Abstract
Kidney allografts possess the ability to enable a short course of immunosuppression to induce tolerance of themselves and of cardiac allografts across a full-MHC barrier in miniature swine. However, the renal element-(s) responsible for kidney-induced cardiac allograft tolerance (KICAT) are unknown. Here we investigated whether MHC disparities between parenchyma versus hematopoietic-derived “passenger” cells of the heart and kidney allografts affected KICAT. Heart and kidney allografts were co-transplanted into MHC-mismatched recipients treated with high-dose tacrolimus for 12 days. Group 1 animals (n=3) received kidney and heart allografts fully MHC-mismatched to each other and to the recipient. Group 2 animals (n=3) received kidney and heart allografts MHC-matched to each other but MHC-mismatched to the recipient. Group 3 animals (n=3) received chimeric kidney allografts whose parenchyma was MHC-mismatched to the donor heart. Group 4 animals (n=3) received chimeric kidney allografts whose passenger leukocytes were MHC-mismatched to the donor heart. Five of six heart allografts in Groups 1 and 3 rejected <40 days. In contrast, heart allografts in Groups 2 and 4 survived >150 days without rejection (p<0.05). These data demonstrate that KICAT requires MHC-matching between kidney allograft parenchyma and heart allografts, suggesting that cells intrinsic to the kidney enable cardiac allograft tolerance.
Introduction
Induction of immunologic tolerance is a long-standing goal of organ transplantation, as it avoids the toxicity and cost associated with chronic administration of immunosuppressive drugs. Some organs, such as kidney and liver, are tolerance-prone, while others, such as heart and lung, are tolerance-resistant. Using MHC-inbred miniature swine (1), we previously showed that hearts in recipients co-transplanted with a kidney allograft from the same full MHC-mismatched donor with a 12-day course of tacrolimus all developed long-term and stable tolerance of both heart and kidney allografts, whereas hearts transplanted alone rejected acutely (2).
The mechanisms underlying kidney-induced cardiac allograft tolerance (KICAT) remain unknown. Previous studies determined that the presence of a juvenile thymus (3) and a radiosensitive kidney cell population (4) appeared necessary for tolerance induction. Cells intrinsic to the kidney, such as renal tubular epithelial cells (RTECs), are susceptible to radiation injury (5,6) and can promote T cell unresponsiveness to self- and allo-antigens in mice and humans (7–14). Alternatively, cells of extra-renal origin which traffic to the kidney, such as passenger leukocytes or plasmacytoid dendritic cells (pDCs) have tolerogenic properties and have been shown to induce tolerance of organ allografts in mice (15–19).
Here, we attempt to distinguish the relative contributions of renal parenchymal cells versus passenger leukocytes to the induction of KICAT. Using kidney allografts from donors previously rendered long-term mixed chimeras by hematopoietic stem cell transplantation, we tested whether MHC-matching of donor kidney and heart parenchyma and/or of passenger leukocytes is essential for the induction of heart allograft tolerance.
Materials and Methods
Animals
Transplant donors and recipients were selected from our herd of partially inbred miniature swine (age, 3–12 months; weight, 15–60 kg). The immunogenetic characteristics of this herd have been described previously (1). In Group 1, MHCdd (class Id/IId) or MHCaa (class Ia/IIa) donor organs were transplanted into MHCcc (class Ic/IIc) recipients to achieve a 2-haplotype, full MHC class I and class II mismatch between donor heart, donor kidney, and recipient (Table 1). In Group 2, MHCdd (class Id/IId) donor organs were transplanted into MHCcc (class Ic/IIc) recipients to achieve a 2-haplotype, full MHC class I and class II mismatch between donor organs and recipient (previously published [2]). In Group 3, MHCac (class Iac/IIac) or MHCcc (class Icc/IIcc) donor hearts were transplanted into MHCad (class Iad/IIad) or MHCaa (class Iaa/IIaa) recipients to achieve a single- or 2-haplotype full MHC class I and class II mismatch, respectively, between donor heart and recipient. In Group 4, MHCad (class Iad/IIad) donor hearts were transplanted into MHCac (class Iac/IIac) recipients to achieve a single-haplotype full MHC class I and class II mismatch between donor heart and recipient. Animals in Group 3 and 4 received kidneys from long-term chimeric animals with haplotypes described below. All recipients demonstrated significant in vitro anti-donor cytotoxic activity (>20% specific lysis) before organ transplantation. All animal care and procedures were approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee and conducted in compliance with the “Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Animal Resources, National Research Council, and published by the National Academy Press.
Table 1.
Histology and survival of cardiac allografts in recipients of co-transplanted heart and kidney allografts
| MHC mismatches |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Donor |
||||||||||||||||||
| Kidney |
Heart allograft histology2 at week |
|||||||||||||||||
| Group | Animal # | Mismatched cell type1 |
Heart | Parenchyma | Passengers | Recipient | 3 | 4 | 10 | 13 | 14 | 15 | 20 | 22 | 25 | >35 | Heart graft survival (days)3 |
Kidney allograft histology4 |
| 1 | 21421 | PCs & PLs | DD | AA | AA | CC | 3R | 28 | 3 | |||||||||
| 21692 | PCs & PLs | AA | DD | DD | CC | 3R | 32 | 1 | ||||||||||
| 21737 | PCs & PLs | AA | DD | DD | CC | 2R | 3R | 35 | 3 | |||||||||
| 25 | 21019 | none | DD | DD | DD | CC | 0 | 0 | 0 | 0 | >295 | 0 | ||||||
| 21018 | none | DD | DD | DD | CC | 0 | 1R | 0 | 0 | >284 | 0 | |||||||
| 20977 | none | DD | DD | DD | CC | 0 | 0 | 0 | 0 | >272 | 0 | |||||||
| 3 | 21407 | PCs alone | AC | AD | AC | AD | 2R | 2R | 1R | 110 | 0 | |||||||
| 21419 | PCs alone | AC | AD | AC | AD | 3R | 40 | 0 | ||||||||||
| 21501 | PCs alone | CC | AA | CC | AA | 3R | 26 | 0 | ||||||||||
| 4 | 21266 | PLs alone | AD | AD | AC | AC | 0 | 0 | 0 | 0 | 0 | 0 | >2546 | 0 | ||||
| 21270 | PLs alone | AD | AD | AC | AC | 1R | 1R | 0 | 0 | >1607 | 0 | |||||||
| 21517 | PLs alone | AD | AD | AC | AC | 0 | 1R | 0 | >150 | 0 | ||||||||
Animal #21407 and #21266 received chimeric kidneys from animal #20311. Animal #21419 and #21270 received chimeric kidneys from animal #20680. Animal #21501 received a chimeric kidney from animal #20407. Animal #21517 received a chimeric kidney from animal #21557.
PC, parenchyma cells; PL, passenger cells.
Grading of acute rejection from 0 (no rejection) to 3 (severe rejection) based on ISHLT scoring system (28).
Heart graft survival is significantly different between groups 1 versus 2; 1 versus 4; and 3 versus 4 (p<0.05, two-tailed Student’s t test). Heart graft survival is not significantly different between groups 1 versus 3 and 2 versus 4 (p>0.05, two-tailed Student’s t test).
Grading of T cell-mediated rejection from 0 (no rejection) to 3 (severe rejection) based on Banff classification (29) on kidney allografts at time of cardiac allograft rejection, kidney allograft removal, or euthanasia.
Previously published results (2).
Underwent donor kidney graftectomy on POD 141. Animal was sacrificed on POD254 to obtain final pathology.
Underwent donor-matched VCA transplantation on POD 131, sacrificed on POD160 since VCA rejected.
Chimeric kidneys used in Groups 3 and 4 were harvested from MHCad (class Iad/IIad) or MHCaa (class Iaa/IIaa) animals who had undergone cytokine-mobilized hematopoietic stem cell (HSC) transplant from MHCac (class Iac/IIac) or bone marrow transplant from MHCcc (class Icc/IIcc) donor animals, respectively, at least 250 days earlier as described previously (21,22) (Table 3). A non-MHC-linked marker, pig allelic antigen (PAA), was used to distinguish between host (PAA−) and HSC donor (PAA+) cells (23). HSC engraftment was confirmed in each chimeric kidney donor by the presence of donor-derived bone marrow colony-forming units over 14 weeks following transplantation. Chimeric animals achieved high levels of donor chimerism in the lymphoid and myeloid lineages prior to kidney procurement (Table 3).
Table 3.
Characterization of chimeric kidney donors
| Peripheral blood chimerism at time of kidney harvest |
||||
|---|---|---|---|---|
| Animal # | HSC recipient MHC | HSC donor MHC1 | Lymphoid (% donor) | Myeloid (% donor) |
| 20311 | AD | AC | ≥98 | ≥98 |
| 20680 | AD | AC | ≥98 | ≥98 |
| 20407 | CC | AA | 30 | 75 |
| 21557 | AD | AC | 85 | ≥98 |
MHC, major histocompatibility complex; pSCF, porcine stem cell factor; HSC, hematopoietic stem cells; HCT, hematopoietic cell transplantation; VCA, vascularized composite allograft; DLI, donor leukocyte infusion; BMT, bone marrow transplantation.
The source of HSC donor cells for all chimeric donors except for #20407 was pIL-3/pSCF-mobilized HSC. Animal #20407 received HSC cells from bone marrow.
Surgical procedures
The surgical procedures used for combined heart/kidney transplantation have been described previously (24–26). Briefly, the recipients underwent bilateral nephrectomies. The aorta and inferior vena cava were used for end-to-side arterial and venous anastomoses for both the heart and kidney, with the heart placed at least 1 cm caudad to the kidney. The kidney transplantation was completed by performing a vesicoureteral anastomosis. Two indwelling silastic central venous catheters were placed surgically into the external and internal jugular veins. The catheters facilitated tacrolimus administration and frequent blood sampling for in vitro assays and for monitoring of renal function and whole blood tacrolimus levels.
Kidney allograft graftectomy was performed in one long-term tolerant animal (#21266) along with the transplantation of a recipent-matched (MHCac) kidney to provide ongoing renal function.
A vascularized composite allograft (VCA) from a donor MHC-matched to the donor heart was transplanted into one long-term tolerant animal (#21270). The VCA, composed of skin, subcutaneous tissue, muscle and its vascular pedicle (femoral artery and femoral vein) was procured from an MHCad (class Iad/IIad) animal and anastomosed to the recipient’s internal jugular vein and internal carotid artery. The animal did not receive immunosuppression post-operatively.
Rejection monitoring
Kidney function was monitored by serial serum creatinine levels. Renal allograft rejection was defined as sustained rise in serum creatinine to >10 mg/dL and/or uremia. Heart function was monitored by daily palpation and electrocardiogram (ECG) using the AliveCor Veterinary Heart Monitor (AliveCor, Inc., San Francisco, CA). Cardiac allograft rejection was defined by either loss of a ventricular impulse on palpation, and/or QRS-wave amplitude of less than 0.3 mV, and/or the lack of ventricular contraction on echocardiography (27). The VCA was monitored for viability by checking capillary refill and monitored for rejection by visual inspection and serial biopsies. VCA rejection was defined as the point at which the skin became necrotic and was confirmed by biopsy.
Routine biopsies were performed on all transplant recipients at predetermined time intervals (POD 20–30, 50–60, 90–100) or if clinical suspicion for rejection arose. Allograft rejection was confirmed histologically in all cases.
Immunosuppression
Tacrolimus (Haorui Pharma-Chem Inc., Irvine, CA) was mixed and administered as an intravenous suspension and given as a continuous infusion at a dose of 0.08–0.20 mg/kg (adjusted to maintain a whole blood level of 30–50 ng/mL) for 12 consecutive days, starting on the day of transplantation (day 0).
Pathology studies
Core needle biopsies were performed on cardiac allografts. Wedge biopsies were performed on kidney allografts. Kidney biopsies were taken at the same time as the heart samples. 6mm punch biopsies and wedge biopsies were performed on the VCA graft. Tissue was fixed in formalin and embedded in paraffin for routine light microscopy (H&E, PAS). Separate portions were frozen for immunofluorescence and immunohistochemical studies. Scoring of rejection was performed without knowledge of the functional status of the graft based on the International Society for Heart and Lung Transplantation System for hearts (28) and the current Banff consensus criteria for kidney (29) and VCA grafts (30).
For assessing tissue chimerism by immunohistochemistry, frozen tissue sections of the chimeric and control kidneys were stained with antibodies to MHC Class Ic (Class Ic, IgM clone: 16.7.E4.2). For assessing tissue chimerism by immunofluorescence, frozen tissue sections of the chimeric and control kidneys were stained with antibodies to porcine allelic antigen (PAA, IgM clone: 1038H-10–9 [23]) detected with goat-anti mouse IgM Alexa Fluor 488 (Invitrogen, USA) and MHC-DR (biotinylated, clone: TH16) detected with Streptavidin Alexa Fluor 594 (Invitrogen, USA). All images were evaluated with a Zeiss A1 Axio Scope Fluorescent Microscope at 100X and merged with ImageJ (Version 1.47, NIH).
Preparation of peripheral blood leukocytes
Freshly heparinized whole blood was diluted approximately 1:2 with HBSS (Gibco BRL, Grand Island, NY), and the mononuclear cells were obtained by means of gradient centrifugation with Histopaque (Sigma, St. Louis, MO). The mononuclear cells were washed once with HBSS, and contaminating red cells were lysed with ammonium chloride potassium lysing buffer (Bio Whittaker, Inc, Walkersville, MD). Cells were then washed with HBSS and resuspended in tissue culture medium. All cell suspensions were kept at 4°CC until used in cellular assays.
Cell-mediated lymphocytotoxicity assay
Cell-mediated lymphocytotoxicity (CML) assays with porcine cells have been described previously (31). Briefly, lymphocyte cultures containing 4×106/mL responder and 4×106/mL stimulator PBMCs (irradiated with 2500 cGy) were incubated for 6 days at 37°C in 5% carbon dioxide and 100% humidity in CML media. Bulk cultures were harvested, and effectors were tested for cytotoxic activity on chromium 51–labeled (Amersham, Arlington Heights, IL) lymphoblast targets generated from phytohemagglutinin (M-form; Life Technologies, Gaithersburg, MD) stimulation. Effector cells were incubated for 5.5 h with target cells at effector/target ratios of 100:1, 50:1, 25:1, and 12.5:1. Two target cells were tested in each assay: (1) PBMCs MHC-matched to the donor and (2) third-party PBMCs. Supernatants were then harvested by using the Skatron collection system (Skatron, Sterling, VA), and 51Cr release was determined on a gamma counter (Micromedics, Huntsville, AL). The results were expressed as a percentage of specific lysis and calculated as follows:
Percentage of specific lysis=((Experimental release [cpm] − Spontaneous release [cpm])/(Maximum release [cpm] − Spontaneous release [cpm]))×100
Mixed lymphocyte reaction assay
Mixed lymphocyte reaction (MLR) assays with porcine cells have been described previously (31). Briefly, cultures containing 4×106 responder and 4×106 irradiated (2500 cGy) stimulator PBMCs were incubated in 200 uL of media in 96-well flat-bottomed plates (Costar Corning; Lowell, MA, USA) for 5 days at 37°C in 5%CO2 and 100% humidity. After the 5-day incubation, 1 uCi of [3H]-thymidine was added to each well, followed by an additional 5-h incubation under the same conditions. [3H]-thymidine incorporation was determined in triplicate samples by beta-scintillation counting. Absolute counts were compensated for background and then expressed as stimulation indices (SI), calculated as SI=average counts per minute for a responder– stimulator pair per c.p.m. of the same responder stimulated by an autologous stimulator.
Assessment of alloantibody
The presence of anti-donor immunoglobulin (IgM and IgG) in the serum of experimental swine was examined by indirect flow cytometry using a Becton Dickinson FACScalibur (Sunnyvale, CA) to determine the MHC-binding specificity of the antibody. FITC-labeled goat anti-swine IgM or IgG polyclonal antibodies were used as secondary reagents (Kirkegaard & Perry Laboratories Inc, Gaithersburg, MD). For staining, 1×106 cells per tube of donor-type PBLs (MHCaa, MHCdd, MHCac, MHCcc, or MHCad) were resuspended in 100 uL HBSS containing 0.1% bovine serum albumin and 0.05% NaN3 and incubated for 30 min at 4°C with 10 uL decomplemented test sera (neat). After two washes, a saturating concentration of FITC-labeled goat anti-swine IgM or IgG was added and incubated for 30 min at 4°C. After a final wash, cells were analyzed by means of flow cytometry with propidium iodide gating to exclude dead cells. Both normal pig serum and pretransplant sera from each experimental animal were used as controls for specific binding.
The presence of cytotoxic antibodies to cell surface antigens in the serum of experimental swine was determined using an antibody-complement reaction, followed by a dye-exclusion assay, and flow cytometry to determine the amount of target cell lysis. Briefly, sera of experimental animals were serially diluted with cytotoxicity media consisting of 100 mL of Media 99 (Mediatech, Manassas, VA, USA) and 2 mL of decomplemented fetal bovine serum in a 96-well plate format. 1.25×105 target cells (MHCaa, MHCcc, MHCdd) were added to each well and incubated for 15 min at 37°C. The cells were washed with cytotoxicity media before adding rabbit complement (Rogers, Arkansas, USA) and incubating for 30 min at 37°C. Prior to acquisition, 7-Aminoactinomycin D (7-AAD), a fluorescent dye that intercalates into double-stranded nucleic acids and is excluded by viable cells, was added to each sample to detect lysed cells.
Statistical analysis
Graft survival times were compared using a two-tailed Student’s t test. Differences in graft survival time were deemed significant when p<0.05.
Results
KICAT required MHC-matching between heart and kidney allografts
To determine whether MHC-matching between donor heart and donor kidney allografts is necessary for KICAT, three MHCcc recipients received heart and kidney grafts from different donors that were fully MHC-mismatched to each other and to the recipient (MHCaa or MHCdd) (Table 1, Group 1). All three recipients rejected their heart allografts by POD 35 (Table 1, Figure 1a–b). In contrast, as we showed previously, recipients that received heart and kidney allografts that were MHC-matched (from the same donor) developed long-term, stable tolerance with indefinite graft survival (Table 1, Group 2) (2).
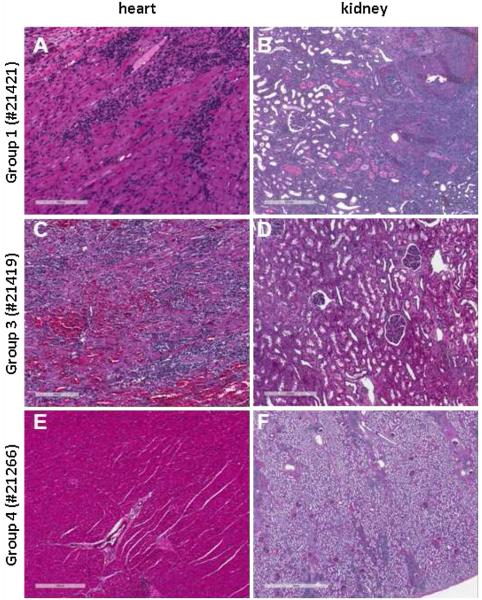
Figure 1. Histology from representative heart and kidney biopsies.
(A) POD31 biopsies on Group 1 animal #21421 showing acute rejection in the heart (ISHLT 3 R), manifested by a diffuse mononuclear infiltrate associated with myocyte necrosis. (B) The kidney biopsy shows acute T cell-mediated rejection with a diffuse mononuclear infiltrate and fibrinoid necrosis of arteries (Banff type 3). (C and D) POD40 biopsies on Group 3 animal #21419 showing acute rejection in the heart (ISHLT 3R) but no rejection in the kidney. (E) POD256 biopsy on Group 4 animal #21266 showing normal myocardium and vessels in the heart (ISHLT 0). (F) POD141 kidney biopsy on #21266 at time of kidney allograftectomy showing a nodular mononuclear infiltrate associated with the arterial tree and mild interstitial fibrosis, without evidence of acute rejection.
Two recipients in Group 1 also showed severe diffuse cellular infiltrate with endarteritis typical of acute cellular rejection on histological analysis of kidney allografts at time of sacrifice (#21421 and #21737, Table 1). Two animals in Group 1 (#21421, #21692) had an initial rise in serum creatinine by POD6; at time of rejection, animal #21421’s creatinine was >20 mg/dL.
Serial CML and MLR assays were performed to assess immune competence in recipients of heart and kidney allografts. The results of the CML and MLR assays were variable (Table 2). In the first animal (#21421), CML and MLR assays showed evidence of donor-responsiveness at the time of rejection (Table 2) and developed specific cytotoxic alloantibody towards MHCaa by POD30 (Figure 2). In the second animal (#21692), MLR showed responsiveness towards heart MHCaa but CML was unresponsive. In the third animal (#21737), CML showed responsiveness towards kidney MHCdd but MLR was unresponsive. Animals #21692 and #21737 did not develop detectable circulating alloantibody (data not shown).
Table 2.
Combined CML and MLR data for heart/kidney recipients
| MHC mismatches |
CML response2 |
MLR response2 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Donor |
||||||||||||
| Kidney |
||||||||||||
| Group | Animal # | Mismatched cell type1 |
Heart | Parenchyma | Passengers | Recipient | Pre-tx | POD25-35 | Rejection/ sacrifice |
Pre-tx | POD25-35 | Rejection/ sacrifice |
| 13 | 21421 | PCs & PLs | DD | AA | AA | CC | +DD/+AA | +DD/−AA | +DD/−AA | +DD/+AA | −DD/+AA | −DD/+AA |
| 21692 | PCs & PLs | AA | DD | DD | CC | +AA/−DD | −AA/−DD | −AA/−DD | +AA/+DD | +AA/−DD | +AA/−DD | |
| 21737 | PCs & PLs | AA | DD | DD | CC | −AA/+DD | −AA/+DD | −AA/+DD | +AA/+DD | −AA/−DD | −AA/−DD | |
| 24 | 21019 | none | DD | DD | DD | CC | +DD | −DD | −DD | +DD | −DD | −DD |
| 21018 | none | DD | DD | DD | CC | +DD | −DD | −DD | +DD | −DD | −DD | |
| 20977 | none | DD | DD | DD | CC | +DD | −DD | −DD | +DD | −DD | −DD | |
| 3 | 21407 | PCs alone | AC | AD | AC | AD | +AC | −AC | +AC | +AC | +AC | −AC |
| 21419 | PCs alone | AC | AD | AC | AD | +AC | −AC | +AC | +AC | −AC | +AC | |
| 21501 | PCs alone | CC | AA | CC | AA | +CC | −CC | −CC | +CC | −CC | −CC | |
| 4 | 21266 | PLs alone | AD | AD | AC | AC | +AD | −AD | −AD5 | +AD | −AD | −AD5 |
| 21270 | PLs alone | AD | AD | AC | AC | +AD | −AD | +AD | +AD | −AD | −AD | |
| 21517 | PLs alone | AD | AD | AC | AC | +AD | −AD | −AD | +AD | −AD | −AD | |
PC, parenchyma cells; PL, passenger cells.
In vitro state of donor-specific responsiveness (+) or donor-specific unresponsiveness (−) at time before transplant, days 25-35 after transplant, and at time of heart allograft rejection or animal sacrifice.
In Group 1 animals who received MHC-disparate heart and kidneys, the response against a particular MHC haplotype (DD vs AA) is indicated.
Previously published results (2).
Animal 21266 showed in vitro evidence of tolerance at POD97; 110 days after kidney graftectomy, the animal showed positive donor response on CML and MLR despite no evidence of cardiac allograft rejection.
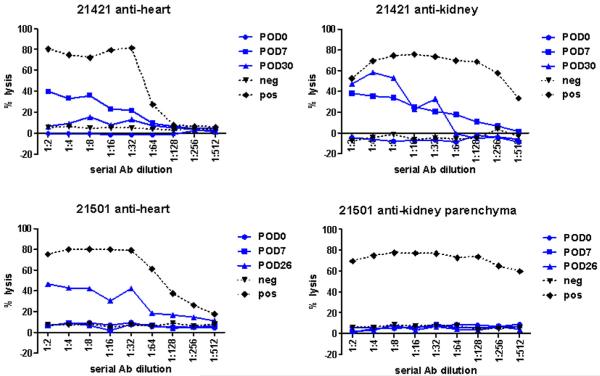
Figure 2. Alloantibody response.
Levels of circulating cytotoxic alloantibody against MHCaa, MHCcc, and MHCdd target cells were measured by flow cytometry in recipients in Groups 1, 3, and 4. Only two animals (Group 1 #21421 and Group 3 #21501) were found to have detectable levels of cytotoxic alloantibody against heart and/or kidney grafts.
Kidneys harvested from chimeric animals contained a mixture of donor- and recipient-derived passenger leukocytes
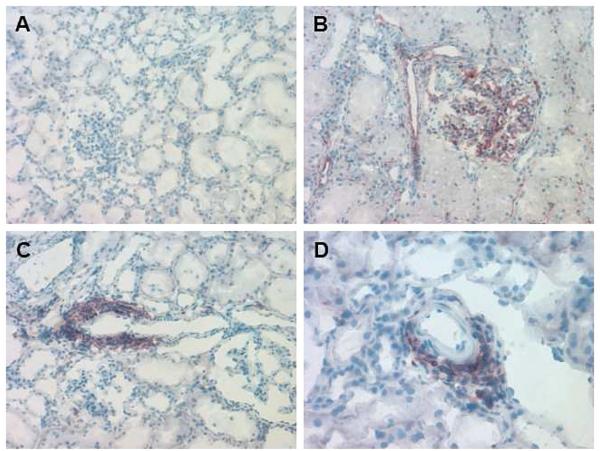
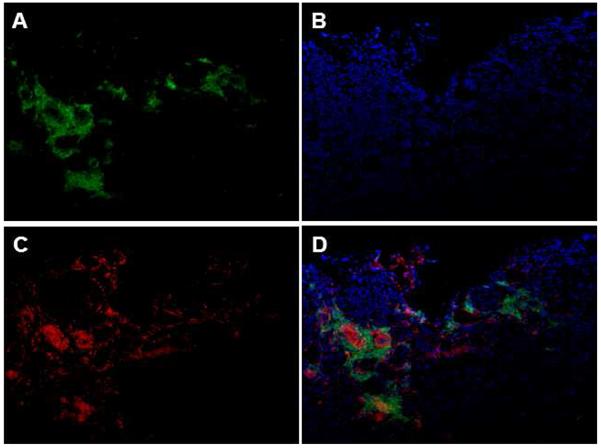
At the time of kidney procurement, the peripheral blood of the chimeric organ donors exhibited high levels of lymphoid and myeloid chimerism (Table 3). Immunofluorescence and immunohistochemical analyses of representative kidney allograft biopsy specimens that were obtained at the time of organ procurement were performed to confirm the presence of HSC donor- versus HSC recipient-derived passenger leukocytes that were present within the donor kidney. As shown in Figures 3 and 4, donor kidneys exhibited a large number of graft-infiltrating leukocytes derived from donor HSC, confirming the chimeric nature of the donor kidney.
Figure 3. Immunohistochemical staining with Class Ic antibody to detect donor kidney chimerism prior to implantation.
(A) MHCdd kidney at 40x used as negative control showing no Class Ic staining. (B) MHCcc kidney at 40x used as positive control showing positive staining predominantly of the endothelium (arteries, glomeruli, capillaries). (C) Representative donor kidney (animal #21557) at 40x showing perivascular aggregates positive for Class Ic staining. (D) Perivascular aggregate from representative animal #21557 at 100x.
Figure 4. Immunofluorescence staining of representative donor kidney (#20311) at 100x showing donor chimerism before implantation.
(A) PAA+ (donor) cells in green. (B) DAPI staining in blue. (C) MHC-DR+ cells in red. (D) Images merged.
Co-transplantation of chimeric kidney allografts whose passenger leukocytes were MHC-matched, but whose parenchymal cells were MHC-mismatched to the donor heart resulted in cardiac allograft rejection. To determine whether MHC-matching between donor heart and donor kidney parenchyma was necessary for the establishment of KICAT, three recipients received heart and kidney grafts whose parenchymal cells were MHC-mismatched but whose passenger leukocytes were MHC-matched (Table 1, Group 3). MHCad recipients #21407 and #21419 received a single-haplotype full-mismatch heart (MHCac) along with a chimeric kidney whose parenchyma expressed MHCad antigens but whose passenger leukocytes expressed MHCac antigens. Similarly, MHCaa recipient #21501 received a two-haplotype full-mismatch heart (MHCcc) along with a chimeric kidney whose parenchyma expressed MHCaa antigens but whose passenger leukocytes expressed MHCcc antigens. All animals in Group 3 rejected their heart allografts within 110 days; two animals rejected their heart allografts within 40 days (Table 1, Figure 1c–d). Creatinine remained normal in Group 3 recipients except for #21501, whose creatinine rose to 5.3 at time of cardiac allograft rejection on POD26. However, none of the renal allografts in Group 3 recipients showed histologic evidence of acute rejection (minimal infiltrates, <5% of the renal cortex, Table 1); the kidney from #21501 had acute tubular injury and mild fibrosis. Serial CML assays for all animals in Group 3 demonstrated loss of donor-specific responsiveness by POD30 (Table 2); 2 of 3 animals demonstrated loss of donor-specific responsiveness by MLR assay by POD30. Two animals (#21407 and #21419) regained donor-responsiveness by CML or MLR at time of cardiac allograft rejection (Table 2). Animal #21501 showed detectable levels of cytotoxic alloantibody by POD26 (Figure 2); animals #21419 and #21407 did not develop detectable serum alloantibody (data not shown).
Of note, to control for inter-animal clinical variability that could affect the nature of the chimeric donor kidney, chimeric animal #20680 donated kidneys to animals in Groups 3 and 4 (#21419 and #21270, respectively) and chimeric animal #20311 donated kidneys to animals in Groups 3 and 4 (#21407 and #21266, respectively).
Co-transplantation of chimeric kidney allografts whose parenchymal cells were MHC-matched, but whose passenger leukocytes were MHC-mismatched to the donor heart, resulted in long-term tolerance of the cardiac allografts. To determine whether MHC-matching between donor heart and donor kidney passenger leukocytes was necessary for the induction of KICAT, three recipients received heart and kidney grafts whose passenger leukocytes were MHC-mismatched but whose parenchymal cells were MHC-matched (Table 1, Group 4). MHCac recipients received a single-haplotype full-mismatch heart (MHCad) along with a chimeric kidney whose parenchyma expressed MHCad antigens but whose passenger leukocytes expressed MHCac antigens. All animals in Group 4 accepted both organs for over 150 days (Table 1). The cardiac allografts maintained strong contractions and exhibited no significant rejection or CAV (Table 1, Fig 1e–f). The creatinine levels in Group 4 recipients rose to between 4.0 and 7.0 mg/dL on POD 4–6 before returning to baseline without intervention by POD10–12.
In one recipient (#21266), donor kidney graftectomy was performed on POD141 with transplantation of self-matched kidney (MHCac) to maintain renal function. Loss of the original donor kidney did not affect cardiac allograft survival as it remained free of rejection, despite the fact that in vitro assays demonstrated return of anti-donor responsiveness. Indeed, serial CML and MLR assays for all animals in Group 4 demonstrated loss of donor-specific responsiveness by POD 30 (Table 2). However, recipient #21266 regained donor-specific responsiveness by postoperative day 33 after kidney graftectomy (data not shown) and recipient #21270 regained donor-specific responsiveness 60 days after initial heart/kidney transplantation by CML but not MLR. None of the animals in Group 4 showed presence of specific cytotoxic alloantibody (data not shown).
Transplantation of a donor-matched vascularized composite allograft (VCA) without immunosuppression did not break KICAT
To test the robustness of KICAT, a donor-matched (MHCad) VCA was transplanted onto animal #21270, a long-term tolerant Group 4 recipient, 131 days after the initial heart/ kidney transplantation (Table 1). The VCA showed visual and histologic signs of rejection by POD14. However, despite VCA rejection, tolerance of the cardiac allograft was maintained as evidenced by lack of rejection on heart biopsy specimens obtained 29 days after VCA transplantation.
Discussion
In this study, we aimed to determine whether an itinerant (passenger leukocyte) or a resident (parenchyma) kidney cell population was necessary for the establishment of kidney-induced cardiac allograft tolerance. We found that KICAT was not induced when the passenger leukocytes of the donor kidney and heart were MHC-matched and the parenchymal cells were MHC-mismatched. In contrast, when the donor kidney and heart parenchymal cells were both MHC-matched, robust and long-term tolerance was established even though the passenger leukocytes were MHC-mismatched (Group 3 versus Group 4 heart graft survival, p<0.05). The results of our study suggest that cells intrinsic to the kidney, such as RTECs or endothelial cells, rather than a migratory population play a dominant role in KICAT.
The competing roles that parenchyma cells versus passenger leukocytes play in tolerance induction and maintenance have been debated extensively. Liver allografts, which have a heavy passenger leukocyte burden, are tolerized via mechanisms that implicate both parenchyma and passenger leukocytes, as shown in rodent and swine models (33–36). The classical experiments of Lechler and Batchelor demonstrated that passenger allogenic dendritic cells were the most immunogenic cells in rat kidney grafts (32). Our study indicates that the parenchymal cells may be the more tolerogenic cells in kidney grafts, modulating the alloreactive T cell response through local and systemic regulation.
Parenchymal cells harbor several qualities that could promote regulation and induce allospecific tolerance. First, RTECs may induce regulatory tolerance via TGFβ-mediated production of Foxp3+ regulatory T cells (7;33;34), which are enriched in the tubules of human renal allografts (35). Second, the kidney endothelium may establish a niche for local immune regulation by the IFNγ-dependent expression of indoleamine 2, 3-dioxygenase, bringing together effector T cells and antigen-presenting cells (36). Indeed, antigen presentation by parenchymal cells, which lack expression of B7 co-stimulatory molecules but express MHC class II molecules when activated, can negate the T effector cell response (37). Third, kidney parenchyma may facilitate the formation of “Treg-rich organized lymphoid structures” (TOLS), defined as nodular infiltrates rich in Foxp3 cells associated with the vasculature of the kidney (38). TOLS were found in kidneys across all experimental groups, though they were relatively sparse in Group 3 recipients. T regulatory cells generated by the kidney parenchyma directly or indirectly could migrate to the heart allograft and shift the balance from rejection to tolerance induction.
While our data demonstrate that allogeneic parenchymal cells may be more important in KICAT than allogeneic passenger cells, they do not negate an important role for host (i.e. recipient) regulatory cells, which may be induced primarily in the kidney and then either migrate to the donor heart or induce a systemic effect on the donor heart. In addition, an alternate explanation of our findings could be that allogeneic passenger cells stimulate alloreactivity by priming the recipient’s immune system; thus, when passenger cells are mismatched to the recipient (Table 1, Group 3), heart rejection ensues whereas when passenger cells are matched to the recipient (Table 1, Group 4), the heart is accepted.
Of note, induction of tolerance to the kidney was disrupted in 2 of 3 animals that received donor heart and kidney grafts that were fully MHC-mismatched to each other (#21421, #21737, Group 1). This was unexpected, because previous work demonstrates that all kidneys alone transplanted across a full MHC mismatch with 12-day course of tacrolimus develop long-term tolerance (39). In the current study, it is possible that the inflammatory state created by heart rejection and the resultant increase in host T-cell activation and proliferation interfered with the “acceptance reaction” that would have otherwise occurred during kidney-enabled tolerance induction (40). Although recipient #21692 in Group 1 showed ACR1 rather than ACR3 in the kidney at the time of heart rejection, with time, the kidney may have progressed to florid rejection.
We tested the strength of the tolerant state induced by KICAT by placing a donor-matched VCA challenge graft on one long-term tolerant heart/kidney recipient (#21270). Although the VCA was rejected, the heart allograft was unaffected, demonstrating the organ-specific robustness of KICAT. Of note, the antigenicity of VCAs, particularly the epidermal skin layer, presents a particular challenge for tolerance induction protocols (41–43).
In conclusion, our data suggest that the mechanism underlying KICAT is dependent upon cells intrinsic to the kidney graft, such as RTECs or endothelial cells, and that the kidney component responsible must be MHC-matched to the donor heart to exert this protective effect. We intend to use these data to further identify and characterize the renal cell type or cell product responsible for inducing tolerance of a co-transplanted organ. If that cell or cell product could be isolated and incorporated into a clinically applicable protocol, tolerance of heart allografts and other tolerance-resistant organs and tissues might be achieved, without the need for whole donor kidney co-transplantation.
Acknowledgments
We acknowledge C06RR020135-01 for construction of the facility utilized for production and maintenance of miniature swine and Novartis for the generous supply of cyclosporine used in preparation of chimeric donors. We are indebted to Mr. J. Scott Arn for management of the swine herd. We thank Nicole Brousaides for preparing biopsy samples for histological analysis. This work was supported in part by grants from the National Heart, Lung, and Blood Institute (P01HL18646) and the National Institute of Allergy and Infectious Disease (R01AI84657) of the National Institutes of Health. Dr. Madariaga is an Edward D. Churchill Surgical Research Fellow, Massachusetts General Hospital and a recipient of a fellowship from the International Society for Heart and Lung Transplantation and a National Research Service Award from the National Heart, Lung, and Blood Institute of the National Institutes of Health (F32HL117540). Dr. Michel is a recipient of the 2013 ASTS-Novartis Scientist Scholarship Grant. Dr. Leonard is a recipient of an AST-Novartis Scientist Grant.
Abbreviations
- ACR
acute cellular rejection
- CAV
cardiac allograft vasculopathy
- CML
cell-mediated lympholysis
- CsA
cyclosporine
- HSC
hematopoietic stem cell
- KICAT
kidney-induced cardiac allograft tolerance
- MHC
major histocompatibility complex
- MLR
mixed lymphocyte reaction
- PBMC
peripheral blood mononuclear cells
- pDCs
plasmacytoid dendritic cells
- POD
postoperative day
- PSL
percent specific lysis
- RTECs
renal tubular epithelial cells
- VCA
vascularized composite allograft
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Sachs DH, Leight G, Cone J, Schwarz S, Stuart L, Rosenberg S. Transplantation in miniature swine. I. Fixation of the major histocompatibility complex. Transplantation. 1976;22:559–567. doi: 10.1097/00007890-197612000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Madariaga ML, Michel SG, Tasaki M, et al. Induction of cardiac allograft tolerance across a full MHC barrier in miniature sqine by donor kidney co-transplantation. Am J Transplant. 2013;13:2558–2566. doi: 10.1111/ajt.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamada K, Choo JK, Allan JS, et al. The effect of thymectomy on tolerance induction and cardiac allograft vasculopathy in a miniature swine heart/kidney transplantation model. Transplantation. 1999;68:485–491. doi: 10.1097/00007890-199908270-00007. [DOI] [PubMed] [Google Scholar]
- 4.Mezrich JD, Yamada K, Lee RS, et al. Induction of tolerance to heart transplants by simultaneous cotransplantation of donor kidneys may depend on a radiation-sensitive renal-cell population. Transplantation. 2003;76:625–631. doi: 10.1097/01.TP.0000079926.80833.42. [DOI] [PubMed] [Google Scholar]
- 5.Williams MV. The cellular basis of renal injury by radiation. Br J Cancer Suppl. 1986;7:257–264. [PMC free article] [PubMed] [Google Scholar]
- 6.Withers HR, Mason KA, Thames HD., Jr Late radiation response of kidney assayed by tubule-cell survival. Br J Radiol. 1986;59:587–595. doi: 10.1259/0007-1285-59-702-587. [DOI] [PubMed] [Google Scholar]
- 7.Frasca L, Marelli-Berg F, Imami N, et al. Interferon-gamma-treated renal tubular epithelial cells induce allospecific tolerance. Kidney Int. 1998;53:679–689. doi: 10.1046/j.1523-1755.1998.00800.x. [DOI] [PubMed] [Google Scholar]
- 8.Singer GG, Yokoyama H, Bloom RD, Jevnikar AM, Nabavi N, Kelley VR. Stimulated renal tubular epithelial cells induce anergy in CD4+ T cells. Kidney Int. 1993;44:1030–1035. doi: 10.1038/ki.1993.345. [DOI] [PubMed] [Google Scholar]
- 9.Neilson EG. Is immunologic tolerance of self modulated through antigen presentation by parenchymal epithelium? Kidney Int. 1993;44:927–931. doi: 10.1038/ki.1993.333. [DOI] [PubMed] [Google Scholar]
- 10.Kirby JA, Rajasekar MR, Lin Y, Proud G, Taylor RM. Interaction between T lymphocytes and kidney epithelial cells during renal allograft rejection. Kidney Int Suppl. 1993;39:S124–S128. [PubMed] [Google Scholar]
- 11.Hagerty DT, Allen PM. Processing and presentation of self and foreign antigens by the renal proximal tubule. J Immunol. 1992;148:2324–2330. [PubMed] [Google Scholar]
- 12.Hadley GA, Rostapshova EA, Bartlett ST. Dominance of tissue-restricted cytotoxic T lymphocytes in the response to allogeneic renal epithelial cell lines. Transplantation. 1996;62:75–83. doi: 10.1097/00007890-199607150-00016. [DOI] [PubMed] [Google Scholar]
- 13.Deckers JGM, Boonstr JG, Van der Kooij SW, Daha MR, van der Woude FJ. Tissue-specific characteristics of cytotoxic graft-infiltrating T cells during renal allograft rejection. Transplantation. 1997;64:178–181. doi: 10.1097/00007890-199707150-00034. [DOI] [PubMed] [Google Scholar]
- 14.Schoop R, Wahl P, Le HM, Heeman U, Wang M, Wuthrich RP. Suppressed T-cell activation by IFN-gamma-induced expression of PD-L1 on renal tubular epithelial cells. Nephrol Dial Transplant. 2004;19:2713–2720. doi: 10.1093/ndt/gfh423. [DOI] [PubMed] [Google Scholar]
- 15.Abe M, Wang Z, de CA, Thomson AW. Plasmacytoid dendritic cell precursors induce allogeneic T-cell hyporesponsiveness and prolong heart graft survival. Am J Transplant. 2005;5:1808–1819. doi: 10.1111/j.1600-6143.2005.00954.x. [DOI] [PubMed] [Google Scholar]
- 16.Heitger A. Regulation of expression and function of IDO in human dendritic cells. Curr Med Chem. 2011;18:2222–2233. doi: 10.2174/092986711795656018. [DOI] [PubMed] [Google Scholar]
- 17.Pallotta MT, Orabona C, Volpi C, et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol. 2011;12:870–878. doi: 10.1038/ni.2077. [DOI] [PubMed] [Google Scholar]
- 18.Hadeiba H, Lahl K, Edalati A, et al. Plasmacytoid dendritic cells transport peripheral antigens to the thymus to promote central tolerance. Immunity. 2012;36:438–450. doi: 10.1016/j.immuni.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ochando JC, Homma C, Yang Y, et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7:652–662. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 20.Sachs DH, Leight G, Cone J, Schwartz S, Stuart L, Rosenberg S. Transplantation in miniature swine. I. Fixation of the major histocompatibility complex. Transplantation. 1976;22:559–567. doi: 10.1097/00007890-197612000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Cina RA, Wikiel KJ, Lee PW, et al. Stable multilineage chimerism without graft versus host disease following nonmyeloablative haploidentical hematopoietic cell transplantation. Transplantation. 2006;81:1677–1685. doi: 10.1097/01.tp.0000226061.59196.84. [DOI] [PubMed] [Google Scholar]
- 22.Leonard DA, Kurtz JM, Mallard C, et al. Vascularized composite allograft tolerance across MHC barriers in a large animal model. Am J Transplant. 2014;14:343–355. doi: 10.1111/ajt.12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuchimoto Y, Huang C, Shimizu A, Seebach J, Arn S, Sachs DH. An allelic non-histocompatibility antigen with wide tissue distribution as a marker for chimerism in pigs. Tissue Antigens. 1999;54:43–52. doi: 10.1034/j.1399-0039.1999.540105.x. [DOI] [PubMed] [Google Scholar]
- 24.Madsen JC, Yamada K, Allan JS, et al. Transplantation tolerance prevents cardiac allograft vasculopathy in major histocompatibility complex class I-disparate miniature swine. Transplantation. 1998;65:304–313. doi: 10.1097/00007890-199802150-00002. [DOI] [PubMed] [Google Scholar]
- 25.Madsen JC, Sachs DH, Fallon JT, Weissman NJ. Cardiac allograft vasculopathy in partially inbred miniature swine. I. Time course, pathology, and dependence on immune mechanisms. J Thorac Cardiovasc Surg. 1996;111:1230–1239. doi: 10.1016/s0022-5223(96)70226-x. [DOI] [PubMed] [Google Scholar]
- 26.Kirkman RL, Colvin MW, Flye GS, et al. Transplantation in miniature swine. VI. Factors influencing survival of renal allografts. Transplantation. 1979;28:18–23. [PubMed] [Google Scholar]
- 27.Avitall B, Payne DD, Connolly RJ, et al. Heterotopic heart transplantation: Electrophysiologic changes during acute rejection. J Heart Transplant. 1988;7:176–182. [PubMed] [Google Scholar]
- 28.Mehra MR, Crespo-Leiro MG, Dipchand A, et al. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy-2010. J Heart Lung Transplant. 2010;29:717–727. doi: 10.1016/j.healun.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 29.Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, et al. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14:272–283. doi: 10.1111/ajt.12590. [DOI] [PubMed] [Google Scholar]
- 30.Cendales LC, Kanitakis J, Schneeberger S, et al. The Banff 2007 working classification of skin-containing composite tissue allograft pathology. Am J Transplant. 2008;8:1396–1400. doi: 10.1111/j.1600-6143.2008.02243.x. [DOI] [PubMed] [Google Scholar]
- 31.Kirkman RL, Colvin RB, Flye MW, Williams GM, Sachs DH. Transplantation in miniature swine. VII. Evidence for cellular immune mechanisms in hyperacute rejection of renal allografts. Transplantation. 1979;28:24–30. [PubMed] [Google Scholar]
- 32.Lechler RI, Batchelor JR. Restoration of immunogenicity to passenger cell-depleted kidney allografts by the addition of donor strain dendritic cells. J Exp Med. 1982;155:31–41. doi: 10.1084/jem.155.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robertson H, Wong WK, Talbot D, Burt AD, Kirby JA. Tubulitis after renal transplantation: Demonstration of an association between CD103+ T cells, transforming growth factor beta1 expression and rejection grade. Transplantation. 2001;71:306–313. doi: 10.1097/00007890-200101270-00024. [DOI] [PubMed] [Google Scholar]
- 34.Zheng SG. The Critical Role of TGF-beta1 in the Development of Induced Foxp3+ Regulatory T Cells. Int J Clin Exp Med. 2008;1:192–202. [PMC free article] [PubMed] [Google Scholar]
- 35.Veronese F, Rotman S, Smith RN, et al. Pathological and clinical correlates of FOXP3+ cells in renal allografts during acute rejection. Am J Transplant. 2007;7:914–922. doi: 10.1111/j.1600-6143.2006.01704.x. [DOI] [PubMed] [Google Scholar]
- 36.Thebault P, Condamine T, Heslan M, et al. Role of IFNgamma in allograft tolerance mediated by CD4 +CD25+ regulatory T cells by induction of IDO in endothelial cells. Am J Transplant. 2007;7:2472–2482. doi: 10.1111/j.1600-6143.2007.01960.x. [DOI] [PubMed] [Google Scholar]
- 37.Marelli-Berg FM, Lechler RI. Antigen presentation by parenchymal cells: A route to peripheral tolerance? Immunol Rev. 1999;172:297–314. doi: 10.1111/j.1600-065x.1999.tb01374.x. [DOI] [PubMed] [Google Scholar]
- 38.Miyajima M, Chase CM, Alessandrini A, et al. Early acceptance of renal allografts in mice is dependent on foxp3(+) cells. Am J Pathol. 2011;178:1635–1645. doi: 10.1016/j.ajpath.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Utsugi R, Barth RN, Lee RS, et al. Induction of transplantation tolerance with a short course of tacrolimus (FK506): I. Rapid and stable tolerance to two-haplotype fully mhc-mismatched kidney allografts in miniature swine. Transplantation. 2001;71:1368–1379. doi: 10.1097/00007890-200105270-00003. [DOI] [PubMed] [Google Scholar]
- 40.Shimizu A, Yamada K, Meehan SM, Sachs DH, Colvin RB. Acceptance reaction: Intragraft events associated with tolerance to renal allografts in miniature swine. J Am Soc Nephrol. 2000;11:2371–2380. doi: 10.1681/ASN.V11122371. [DOI] [PubMed] [Google Scholar]
- 41.Berli JU, Broyles JM, Lough D, et al. Current concepts and systematic review of vascularized composite allotransplantation of the abdominal wall. Clin Transplant. 2013;27:781–789. doi: 10.1111/ctr.12243. [DOI] [PubMed] [Google Scholar]
- 42.Horner BM, Randolph MA, Huang CA, Butler PE. Skin tolerance: In search of the Holy Grail. Transpl Int. 2008;21:101–112. doi: 10.1111/j.1432-2277.2007.00559.x. [DOI] [PubMed] [Google Scholar]
- 43.Leonard DA, Kurtz JM, Cetrulo CL., Jr Vascularized composite allotransplantation: towards tolerance and the importance of skin-specific immunobiology. Curr Opin Organ Transplant. 2013;18:645–651. doi: 10.1097/MOT.0000000000000022. [DOI] [PubMed] [Google Scholar]