Abstract
Polycystic ovary syndrome (PCOS) is currently considered a predominantly hyperandrogenic syndrome. In theory, hyperandrogenism can be caused by high level of testosterone (T) as well as by enhanced androgen receptor (AR) activity. C-Terminal binding protein 1 antisense (CTBP1-AS) was a novel long noncoding RNA (lncRNA) to regulate AR activity. In this study, we found that expression level of CTBP1-AS in peripheral blood leukocytes was significantly higher in women with PCOS than that in controls after adjustment for age and body mass index (BMI). Individuals having higher expression of CTBP1-AS had significantly greater disease risk than those having lower expression. We also identified expression of CTBP1-AS as an independent risk factor for PCOS. A positive correlation was observed between the CTBP1-AS expression and the total T (TT) concentration either unadjusted or after adjusting for age, BMI, and homeostatic model assessment insulin resistance. Taken together, our current study presented the first evidence that the lncRNA CTBP1-AS, a novel AR modulator, is associated with PCOS in Chinese population and established the possibility that abnormal CTBP1-AS expression is a risk factor for PCOS and it is a predictor of variability in serum TT level in Chinese women with PCOS.
Keywords: androgen receptor, lncRNA, CTBP1-AS, polycystic ovary syndrome, hyperandrogenism
Introduction
Polycystic ovary syndrome (PCOS) is one of the most frequent metabolic and reproductive diseases affecting approximately 10% of reproductive-aged women worldwide.1 Although the clinical manifestations of the syndrome are highly variable including reproductive, metabolic, and psychological abnormalities, androgenic effects constitute the common mechanism responsible for its phenotype.2 Although the pathophysiology of PCOS is complex and remains largely unclear, the disorder is currently considered a predominantly hyperandrogenic syndrome.3
Androgenic effects are exerted via the androgen receptor (AR), a nuclear transcription factor and member of the steroid receptors subgroup, which controls the expression of a large number of downstream target genes.4 In theory, hyperandrogenism can be caused by high level of testosterone (T) as well as by enhanced AR activity. Notably, a hyperactive AR at the levels of the gonadotropin-releasing hormone pulse generator in the hypothalamus5 and at the granulosa cells in the ovary,6,7 skeletal muscles, or adipocytes8,9 can make initially normal T and dihydrotestosterone as biochemically hyperandrogenic.
In the past decades, various clinical studies established that the transcriptional activity of AR is mainly mediated by AR gene CAG repeat polymorphism and the epigenetic effect of X chromosome inactivation (XCI).10–14 However, other existing studies have produced conflicting and inconsistent results15–21 and even yielded paradoxical result.22 Therefore, we must evaluate AR as a dynamic heterocomplex because there are a large number of AR coactivators and corepressors, which influence androgen action via cross talk with AR.23 In addition, it has been suggested that abnormalities in the expression of these factors may be involved in the development and progression of some diseases such as prostate cancer, hirsutism, and androgen insensitivity syndrome.24–26 In theory, it is plausible for the hypothesis that variation in expression or function of these modulators might modify the activity of AR and be involved in the pathogenesis of hyperandrogenism and thus contribute to development of PCOS. However, until now, there have been no studies on the association between AR modulators and PCOS.
In a very recent study, Takayama et al27,28 uncovered that CTBP1-AS was a novel androgen-regulated long noncoding RNA (lncRNA) associated with the AR signaling pathway, serving as AR modulator. C-Terminal binding protein 1 antisense (CTBP1-AS) is located in the antisense (AS) region of CTBP1, which is a corepressor for AR. The lncRNA CTBP1-AS directly represses the expression of CTBP1 by recruitment of the RNA-binding transcriptional repressor PTB-associated splicing factor (PSF) and histone deacetylases, which in turn promotes transcriptional activity of AR. Additionally, upregulated CTBP1-AS also inhibits other endogenous tumor-suppressor genes and promotes both hormone-dependent and castration-resistant tumor growth in prostate cancer.29 Thus, the possibility exists that CTBP1-AS like CAG repeat polymorphism is involved in the pathogenesis of hyperandrogenism such as PCOS. However, whether the lncRNA CTBP1-AS as AR modulator is associated with PCOS is unknown.
In this study, we address the expression profiling of CTBP1-AS in women with PCOS and its relation to the key clinical characteristics of PCOS. First, we measured the expression of CTBP1-AS in the peripheral blood leukocytes in patients with PCOS and healthy control women. Second, the correlation between CTBP1-AS and the disease as well as multiple key endocrine parameters of PCOS was analyzed using multiple statistical methods.
Materials and Methods
Study Patients
The study protocol was approved by the Institutional Ethical Review Board of Yuhuangding Hospital of Yantai, Affiliated Hospital of Qingdao Medical University. Written informed consent was obtained from all patients. All the study evaluations and procedures were conducted in accordance with the guidelines of the Declaration of Helsinki on human experimentation. The study was initiated on March, 2013 and completed in March, 2014. We recruited 23 patients with PCOS at Department of Reproductive Medicine of Yuhuangding Hospital of Yantai from March 2013 to March 2014. The diagnosis of PCOS was based on the 2003 Rotterdam criteria30 and required 2 or more of the following 3 features: menstrual disorders (oligomenorrhoea or amenorrhea), clinical or biochemical evidence of hyperandrogenism, and polycystic ovaries visible on ultrasound examination (at least 12 follicles measuring 2-9 mm or volume of the ovary >10 cm3). Hyperandrogenism was assessed by the presence of hirsutism and/or acne and/or by elevated androgen levels. Hirsutism was defined by Ferriman-Gallwey index score >5.15 We excluded patients with Cushing syndrome, congenital adrenal hyperplasia, and androgen-secreting tumors. The control group consisted of age-, race-, sex-, and BMI-matched 17 healthy women before entering in vitro fertilization program due to male factor infertility. They had no evidence of clinical or biochemical hyperandrogenism, no menstrual cycle irregularities, or other endocrine disorders related to PCOS. None of the patients had taken any hormonal drugs, such as oral contraceptives, antiandrogens, or insulin sensitizers for at least 6 months before the study. All patients and controls were unrelated Han Chinese women from the same geographical area.
Standardized anthropometric measurements for all patients were carried out in the early follicular phase of the menstrual cycle (days 3-5) or randomly in patients with amenorrhea between 09:00 AM and 11:00 AM including body weight, height, waist circumference, and waist/hip ratio (WHR). The body mass index (BMI) was calculated as weight/height2 (kg/m2). Blood pressure (BP) was measured in the sitting position after a 10-minute rest. All patients had the ultrasound examinations performed by experienced reproductive gynecologists performing a transvaginal scan on cycle days 12 to 14. Transvaginal scans were reevaluated by one of the authors (CH).
Hormone Assays
After an overnight 12-hour fasting, antecubital intravenous blood samples (˜5 mL) were collected from all participants at an early follicular phase (days 3-5) of a spontaneous menstrual cycle or progesterone (P)-induced withdrawal bleed between 08:00 AM and 10:00 AM. Serum luteinizing hormone (LH), follicle-stimulating hormone (FSH), prolactin (PRL), estradiol (E2), P, total T (TT) concentrations, fasting plasma glucose, and fasting insulin were determined by chemiluminescent immunometric assays (Ortho Clinical Diagnostics, Johnson & Johnson, Rochester, New York). Total cholesterol, triglycerides, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and lipoprotein (a; LP (a)) were measured by colorimetric enzymatic methods (Siemens Advia System, Deerfield, Illinois). The intra-assay and interassay coefficients of variation ranged between 1.8% and 6.8%. Fasting glucose and insulin levels served for homeostasis model assessment of insulin resistance (HOMA-IR), calculated by multiplying fasting insulin (mU/L) by fasting glucose (mmol/L) and dividing this product by 22.5.
Isolation of Peripheral Blood Leukocytes
Blood samples were obtained from patients with PCOS (n = 23) and normal healthy donors (n = 17). The blood in heparin tubes (5 mL per patient) was diluted with equal volumes of phosphate-buffered saline and then overlaid on a Ficoll-Paque Plus kit (GE Healthcare Life Sciences, Pittsburgh, Pennsylvania) at a 1:1 ratio and centrifuged at 800g for 30 minutes at 20°C. The peripheral blood mononuclear cell layer was harvested and washed with phosphate-buffered saline 2 times to remove plasma and Ficoll (Axis-Shield, Oslo, Norway). Then, these samples were stored at −80°C until further analyses.
RNA Isolation and Real-Time Polymerase Chain Reaction
Total RNA was harvested from the above-mentioned peripheral blood leukocytes using the RNeasy Mini kit (QIAGEN, Hilden, Germany) according to the manufacturers’ instructions. After evaluation of concentration and purity of RNA using the ND-1000 NanoDrop spectrophotometer (NanoDrop, Wilmington, Delaware) and denaturing gel electrophoresis, the RNA reverse-transcribed into complementary DNA using PrimeScript RT Reagent Kit (TaKaRa, Shiga, Japan) in the Rotor-Gene 3000 Real-time PCR system (Corbett Research, Australia), according to the manufacturers’ protocol.
Measurement of CTBP1-AS Gene Expression
In this process, the levels of CTBP1-AS were quantified by quantitative real-time polymerase chain reaction (qRT-PCR) using the SYBR Premix Ex Taq II (TaKaRa) following the manufacturer’s protocol. In brief, reactions were conducted using the Rotor-Gene 3000 Real-time PCR system (Corbett Research) with the following reaction profile: predenaturation for 30 seconds at 95°C and PCR amplification for 45 cycles with 5 seconds at 95°C and 60 seconds at 60°C. The PCR was followed by a melt curve analysis to determine the reaction specificity. Agarose gel electrophoresis was performed to confirm the size of PCR product. The levels of CTBP1-AS were analyzed by relative quantification using the comparative threshold (Ct) cycle method relative to the reference gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression level.31 It is best to calculate the mean ± standard error of the mean (SEM) for the change in gene expression in each group as individual data points using equation 2−ΔCt.32 Each set of qRT-PCR reactions was repeated 3 times.
The following primers were used to quantify the lncRNA CTBP1-AS levels: CTBP1-AS (GenBank: NR_104331.1), 5′-ACAACACAAAGCCCCGGAA-3′ (forward) and 5′-AGTGAAGAATGGTCTCGCCC-3′ (reverse), fragment length: 121; GAPDH RNA was quantified as a control to normalize differences in total RNA levels using the following primers (GenBank: NM_002046): 5′-GGGAAACTGTGGCGTGAT-3′ (forward) and 5′-GAGTGGGTGTCGCTGTTGA-3′ (reverse), fragment length: 202.
Statistical Analysis
The results are expressed as means ± SEM or median (interquartile range). Normal distribution of the continuous variables was tested by the Shapiro-Wilk statistic. The expression level of CTBP1-AS was natural logarithm-transformed (-ln) because of a skewed distribution. Characteristics of patients with PCOS and healthy controls were compared using either Student t test for quantitative data with Gaussian distribution or Mann-Whitney U test for data with non-Gaussian distribution. The distributions of the CTBP1-AS expression were divided into binary groups among the patients with PCOS and control participants. Limits for the binary groups of this lncRNA expression were derived from the control group as follows: <3.757 for the low expression group and ≥3.757 for the high expression group. In a backward stepwise binomial logistic regression analysis, continuous parameters independently predicting PCOS were identified. Predictors that did not contribute to the model were excluded (exclusion threshold P > .04). Separate Pearson or Spearman rank correlation coefficients were calculated to analyze whether there were linear relations between the expression of CTBP1-AS and certain clinical biochemical traits. To explore the effect of the CTBP1-AS expression on TT concentration, a multiple linear repression model was constructed with TT as a dependent variable and age, BMI, HOMA-IR, and CTBP1-AS expression level as independent variables. Logistic regression model was conducted to explore the association of the expression of CTBP1-AS with hirsutism in patients with PCOS, adjusting for TT as independent variable.
SPSS software version 20.0 (SPSS Inc., Chicago, Illinois) was used for analyses. Data were considered statistically significant at P < .05.
Results
Clinical Characteristics of Patients
The clinical, metabolic, and hormonal features of the controls and patients with PCOS are shown in Table 1. As expected from design, we found no differences in age and BMI between controls and patients with PCOS. Compared to healthy controls, patients with PCOS had higher hirsutism score, higher levels of TT, LH, LH/FSH, fasting insulin, and HOMA-IR (P < .05). The differences in WHR, systolic BP and diastolic BP, and the levels of E2, FSH, PRL, fasting glucose, triglycerides, cholesterol, HDL cholesterol, LDL cholesterol, and LP(a) between controls and patients with PCOS did not reach statistical significance. These differences persisted after adjustment for BMI.
Table 1.
Clinical Characteristic of Controls and Patients With PCOS.a
| Variables | Controls | Patients With PCOS | P Value |
|---|---|---|---|
| Number | 17 | 23 | |
| Age, years | 29.71 ± 0.44 | 28.44 ± 0.37 | .051 |
| BMI, kg/m2 | 27.01 ± 1.1 | 28.88 ± 1.56 | .011 |
| WHR | 0.84 ± 0.01 | 0.88 ± 0.01 | .020 |
| Hirsutism score | 2 (0-3) | 4 (1-6) | <.001 |
| Systolic BP, mm Hg | 119.00 ± 1.56 | 119.61 ± 3.36 | .884 |
| Diastolic BP, mm Hg | 76.00 ± 1.57 | 76.13 ± 2.12 | .963 |
| Total T, ng/mL | 0.24 ± 0.02 | 0.41 ± 0.04 | .001 |
| E2, pg/mL | 43.67 ± 4.76 | 47.17 ± 3.53 | .550 |
| FSH, mIU/mL | 6.45 ± 0.37 | 5.63 ± 0.27 | .077 |
| LH, mIU/mL | 5.27 ± 0.44 | 11.43 ± 1.35 | .001 |
| LH/FSH | 0.81 ± 0.07 | 2.04 ± 0.24 | <.001 |
| PRL, ng/mL | 14.94 ± 1.25 | 14.54 ± 1.11 | .814 |
| Fasting glucose, mg/dL | 4.65 ± 0.08 | 5.07 ± 0.14 | .030 |
| Fasting insulin, mIU/mL | 8.29 ± 0.52 | 16.00 ± 1.12 | .004 |
| HOMA-IR | 1.72 ± 0.12 | 4.43 ± 0.89 | .013 |
| Cholesterol, mmol/L | 5.02 ± 0.26 | 5.06 ± 0.16 | .892 |
| Triglycerides, mmol/L | 0.66 ± 0.05 | 1.13 ± 0.14 | .008 |
| HDL cholesterol, mmol/L | 1.62 ± 0.10 | 1.41 ± 0.06 | .075 |
| LDL cholesterol, mmol/L | 2.55 ± 0.17 | 3.02 ± 0.19 | .083 |
| LP(a), mg/L | 94.60 (46.6-370.8) | 167.3 (50.00-598.6) | .092 |
Abbreviations: BMI, body mass index; WHR, waist to hip ratio; BP, blood pressure; T, testosterone; E2, estradiol; FSH, follicle-stimulating hormone; LH, luteinizing hormone; PRL, prolactin; HOMA-IR, homeostasis model assessment; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LP(a), lipoprotein(a); SEM, standard error of the mean.
aValues are expressed as mean ± SEM or median (interquartile range). P values were obtained from unpaired 2-tailed Student t test or Mann-Whitney U test. Clinical indexes with significant differences (P < .05) are in bold.
Expression of CTBP1-AS in Controls and Women With PCOS
As shown in Figure 1, mean CTBP1-AS expression level in peripheral blood leukocytes was significantly higher in the women with PCOS than that in the controls (-lnCTBP1-AS, 5.323 ± 0.205 vs 2.059 ± 0.210, P = .023) after age and BMI adjustment. The experiment was performed 3 times with similar results.
Figure 1.
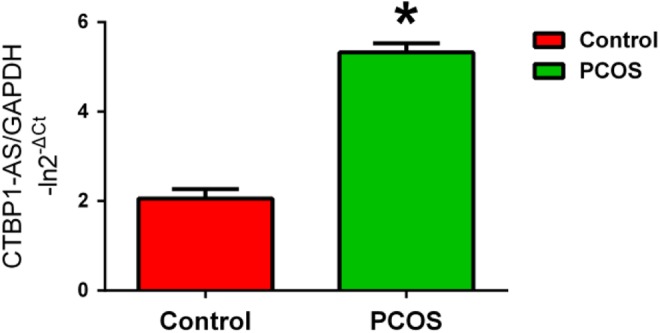
The expression of CTBP1-AS in peripheral blood leukocytes in controls and patients with PCOS measured using quantitative real-time PCR after adjustment for age and BMI. Data are expressed as means ± SEM. *P < .05, Student t test. The experiment was performed 3 times with similar results. CTBP1-AS indicates C-terminal binding protein 1 antisense; PCOS, polycystic ovary syndrome; PCR, polymerase chain reaction; BMI, body mass index; SEM, standard error of the mean.
Association Between CTBP1-AS and the Presence of PCOS
We sought to further analyze the relationship between different expression levels of CTBP1-AS and risk of PCOS. Table 2 shows the risk of PCOS in individuals with different CTBP1-AS expression levels. Individuals having higher expression had significantly greater disease risk than those having lower expression (odds ratio = 1.822, 95% confidence interval = 1.223-2.225, P = .005) after adjustment for BMI.
Table 2.
Odds Ratio of PCOS Events by Expression of CTBP1-AS.a
| Controls (n = 17) | Patients With PCOS (n = 23) | OR | 95%CI | P Value | |||
|---|---|---|---|---|---|---|---|
| Low | High | Low | High | ||||
| No adjustment | 8 (47.1%) | 9 (52.9%) | 8 (34.8%) | 15 (65.2%) | 1.667 | 1.163-1.906 | .014 |
| Adjusted for BMI | 1.822 | 1.223-2.225 | .005 | ||||
Abbreviations: BMI, body mass index; CTBP1-AS; C-terminal binding protein 1 antisense; PCOS, polycystic ovary syndrome; OR, odds ratio; 95% CI, confidence intervals.
aData are n (%), unless otherwise indicated. CTBP1-AS expression for binary groups cutoffs were <3.757 for the low expression, ≥3.757 for the high expression. The OR, 95% CI, and P value were estimated for PCOS events in the high expression group compared to the low expression using logistic regression models. P values < .05 are in bold.
Expression of CTBP1-AS as an Independent Risk Factor for PCOS
We investigated the role of CTBP1-AS expression level in context of established risk factors for PCOS (Table 3). To this end, we included BMI, serum LH, HOMA-IR, and expression of CTBP1-AS in a backward stepwise binomial regression analysis and identified CTBP1-AS expression as an independent risk factor for PCOS (P < .05).
Table 3.
Expression of CTBP1-AS as an Independent Factor for PCOS.a
| Risk Factor | P Valueb |
|---|---|
| Elevated TT | .013 |
| Elevated LH | .021 |
| CTBP1-AS expression | .015 |
Abbreviations: CTBP1-AS; C-terminal binding protein 1 antisense; LH, luteinizing hormone; PCOS, polycystic ovary syndrome; TT, total testosterone.
a P < .05 are in bold.
bBackward stepwise binomial regression analysis.
Correlation Between CTBP1-AS Level and Clinical Parameters in PCOS
In order to analyze whether there was an association between the expression of CTBP1-AS and certain clinical biochemical traits, separate Pearson or Spearman rank correlation coefficients were calculated. We included the expression of CTBP1-AS as dependent variables, and clinical characteristics in both controls and patients with PCOS as independent variables. As shown in Table 4, there was a significant correlation between CTBP1-AS and TT only in the PCOS groups (r = .453, P = .027) after adjustment for age and BMI. However, there was no association between CTBP1-AS and other clinical characteristics.
Table 4.
Partial Pearson or Spearman Rank Correlation Coefficients of the Expression of CTBP1-AS and Patients’ Characteristics.a
| Controls | Women With PCOS | |||
|---|---|---|---|---|
| r | P Value | r | P Value | |
| Age, years | ||||
| BMI, kg/m2 | ||||
| WHR | −.191 | .464 | −.079 | .721 |
| Hirsutism score | −.361 | .154 | −.064 | .773 |
| Systolic BP, mm Hg | −.101 | .699 | −.077 | .726 |
| Diastolic BP, mm Hg | −.029 | .912 | −.108 | .623 |
| Total T, ng/mL | .415 | .057 | .453 | .027 |
| E2, pg/mL | −.059 | .822 | −.046 | .836 |
| FSH, mIU/mL | −.277 | .102 | −.306 | .156 |
| LH, mIU/mL | .294 | .252 | −.073 | .742 |
| LH/FSH | −.267 | .301 | .059 | .788 |
| PRL, ng/mL | .005 | .984 | .270 | .213 |
| Fasting glucose, mg/dL | .179 | .492 | .023 | .918 |
| Fasting insulin, mIU/mL | −.247 | .339 | −.032 | .883 |
| HOMA-IR | −.155 | .552 | .284 | .167 |
| Cholesterol, mmol/L | −.180 | .490 | .018 | .933 |
| Triglycerides, mmol/L | −0.091 | .728 | .127 | .563 |
| HDL, mmol/L | .091 | .729 | .106 | .630 |
| LDL, mmol/L | −.041 | .877 | −.050 | .822 |
| LP(a), mg/L | −.408 | .067 | −.101 | .647 |
Abbreviations: BMI, body mass index; CTBP1-AS; C-terminal binding protein 1 antisense; WHR, waist to hip ratio; BP, blood pressure; T, testosterone; E2, estradiol; FSH, follicle-stimulating hormone; LH, luteinizing hormone; PRL, prolactin; HOMA-IR, homeostasis model assessment insulin resistance; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LP(a), lipoprotein(a).
aThe correlation coefficient (r) and P value were adjusted for age and BMI. P values <.05 are in bold.
The Effect of CTBP1-AS Expression on TT
A positive correlation was observed between the expression of CTBP1-AS and the TT concentration either unadjusted or after adjusted for age, BMI, and HOMA-IR (Table 5). A model using the expression of CTBP1-AS, age, BMI, and HOMA-IR as predictors explained 9.2% (adjusted R 2) of the variability in the serum TT levels (P = .017). The same analysis in the control group revealed no significant difference in any biochemical parameter (data not shown).
Table 5.
Multiple Linear Regression Models With TT as the Dependent Variable in PCOS.a
| Variable | Standardized β coefficient | P Value |
|---|---|---|
| Unadjusted model (R 2 = .051) | ||
| CTBP1-AS expression | .231 | .018 |
| Adjusted model (R 2 = .081) | ||
| CTBP1-AS expression | .209 | .023 |
| Age | −.140 | .129 |
| BMI | .101 | .319 |
| Adjusted model (R 2 = .092) | ||
| CTBP1-AS expression | .239 | .017 |
| Age | −.138 | .131 |
| BMI | .125 | .213 |
| HOMA-IR | −.102 | .317 |
Abbreviations: BMI, body mass index; CTBP1-AS, C-terminal binding protein 1 antisense; HOMA-IR, homeostasis model assessment of insulin resistance; PCOS, polycystic ovary syndrome.
a P < 0.05 are in bold.
Association of the CTBP1-AS Expression With Hirsutism in PCOS
To explore the association of the CTBP1-AS expression with clinical androgen trait, hirsutism, in PCOS, a logistic regression model was constructed with hirsutism (yes/no) as dependent variable, CTBP1-AS expression (high/low) as independent variable, and TT as covariate. The result was not significant (P = .122). The TT was also not found to have a significant effect on hirsutism (P = .104; Table 6).
Table 6.
Association of Expression of CTBP1-AS With Hirsutism in PCOS.a
| Low Expression | High Expression | ||||
|---|---|---|---|---|---|
| Hirsutism (yes) | 2 | 9 | OR | 95% CI | P value |
| Hirsutism (no) | 6 | 6 | 0.222 | 0.033 -1.493 | .122 |
| Adjusted for TT | 0.213 | 0.026 -1.485 | .104 |
Abbreviations: CI, confidence interval; CTBP1-AS, C-terminal binding protein 1 antisense; OR, odds ratio; PCOS, polycystic ovary syndrome; TT, total testosterone.
aA logistic regression model was constructed with hirsutism (yes/no) as dependent variable, CTBP1-AS expression (high/low) as independent variable, and TT as covariate.
Discussion
To our knowledge, this is the first study to determine the association of lncRNA CTBP1-AS, a novel AR modulator, and PCOS. Patients with PCOS tended to have a significantly higher CTBP1-AS expression than healthy controls. Individuals with more elevated CTBP1-AS expression had significantly greater disease risk than those having the lower expression after adjustment for BMI, indicating that CTBP1-AS seems to be a major determinant of PCOS. Our results are consistent with a previous functional study, which found upregulated CTBP1-AS can promote AR-mediated transcriptional activity to facilitate the expression of androgen-responsive genes.27
There are several lines of evidence demonstrating that hyperandrogenism, which is a central feature for PCOS, determines the characteristic phenotype of the syndrome, that is, menstrual cycle dysfunction, hirsutism, and polycystic ovarian morphology.10,33,34 In theory, hyperandrogenism can be caused by high level of TT as well as by enhanced AR activity. Therefore, more active AR can cause a hyperandrogenic phenotype in absence of markedly elevated androgens. In view of this, a number of studies have reported that increased androgenic activity could amplify IR as well as T in physiological concentrations in differentiated rat skeletal muscle myotubes and cultured subcutaneous adipocytes of women.8,9 Amplified transcriptional activity of AR promotes intraovarian androgenic microenvironment which presumably stimulates early follicular growth and contributes to mechanisms of follicular arrest found in PCOS.35
Although the association of AR activity with CAG polymorphism and XCI has been extensively studied, they have produced conflicting results.10,11,13,15,17–19,21,22,36,37 There is little information on AR coregulators in the pathogenesis of PCOS, although recent advances in molecular biology have identified several AR coregulators that modulate the androgen action via cross talk with AR.35,38 The dependency of the AR on its coregulators suggests a key role for these regulatory molecules in the development and maintenance of disorders associated with hyperandrogenism in humans.
It is noteworthy that Takayama et al27 recently reported the identification of a novel androgen-dependent lncRNA CTBP1-AS as AR activity modulator, whose expression is inversely correlated with the expression of CTBP1, a novel AR corepressor. More importantly, epigenetic modifications of CTBP1-AS provide new insight into the regulated mechanism of AR activity without changing the DNA code.39 Notably, CTBP1-AS as an important modulator of AR activity appears to be clinically relevant in some diseases closely associated with abnormal AR activity. Thus, it is possible that CTBP1-AS might play a role in the pathogenesis of PCOS-related hyperandrogenism.
According to our data, there is a significant elevation in CTBP1-AS expression in patients with PCOS compared to healthy controls. Furthermore, higher expression of CTBP1-AS was more frequent in PCOS possibly enhancing androgenic effects in these patients, while lower expression of the modulator was more frequent in the control group, presumably exerting a protective effect. This indicated that women with higher expression of CTBP1-AS have greater disease risk which was consistent with a role for androgen hyperactivity in the development of the PCOS. Additionally, we report novel evidence that CTBP1-AS influenced the PCOS phenotype by serving as an independent risk factor for PCOS, comparable to established markers of PCOS, for example, elevated LH and increased BMI (Table 3). Yeh et al40 had shown that the AR coactivator had been shown to enhance AR activity up to 10-fold, a level that AR alone cannot reach. Hence, we would infer that even a modest change in CTBP1-AS level could have a large or cumulative effect on AR sensitivity. The mechanisms in the background of these findings remain to be elucidated.
Moreover, the more detailed analysis showed that the CTBP1-AS expression was associated with a linear increase in serum TT level only in the group with PCOS. To obtain a reliable conclusion, a multiple linear regression model was performed. A positive correlation was observed between CTBP1-AS expression and serum TT, either unadjusted or after the adjustment for age, BMI, and HOMA-IR. Testosterone is the major circulating androgen in women. It is likely that change in androgen sensitivity could result in altered androgen production, which mimics altered insulin secretion when insulin resistance is present. This trend is in agreement with Ibanez et al’s observation which reported that the increased androgen activity has a stimulatory effect on ovarian androgen production.41 Intriguingly, our findings are consistent with that of many prior genetic studies that found an association between the CAG repeats and serum T in patients with PCOS,13,19,21,22 suggesting CTBP1-AS might modulate the transcriptional activity of AR by interacting CAG polymorphism. Hsiao et al42 demonstrated that ARA 24, an AR coactivator, can interact with the AR N-terminal polyglutamine region (CAG) and enhance AR transactivation. They concluded that the interaction of AR coactivator with the CAG repeat could cooperate to modulate the AR activity. Accordingly, we speculated that CTBP1-AS might modulate AR transcriptional activity alone but also functionally interact with CAG polymorphism to regulate AR activity, which remains to be elucidated.
Although we have found a significant positive correlation between CTBP1-AS and TT, it is only a modest correlation (r = .453, P = .027), and the adjusted model explains only 9.2% of the variability in serum TT. Theoretically, hyperandrogenism in PCOS results from the interaction of multifactors, therefore other factors might confound the true effect of CTBP1-AS on TT in our study. The transcriptional activity of AR is affected not only by AR coregulators such as CTBP1-AS 27 but also by polymorphisms in the AR gene,43 E2,44 and insulin-like growth factor.45 Thus, it may highlight that multiple mechanisms affect the results.
In addition, it is expected that because of a higher receptor activity, women with higher CTBP1-AS will express a significantly clinical androgen trait such as hirsutism. However, it was not borne out in our study. Level of TT was not found to be the predictor of hirsutism as well. Regarding the hirsutism of PCOS, some reported that short CAG repeat was associated with hirsutism and acne.46 Thus, hirsutism in patients with PCOS may be affected by the multiple and complex factors, and this could explain why hirsutism was not correlated with CTBP1-AS level in PCOS. Further study is needed to explore the mechanism by which CTBP1-AS influences phenotype of PCOS.
As far as we know, data on the functional importance of the lncRNA CTBP1-AS on AR activity are sparse and mainly focus on prostate cancer. Nevertheless, it has been identified as critical to the androgen-mediated transcriptional regulation.27 Abnormal AR activity regulated by coregulators may predispose an individual to the development of PCOS. Association of AR activity with other coregulators in some diseases that influence target organ response to androgens further supports this hypothesis.23 Additionally, in a very recent study, Yang et al47 found lncRNA-dependent mechanisms of AR-regulated transcriptional activity in prostate cancer cells, revealing that lncRNAs may be part of AR transcription regulatory network. Our present findings will also have an impact on the research field of steroid hormone receptors, as we still have limited knowledge with regard to those receptors’ lncRNA coregulators.
One limit of the current research is the relatively small sample size, but our cohort with PCOS appeared to be well suited for the investigations performed, since the cohort was rather homogenous. Another limitation is the use of peripheral blood leukocytes. Gene analysis in peripheral blood leukocytes does not necessarily reflect CTBP1-AS expression status in specific target tissues such as the ovary, and further work would be required to validate the use of peripheral blood leukocytes for understanding the gene expression status in a hormonally regulated tissue. We adjusted for age and BMI when assessing association with PCOS. Obesity is thought to promote the development of PCOS.48 There is a normal age-related decline in androgen levels.49 Insulin promotes the synthesis of androgen in thecal cells directly.50 This is the reason why BMI, HOMA-IR, and age should be adjusted when establishing the presence of hyperandrogenemia in women.
Conclusion
In conclusion, in a perspective of AR coregulators, our study differs from previous genetic investigations about the association between CAG repeat polymorphism and PCOS. Our current study established the possibility that abnormal CTBP1-AS expression is a risk factor for PCOS in the Chinese population, and it is a predictor of serum TT level variability in Chinese women with PCOS. Accordingly, although there is as yet no evidence for a causal link between CTBP1-AS and PCOS, it is conceivable that CTBP1-AS might contribute, at least to some extent, to the etiology of PCOS. In light of these findings, our next focus will be to investigate the biological functions and mechanisms of CTBP1-AS in vivo and whether there is a causal link between lncRNA CTBP1-AS and PCOS in other cell lines and large other ethnic groups.
Acknowledgments
The authors would like to gratefully thank Xin Huang, Ning Li, Xuebo Wang, and Xiaofang Shen for advice on qPCR technology from the Central Laboratory of Yuhuangding Hospital of Yantai.
Footnotes
Authors’ Note: Zhenteng Liu and Cuifang Hao contributed equally to this work. This work was performed at the Central Laboratory of Yuhuangding Hospital of Yantai and Medicine and Pharmacy Research center of Binzhou Medical University, Yantai, Shandong, China.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Basic Research Program of China (grant 81170622).
References
- 1. Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7 (4):219–231. [DOI] [PubMed] [Google Scholar]
- 2. Sung YA, Oh JY, Chung H, Lee H. Hyperandrogenemia is implicated in both the metabolic and reproductive morbidities of polycystic ovary syndrome. Fertil Steril. 2014;101 (3):840–845. [DOI] [PubMed] [Google Scholar]
- 3. Azziz R, Carmina E, Dewailly D, et al. Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab. 2006;91 (11):4237–4245. [DOI] [PubMed] [Google Scholar]
- 4. Gelmann EP. Molecular biology of the androgen receptor. J Clin Oncol. 2002;20 (13):3001–3015. [DOI] [PubMed] [Google Scholar]
- 5. Blank SK, McCartney CR, Helm KD, Marshall JC. Neuroendocrine effects of androgens in adult polycystic ovary syndrome and female puberty. Semin Reprod Med. 2007;25 (5):352–359. [DOI] [PubMed] [Google Scholar]
- 6. Sen A, Hammes SR. Granulosa cell-specific androgen receptors are critical regulators of ovarian development and function. Mol Endocrinol. 2010;24 (7):1393–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walters KA, Middleton LJ, Joseph SR, et al. Targeted loss of androgen receptor signaling in murine granulosa cells of preantral and antral follicles causes female subfertility. Biol Reprod. 2012;87 (6):151. [DOI] [PubMed] [Google Scholar]
- 8. Corbould A. Chronic testosterone treatment induces selective insulin resistance in subcutaneous adipocytes of women. J Endocrinol. 2007;192 (3):585–594. [DOI] [PubMed] [Google Scholar]
- 9. Allemand MC, Irving BA, Asmann YW, et al. Effect of testosterone on insulin stimulated IRS1 Ser phosphorylation in primary rat myotubes--a potential model for PCOS-related insulin resistance. PLoS One. 2009;4 (1): e4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schuring AN, Welp A, Gromoll J, et al. Role of the CAG repeat polymorphism of the androgen receptor gene in polycystic ovary syndrome (PCOS). Exp Clin Endocrinol Diabetes. 2012;120 (2):73–79. [DOI] [PubMed] [Google Scholar]
- 11. Shah NA, Antoine HJ, Pall M, Taylor KD, Azziz R, Goodarzi MO. Association of androgen receptor CAG repeat polymorphism and polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93 (5):1939–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vottero A, Stratakis CA, Ghizzoni L, Longui CA, Karl M, Chrousos GP. Androgen receptor-mediated hypersensitivity to androgens in women with nonhyperandrogenic hirsutism: skewing of X-chromosome inactivation. J Clin Endocrinol Metab. 1999;84 (3):1091–1095. [DOI] [PubMed] [Google Scholar]
- 13. Mifsud A, Ramirez S, Yong EL. Androgen receptor gene CAG trinucleotide repeats in anovulatory infertility and polycystic ovaries. J Clin Endocrinol Metab. 2000;85 (9):3484–3488. [DOI] [PubMed] [Google Scholar]
- 14. Peng CY, Xie HJ, Guo ZF, et al. The association between androgen receptor gene CAG polymorphism and polycystic ovary syndrome: a case-control study and meta-analysis. J Assist Reprod Genet. 2014;31 (9):1211–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dasgupta S, Sirisha PV, Neelaveni K, et al. Androgen receptor CAG repeat polymorphism and epigenetic influence among the south Indian women with Polycystic Ovary Syndrome. PLoS One. 2010;5 (8):e12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ferk P, Perme MP, Teran N, Gersak K. Androgen receptor gene (CAG)n polymorphism in patients with polycystic ovary syndrome. Fertil Steril. 2008;90 (3):860–863. [DOI] [PubMed] [Google Scholar]
- 17. Jaaskelainen J, Korhonen S, Voutilainen R, Hippelainen M, Heinonen S. Androgen receptor gene CAG length polymorphism in women with polycystic ovary syndrome. Fertil Steril. 2005;83 (6):1724–1728. [DOI] [PubMed] [Google Scholar]
- 18. Liu Q, Hong J, Cui B, et al. Androgen receptor gene CAG(n) trinucleotide repeats polymorphism in Chinese women with polycystic ovary syndrome. Endocrine. 2008;33 (2):165–170. [DOI] [PubMed] [Google Scholar]
- 19. Skrgatic L, Baldani DP, Cerne JZ, Ferk P, Gersak K. CAG repeat polymorphism in androgen receptor gene is not directly associated with polycystic ovary syndrome but influences serum testosterone levels. J Steroid Biochem Mol Biol. 2012;128 (3-5):107–112. [DOI] [PubMed] [Google Scholar]
- 20. Rajender S, Carlus SJ, Bansal SK, et al. Androgen receptor CAG repeats length polymorphism and the risk of polycystic ovarian syndrome (PCOS). PLoS One. 2013;8 (10):e75709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim JJ, Choung SH, Choi YM, Yoon SH, Kim SH, Moon SY. Androgen receptor gene CAG repeat polymorphism in women with polycystic ovary syndrome. Fertil Steril. 2008;90 (6):2318–2323. [DOI] [PubMed] [Google Scholar]
- 22. Hickey T, Chandy A, Norman RJ. The androgen receptor CAG repeat polymorphism and X-chromosome inactivation in Australian Caucasian women with infertility related to polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87 (1):161–165. [DOI] [PubMed] [Google Scholar]
- 23. Heemers HV, Tindall DJ. Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocr Rev. 2007;28 (7):778–808. [DOI] [PubMed] [Google Scholar]
- 24. Ravindranathan P, Lee TK, Yang L, et al. Peptidomimetic targeting of critical androgen receptor-coregulator interactions in prostate cancer. Nat Commun. 2013;4:1923. [DOI] [PubMed] [Google Scholar]
- 25. Urbanucci A, Waltering KK, Suikki HE, Helenius MA, Visakorpi T. Androgen regulation of the androgen receptor coregulators. BMC Cancer. 2008;8:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vija L, Meduri G, Comperat E, et al. Expression and characterization of androgen receptor coregulators, SRC-2 and HBO1, during human testis ontogenesis and in androgen signaling deficient patients. Mol Cell Endocrinol. 2013;375 (1-2):140–148. [DOI] [PubMed] [Google Scholar]
- 27. Takayama K, Horie-Inoue K, Katayama S, et al. Androgen-responsive long noncoding RNA CTBP1-AS promotes prostate cancer. EMBO J. 2013;32 (12):1665–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Takayama K, Tsutsumi S, Katayama S, et al. Integration of cap analysis of gene expression and chromatin immunoprecipitation analysis on array reveals genome-wide androgen receptor signaling in prostate cancer cells. Oncogene. 2011;30 (5):619–630. [DOI] [PubMed] [Google Scholar]
- 29. Sung YY, Cheung E. Antisense now makes sense: dual modulation of androgen-dependent transcription by CTBP1-AS. EMBO J. 2013;32 (12):1653–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19 (1):41–47. [DOI] [PubMed] [Google Scholar]
- 31. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29 (9):e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3 (6):1101–1108. [DOI] [PubMed] [Google Scholar]
- 33. Dewailly D, Catteau-Jonard S, Reyss AC, Leroy M, Pigny P. Oligoanovulation with polycystic ovaries but not overt hyperandrogenism. J Clin Endocrinol Metab. 2006;91 (10):3922–3927. [DOI] [PubMed] [Google Scholar]
- 34. Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89 (6):2745–2749. [DOI] [PubMed] [Google Scholar]
- 35. McEwan IJ, McGuinness D, Hay CW, Millar RP, Saunders PT, Fraser HM. Identification of androgen receptor phosphorylation in the primate ovary in vivo. Reproduction. 2010;140 (1):93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xia Y, Che Y, Zhang X, et al. Polymorphic CAG repeat in the androgen receptor gene in polycystic ovary syndrome patients. Mol Med Rep. 2012;5 (5):1330–1334. [DOI] [PubMed] [Google Scholar]
- 37. Wang R, Goodarzi MO, Xiong T, Wang D, Azziz R, Zhang H. Negative association between androgen receptor gene CAG repeat polymorphism and polycystic ovary syndrome? A systematic review and meta-analysis. Mol Hum Reprod. 2012;18 (10):498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee HJ, Chang C. Recent advances in androgen receptor action. Cell Mol Life Sci. 2003;60 (8):1613–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Weichenhan D, Plass C. The evolving epigenome. Hum Mol Genet. 2013;22 (R1):R1–R6. [DOI] [PubMed] [Google Scholar]
- 40. Yeh S, Chang C. Cloning and characterization of a specific coactivator, ARA70, for the androgen receptor in human prostate cells. Proc Natl Acad Sci U S A. 1996;93 (11):5517–5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ibanez L, Ong KK, Mongan N, et al. Androgen receptor gene CAG repeat polymorphism in the development of ovarian hyperandrogenism. J Clin Endocrinol Metab. 2003;88 (7):3333–3338. [DOI] [PubMed] [Google Scholar]
- 42. Hsiao PW, Lin DL, Nakao R, Chang C. The linkage of Kennedy’s neuron disease to ARA24, the first identified androgen receptor polyglutamine region-associated coactivator. J Biol Chem. 1999;274 (29):20229–20234. [DOI] [PubMed] [Google Scholar]
- 43. Tut TG, Ghadessy FJ, Trifiro MA, Pinsky L, Yong EL. Long polyglutamine tracts in the androgen receptor are associated with reduced trans-activation, impaired sperm production, and male infertility. J Clin Endocrinol Metab. 1997;82 (11):3777–3782. [DOI] [PubMed] [Google Scholar]
- 44. McAbee MD, Doncarlos LL. Estrogen, but not androgens, regulates androgen receptor messenger ribonucleic acid expression in the developing male rat forebrain. Endocrinology. 1999;140 (8):3674–3681. [DOI] [PubMed] [Google Scholar]
- 45. Gupta C. Modulation of androgen receptor (AR)-mediated transcriptional activity by EGF in the developing mouse reproductive tract primary cells. Mol Cell Endocrinol. 1999;152 (1-2):169–178. [DOI] [PubMed] [Google Scholar]
- 46. Van Nieuwerburgh F, Stoop D, Cabri P, Dhont M, Deforce D, De Sutter P. Shorter CAG repeats in the androgen receptor gene may enhance hyperandrogenicity in polycystic ovary syndrome. Gynecol Endocrinol. 2008;24 (12):669–673. [DOI] [PubMed] [Google Scholar]
- 47. Yang L, Lin C, Jin C, et al. lncRNA-dependent mechanisms of androgen-receptor-regulated gene activation programs. Nature. 2013;500 (7464):598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Moran C, Arriaga M, Rodriguez G, Moran S. Obesity differentially affects phenotypes of polycystic ovary syndrome. Int J Endocrinol. 2012;2012:317241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Spencer JB, Klein M, Kumar A, Azziz R. The age-associated decline of androgens in reproductive age and menopausal Black and White women. J Clin Endocrinol Metab. 2007;92 (12):4730–4733. [DOI] [PubMed] [Google Scholar]
- 50. Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33 (6):981–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]


