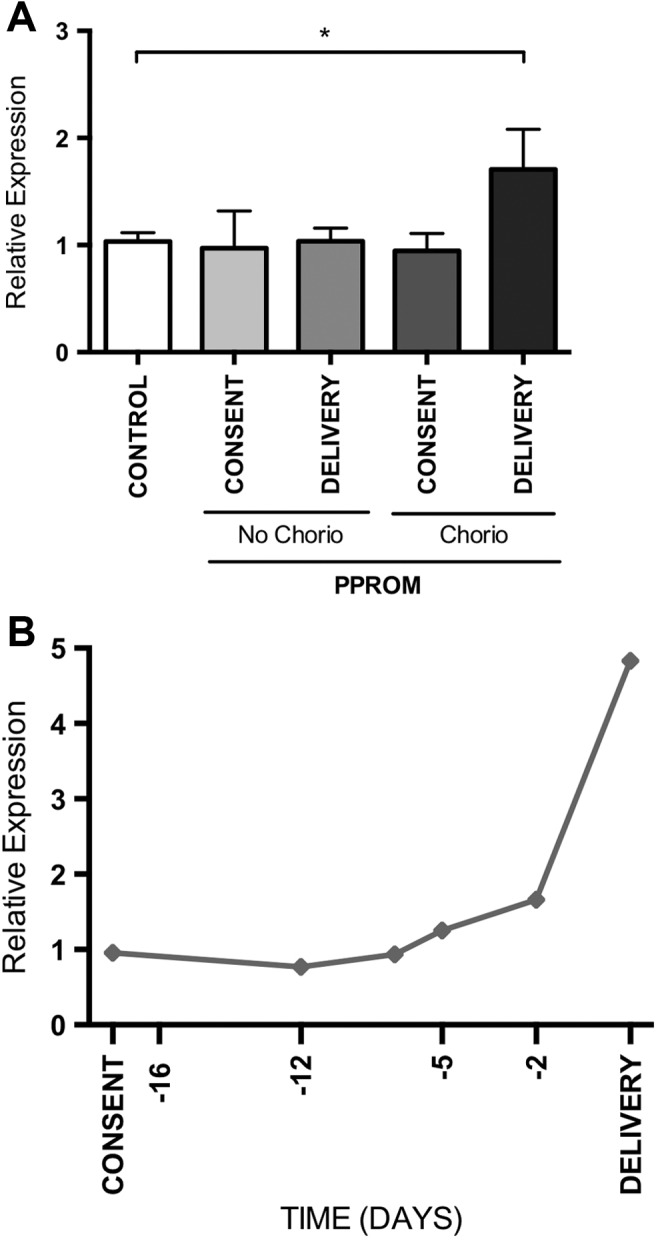
Expression of IL1B is elevated in women with chorioamnionitis at the time of delivery. A, Expression of IL1B in maternal blood measured in a control group (n = 12), women without chorioamnionitis at consent (n = 2) and delivery (n = 4), and in women with chorioamnionitis at consent (n = 5) and delivery (n = 11). Mean and SEM are graphed, *P < .05. B, Expression measured over time from consent until delivery of IL1B in a patient with chorioamnionitis. This patient developed Escherichia coli septicemia as did her baby. IL1B indicates interleukin 1β; SEM, standard error of the mean.
We measured levels of mRNA IL1B in blood taken longitudinally for 1 case where both the mother and the baby developed florid Escherichia coli septicemia. Both were unwell after birth, where the mother required admission to the high-dependency unit and the baby was admitted to the neonatal intensive care unit. In these longitudinal maternal blood samples collected antenatally before birth, IL1B mRNA expression rose markedly. Furthermore, it appears that the rise preceded the development of overt clinical evidence of infection which triggered delivery (Figure 1B).
Expression of Inflammation-Associated mRNA in Maternal Blood and Placenta in Patients With and Without Chorioamnionitis
Having identified upregulation of only 1 proinflammatory cytokine in maternal blood with chorioamnionitis of our panel of 6 genes, we widened our search by performing a PCR array. We chose genes that code inflammatory cytokines and genes involved in the immune response to infection.
We performed the PCR array on RNA extracted from blood samples of the following women selected from the PPROM cohort: with chorioamnionitis (Chorio, n = 7) and without chorioamnionitis (No Chorio, n = 4). In relation to the blood samples, we examined samples taken on the day of presentation when consent to participate was obtained (named CONSENT samples) and on the day of delivery where often a suspicion of clinical chorioamnionitis triggers the decision to deliver (DELIVERY samples; see Figure 2). These were compared to a gestationally matched control group (CONTROL, n = 8).
Figure 2.
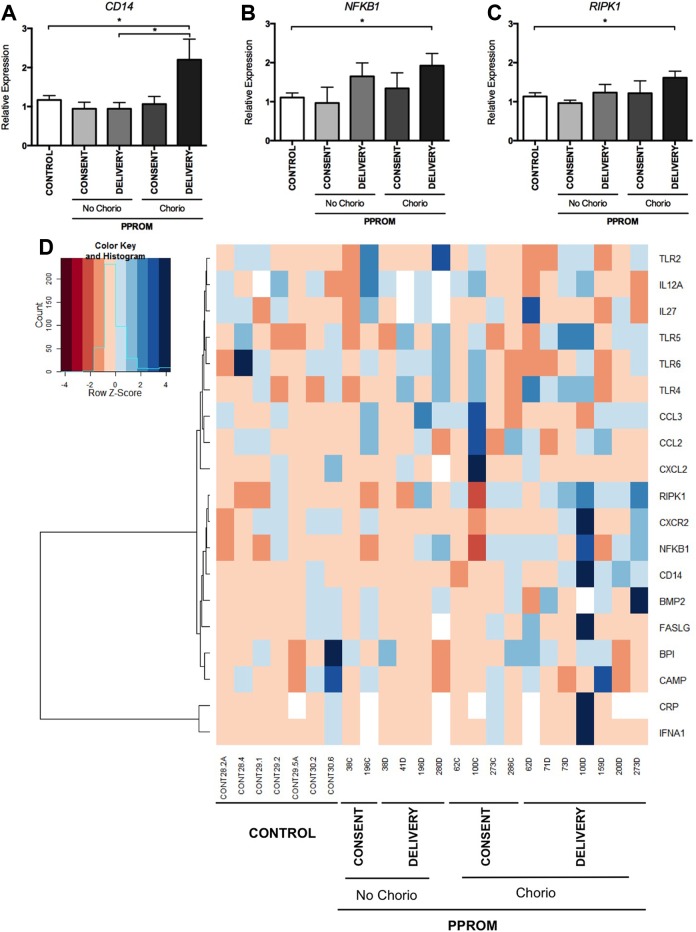
Expression of proinflammatory genes associated with chorioamnionitis measured by PCR array. The expression of (A) CD14, (B) NFKB1, and (C) RIPK1 mRNA is increased at delivery in women with chorioamnionitis (n = 7) compared to gestational controls (n = 8). Mean and SEM are graphed, *P < .05. D, Cluster analysis and heatmap of 19 detectable proinflammatory genes in the maternal blood of women with the diagnosis of PPROM at delivery with and without chorioamnionitis and a control group. PCR indicates polymerase chain reaction; NFKB1, nuclear factor-κB1; RIPK1, TNF receptor super family-interacting serine–threonine kinase 1; mRNA, messenger RNA; SEM, standard error of the mean; PPROM, preterm prelabor rupture of the fetal membranes.
Of the 24 genes examined on the custom PCR array, 19 were detectable in peripheral maternal blood samples and 3 were significantly upregulated (P < .05) in the blood of women with chorioamnionitis when compared to controls. These were CD14, nuclear factor-κB1 (NFKB1), and TNF receptor super family-interacting serine–threonine kinase 1 (RIPK1; Figure 2A-C). Only CD14 was found to be significantly upregulated in women with chorioamnionitis compared to women without chorioamnionitis. A heatmap of the expression data suggests a global upregulation of the genes examined, but there was no suggestion of a pattern of differential expression of the genes examined between the clinical cohorts (Figure 2D). No difference in expression of inflammatory genes across the 3 cohorts was found in placental tissue samples.
Copy Number of Inflammation-Associated mRNA in Maternal Blood Measured by Digital PCR
Thus far, we have undertaken a hypothesis-based screen of 6 candidate inflammatory genes and identified IL1B as differentially regulated in the blood of women with PPROM and chorioamnionitis compared to controls. We also screened a further 24 genes on a PCR array and identified 3 additional candidate blood mRNA biomarkers, that is, CD14, NFKB1, and RIPK1.
We now sought to validate these findings that mRNA coding IL1B, CD14, NFKB1, and RIPK1 are elevated in the blood of women with chorioamnionitis, taken on the day of delivery. We performed this validation on a larger cohort, which also includes the samples analyzed in the previous experiments.
Notably, to undertake this validation, we used both conventional PCR and digital PCR. Digital PCR uses new technology platforms where mRNA quantification is provided as absolute copy number, rather than expression relative to housekeeping genes. It has advantages to conventional qPCR in with greater precision10 and it does not require the use of housekeeping genes. Thus, our rationale for undertaking this experiment is to (1) validate our findings from the PCR array and the IL1B results obtained from the initial cohort and (2) compare the performance of conventional qualitative PCR to digital PCR.
This larger validation cohort confirmed our previous findings that IL1B was significantly upregulated in women with chorioamnionitis at delivery (n = 19) when compared to gestationally matched controls (n = 12; Figure 3A) using conventional qPCR. Disappointingly, in this larger validation cohort, we did not find any of the 3 other genes, CD14, NFKB1, and RIPK1, to be differentially expressed across the groups (Figure 3C, E, and G).
Figure 3.
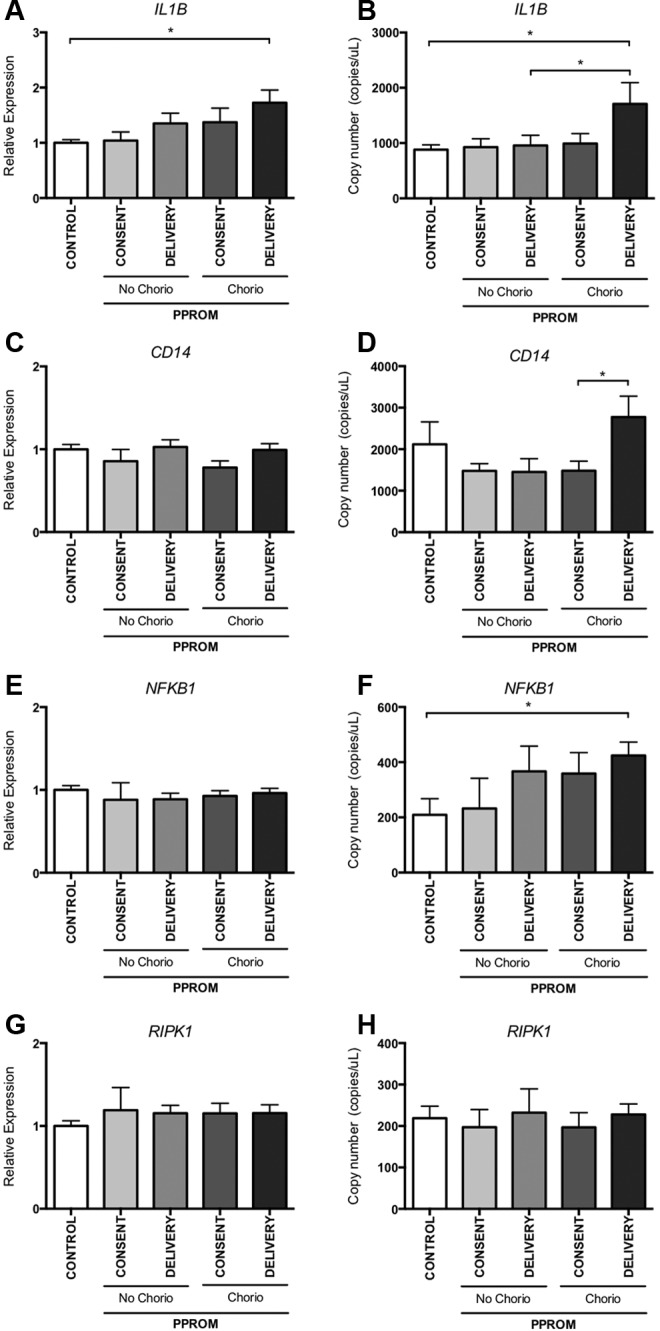
Expression of IL1B, CD14, NFKB1, and RIPK1 measured by quantitative and digital PCR. Expression of the identified proinflammatory genes was measured in a larger cohort; women with chorioamnionitis (at consent [n = 10] and at delivery [n = 19]), PPROM no chorioamnionitis (at consent [n = 5] and at delivery [n = 8]), and gestational-matched controls (n = 12) by quantitative PCR (A, C, E, and G) and digital PCR (B, D, F, and H). Mean and SEM are graphed, *P < .05. IL1B indicates interleukin 1β; NFKB1, nuclear factor-κB1; RIPK1, TNF receptor super family-interacting serine–threonine kinase 1; PCR, polymerase chain reaction; PPROM, preterm prelabor rupture of the fetal membranes; SEM, standard error of the mean.
Confirming the results from qPCR, digital PCR analysis of IL1B showed significantly increased expression of IL1B in the blood of women with chorioamnionitis taken at the time of delivery compared to controls (Figure 3A). Most importantly, digital PCR also showed a significant difference in IL1B mRNA expression between women with and without chorioamnionitis at delivery (Figure 3B). Furthermore, CD14 levels in blood at delivery, in this chorioamnionitis cohort, were significantly higher compared to levels in blood taken at consent from the same women (Figure 3D). Digital PCR also revealed that NFKB1 was upregulated in women with chorioamnionitis compared to control, whereas conventional qPCR did not detect a difference (Figure 3B and F).
Discussion
A test that could improve diagnostic certainty regarding the presence of chorioamnionitis would help inform the decision about when to deliver women with PPROM. Diagnostic tests, such as C-reactive protein or the white cell count, have proven to be either nonspecific or insensitive such that their use cannot be recommended as tests to determine clinical management.11,12
Other markers of inflammation detectable in the amniotic fluid or fetal membranes have previously been examined in the setting of suspected intrauterine infection; however, to date they have not had adequate utility to translate into regular clinical practice.8,13,14 Sampling these tissues requires invasive procedures likely to be accepted by a limited number of women concerned about the potential harm from such procedures.15,16
We undertook a prospective study where we set out to identify an mRNA-based biomarker in maternal blood that can identify the presence of chorioamnionitis in utero, among women who had PPROM. To do this, we performed a combination of hypothesis-based screening of 6 genes and a discovery-based approach for new targets by examining 24 genes using a custom PCR array. We then performed a validation using both conventional and digital PCR.
Of all the cytokines examined, we found that expression of IL1B mRNA in maternal blood was increased among women who have chorioamnionitis, compared to (1) women who had PPROM but did not have evidence of an intrauterine infection at delivery and (2) a gestation-matched cohort of women with normal pregnancies who progressed to a healthy delivery at term.
Interleukin 1β is a key regulator of mechanisms that underlie innate immunity and inflammation. It induces the expression of cyclooxygenase type 2, type 2 phospholipase A, and inducible nitric oxide synthase and increases the expression of endothelial adhesion molecules promoting the movement of inflammatory cells from intravascular into extravascular spaces and tissues. It increases the production of other cytokines including IL-6 leading to further cascades of proinflammatory molecules and mediators.17,18 The role of IL1B in parturition, particularly preterm labor associated with inflammation, is established in observational and animal studies.19,20 In general elevated levels of IL1B associated with parturition have been found in gestational tissues, which limits its use as a diagnostic biomarker. However, in our study, the use of the PAXgene system for stabilization of nucleic acids enables the measurement of leukocyte and/or fetoplacental mRNA from a peripheral blood sample.
We have shown that expression of IL1B mRNA as measured in the maternal blood especially using copy number with digital PCR has the potential to diagnose women with PPROM and chorioamnionitis and distinguish them from women without chorioamnionitis. Although the performance of IL1B expression as a single biomarker to predict chorioamnionitis may be modest, it is likely that with the addition of other markers, which are the subject of ongoing discovery and investigation, it will be an important component in a noninvasive test to diagnose chorioamnionitis.
Although expression of IL1B had a clear association with chorioamnionitis, it was disappointing that the other genes examined which have previously been associated with inflammation and infection were not found to be sufficiently different when measured in the peripheral blood of women with chorioamnionitis.
Besides identifying a putative mRNA biomarker, our other main finding is that our data suggest measuring circulating mRNA in blood by digital PCR may be a more accurate and promising approach to identify biomarkers compared to using conventional qPCR.
The measurement of free fetoplacental mRNA in the maternal circulation has been studied by others but usually at a single time point as a biomarker for particular pregnancy morbidities such as preeclampsia or growth restriction.21–23 We have previously proposed, and this study supports the proposition that measuring dynamic changes in mRNA expression provides an advantage in monitoring pathologies which evolve over time where a threshold may be crossed, where the likelihood of adverse outcome increases sufficiently to justify iatrogenic prematurity.24 There are some important advantages with using the digital PCR technology, namely, it provides strong reproducibility and precision and normalization is not required. Furthermore, digital PCR has particular utility in measuring genes of low copy number or where a housekeeping gene with which to normalize expression to is not known or reliable.25 The expression of IL1B mRNA measured by digital PCR yielded more acute and significant differences in expression between key clinical groups compared to conventional qPCR. Thus, for mRNA blood biomarker research, we propose digital PCR should be considered, at least in parallel, and perhaps in place of conventional PCR.
This work is an initial exploratory study and is constrained by the small sample size which is, in part, a consequence of focusing on earlier gestations where cases occur less frequently. However, as the dilemma of choosing between the risk of exacerbating infection with ongoing expectant management or iatrogenic preterm birth is greatest earlier in pregnancy, it is here that we have concentrated our attention. As a result of the smaller size of our study, the ability to draw conclusions about the influence of demographic factors or the differential effects of certain microbes is also necessarily limited. Such factors would require a much larger study in order to be adequately powered to detect a meaningful impact, which we hope to address in the future.
Conclusion
The copy number of IL1B mRNA in the maternal blood measured using digital PCR is significantly upregulated in women with PPROM and chorioamnionitis, compared to those with PPROM but without chorioamnionitis. However, it will need to be combined with other biomarkers to be sufficiently sensitive and specific to serve as a clinically useful test. We also conclude that future studies examining the biomarker potential of mRNA in the maternal circulation should consider measuring mRNA transcripts using digital PCR either in parallel or instead in conventional PCR.
Acknowledgments
We wish to thank the Medical Research Foundation for Women and Babies for funding this work. The authors acknowledge Clinical Research midwives Debra Jinks, Rachel Murdoch and Genevieve Christophers and the Obstetric, midwifery staff and patients at the Mercy Hospital for Women (Heidelberg) for their provision of placental tissue and blood samples.
Footnotes
Authors’ Note: The authors Natalie J Hannan and Stephen Tong contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: NHMRC provided salary support to ST, NJH and TKL, and a postgraduate scholarship to OS.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Grant Support: This study was supported by the Medical Research Foundation for Women and Babies. The National Health and Medical Research Council of Australia provided scholarship; OS #1055795 and salary support; TKL #1062418, NJH #628927 and ST #1050765.
References
- 1. Goldenberg RL, Culhane JF, Iams JD, Romero R. Preterm Birth 1 Epidemiology and causes of preterm birth. Lancet. 2008;371 (9606):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Czikk MJ, McCarthy FP, Murphy KE. Chorioamnionitis: from pathogenesis to treatment. Clin Microbiol Infect. 2011;17 (9):1304–1311. doi:10.1111/j.1469-0691.2011.03574.x. [DOI] [PubMed] [Google Scholar]
- 3. Ramsey PS, Lieman JM, Brumfield CG, Carlo W. Chorioamnionitis increases neonatal morbidity in pregnancies complicated by preterm premature rupture of membranes. Am J Obstet Gynecol. 2005;192 (4):1162–1166. doi:10.1016/j.ajog.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 4. Andrews WW, Cliver SP, Biasini F, et al. Early preterm birth: association between in utero exposure to acute inflammation and severe neurodevelopmental disability at 6 years of age. Am J Obstet Gynecol. 2008;198(4):466.e1–466.e11. doi:10.1016/j.ajog.2007.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shatrov JG, Birch SCM, Lam LT. Chorioamnionitis and Cerebral Palsy. Obstet Gynecol. 2010;116 (2):387–392. [DOI] [PubMed] [Google Scholar]
- 6. Kallapur SG, Presicce P, Chougnet CA, Rueda CM, Jobe AH. Fetal Immune Response to Chorioamnionitis. Semin Reprod Med. 2014;32 (1):56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ng EKO, Tsui NBY, Lau TK, et al. mRNA of placental origin is readily detectable in maternal plasma. Proc Natl Acad Sci U S A. 2003;100 (8):4748–4753. doi:10.1073/pnas.0637450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim YM, Romero R, Chaiworapongsa T, et al. Toll-like receptor-2 and -4 in the chorioamniotic membranes in spontaneous labor at term and in preterm parturition that are associated with chorioamnionitis. Am J Obstet Gynecol. 2004;191 (4):1346–1355. doi:10.1016/j.ajog.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 9. Menon R, Taylor RN, Fortunato SJ. Chorioamnionitis--a complex pathophysiologic syndrome. Placenta. 2010;31 (2):113–120. doi:10.1016/j.placenta.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 10. Whale AS, Huggett JF, Cowen S, et al. Comparison of microfluidic digital PCR and conventional quantitative PCR for measuring copy number variation. Nucleic Acids Res. 2012;40 (11):e82 doi:10.1093/nar/gks203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Trochez-Martinez RD, Smith P, Lamont RF. Use of C-reactive protein as a predictor of chorioamnionitis in preterm prelabour rupture of membranes: a systematic review. BJOG. 2007;114 (7):796–801. doi:10.1111/j.1471-0528.2007.01385.x. [DOI] [PubMed] [Google Scholar]
- 12. Van de Laar R, van der Ham DP, Oei SG, Willekes C, Weiner CP, Mol BW. Accuracy of C-reactive protein determination in predicting chorioamnionitis and neonatal infection in pregnant women with premature rupture of membranes: a systematic review. Eur J Obs Gynecol Reprod Biol. 2009;147 (2):124–129. doi:10.1016/j.ejogrb.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 13. Gulati S, Agrawal S, Raghunandan C, et al. Maternal serum interleukin-6 and its association with clinicopathological infectious morbidity in preterm premature rupture of membranes: a prospective cohort study. J Matern Fetal Neonatal Med. 2012;25 (8):1428–1432. doi:10.3109/14767058.2011.638952. [DOI] [PubMed] [Google Scholar]
- 14. Holst RM, Laurini R, Jacobsson B, et al. Expression of cytokines and chemokines in cervical and amniotic fluid: relationship to histological chorioamnionitis. J Matern Fetal Neonatal Med. 2007;20 (12):885–893. doi:10.1080/14767050701752601. [DOI] [PubMed] [Google Scholar]
- 15. Lacerte M, Bujold E, Audibert F, Mayrand M-H. Amniocentesis for PPROM management: a feasibility study. J Obstet Gynaecol Can. 2008;30(8):659–664. Web site http://www.ncbi.nlm.nih.gov/pubmed/18786287. Accessed July 15, 2014. [DOI] [PubMed] [Google Scholar]
- 16. McIntosh JJ, McHugh K, Haas DM. Difficulties in establishing routine amniocentesis for preterm labor evaluation. J Matern Fetal Neonatal Med. 2012;25 (3):313–314. doi:10.3109/14767058.2011.573826. [DOI] [PubMed] [Google Scholar]
- 17. Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi:10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 18. Van de Veerdonk FL, Netea MG, Dinarello CA, Joosten LA. Inflammasome activation and IL-1β and IL-18 processing during infection. Trends Immunol. 2011;32 (3):110–116. doi:10.1016/j.it.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 19. Bowen JM, Chamley L, Keelan JA, Mitchell MD. Cytokines of the placenta and extra-placental membranes: roles and regulation during human pregnancy and parturition. Placenta. 2002;23 (4):257–273. doi:10.1053/plac.2001.0782. [DOI] [PubMed] [Google Scholar]
- 20. Sadowsky DW, Adams KM, Gravett MG, Witkin SS, Novy MJ. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am J Obstet Gynecol. 2006;195 (6):1578–1589. doi:10.1016/j.ajog.2006.06.072. [DOI] [PubMed] [Google Scholar]
- 21. Ashur-Fabian O, Yerushalmi GM, Mazaki-Tovi S, et al. Cell free expression of hif1alpha and p21 in maternal peripheral blood as a marker for preeclampsia and fetal growth restriction. PLoS One. 2012;7 (5):e37273 doi:10.1371/journal.pone.0037273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hahn S, Rusterholz C, Hosli I, Lapaire O. Cell-free nucleic acids as potential markers for preeclampsia. Placenta. 2011;32 suppl:S17–S20. doi:10.1016/j.placenta.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 23. Pang WW, Tsui MH, Sahota D, et al. A strategy for identifying circulating placental RNA markers for fetal growth assessment. Prenat Diagn. 2009;29 (5):495–504. doi:10.1002/pd.2230. [DOI] [PubMed] [Google Scholar]
- 24. Whitehead C, Teh WT, Walker SP, et al. Quantifying circulating hypoxia-induced RNA transcripts in maternal blood to determine in utero fetal hypoxic status. BMC Med. 2013;11:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lanoix D, Lacasse A-A, St-Pierre J, et al. Quantitative PCR pitfalls: the case of the human placenta. Mol Biotechnol. 2012;52 (3):234–243. doi:10.1007/s12033-012-9539-2. [DOI] [PubMed] [Google Scholar]