Abstract
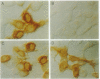
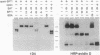
Bovine pancreatic trypsin inhibitor (BPTI) binds to trypsin and anhydrotrypsin (an enzymatically inactive derivative of trypsin) with affinities of 6 x 10(-14) and 1.1 x 10(-13) M, respectively. We have taken advantage of the high affinity and specificity of this binding reaction to develop a protein tagging system in which biotinylated trypsin or biotinylated anhydrotrypsin is used as the reagent to detect recombinant fusion proteins into which BPTI has been inserted. Two proteins, opsin and growth hormone, were used as targets for insertional mutagenesis with BPTI. In each case, both domains of the fusion protein appear to be correctly folded. The fusion proteins can be specifically and efficiently detected by biotinylated trypsin or biotinylated anhydrotrypsin, as demonstrated by staining of transfected cells, protein blotting, affinity purification, and a mobility shift assay in SDS/polyacrylamide gels.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ako H., Foster R. J., Ryan C. A. The preparation of anhydro-trypsin and its reactivity with naturally occurring proteinase inhibitors. Biochem Biophys Res Commun. 1972 Jun 28;47(6):1402–1407. doi: 10.1016/0006-291x(72)90228-8. [DOI] [PubMed] [Google Scholar]
- Cunningham B. C., Wells J. A. High-resolution epitope mapping of hGH-receptor interactions by alanine-scanning mutagenesis. Science. 1989 Jun 2;244(4908):1081–1085. doi: 10.1126/science.2471267. [DOI] [PubMed] [Google Scholar]
- DeNoto F. M., Moore D. D., Goodman H. M. Human growth hormone DNA sequence and mRNA structure: possible alternative splicing. Nucleic Acids Res. 1981 Aug 11;9(15):3719–3730. doi: 10.1093/nar/9.15.3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg D. P. Kinetic analysis of the folding and unfolding of a mutant form of bovine pancreatic trypsin inhibitor lacking the cysteine-14 and -38 thiols. Biochemistry. 1988 Apr 5;27(7):2481–2489. doi: 10.1021/bi00407a034. [DOI] [PubMed] [Google Scholar]
- Hodges R. S., Heaton R. J., Parker J. M., Molday L., Molday R. S. Antigen-antibody interaction. Synthetic peptides define linear antigenic determinants recognized by monoclonal antibodies directed to the cytoplasmic carboxyl terminus of rhodopsin. J Biol Chem. 1988 Aug 25;263(24):11768–11775. [PubMed] [Google Scholar]
- Huber R., Kukla D., Bode W., Schwager P., Bartels K., Deisenhofer J., Steigemann W. Structure of the complex formed by bovine trypsin and bovine pancreatic trypsin inhibitor. II. Crystallographic refinement at 1.9 A resolution. J Mol Biol. 1974 Oct 15;89(1):73–101. doi: 10.1016/0022-2836(74)90163-6. [DOI] [PubMed] [Google Scholar]
- Kolodziej P. A., Young R. A. Epitope tagging and protein surveillance. Methods Enzymol. 1991;194:508–519. doi: 10.1016/0076-6879(91)94038-e. [DOI] [PubMed] [Google Scholar]
- Leung D. W., Spencer S. A., Cachianes G., Hammonds R. G., Collins C., Henzel W. J., Barnard R., Waters M. J., Wood W. I. Growth hormone receptor and serum binding protein: purification, cloning and expression. Nature. 1987 Dec 10;330(6148):537–543. doi: 10.1038/330537a0. [DOI] [PubMed] [Google Scholar]
- Levilliers N., Péron M., Arrio B., Pudles J. On the mechanism of action of proteolytic inhibitors. IV. Effect of 8 M urea on the stability of trypsin in trypsin-inhibitor complexes. Arch Biochem Biophys. 1970 Oct;140(2):474–483. doi: 10.1016/0003-9861(70)90091-3. [DOI] [PubMed] [Google Scholar]
- Nathans J. Determinants of visual pigment absorbance: role of charged amino acids in the putative transmembrane segments. Biochemistry. 1990 Jan 30;29(4):937–942. doi: 10.1021/bi00456a013. [DOI] [PubMed] [Google Scholar]
- Nathans J., Hogness D. S. Isolation, sequence analysis, and intron-exon arrangement of the gene encoding bovine rhodopsin. Cell. 1983 Oct;34(3):807–814. doi: 10.1016/0092-8674(83)90537-8. [DOI] [PubMed] [Google Scholar]
- Vincent J. P., Lazdunski M. Trypsin-pancreatic trypsin inhibitor association. Dynamics of the interaction and role of disulfide bridges. Biochemistry. 1972 Aug 1;11(16):2967–2977. doi: 10.1021/bi00766a007. [DOI] [PubMed] [Google Scholar]
- Vincent J. P., Peron-Renner M., Pudles J., Lazdunski M. The association of anhydrotrypsin with the pancreatic trypsin inhibitors. Biochemistry. 1974 Sep 24;13(20):4205–4211. doi: 10.1021/bi00717a023. [DOI] [PubMed] [Google Scholar]
- de Vos A. M., Ultsch M., Kossiakoff A. A. Human growth hormone and extracellular domain of its receptor: crystal structure of the complex. Science. 1992 Jan 17;255(5042):306–312. doi: 10.1126/science.1549776. [DOI] [PubMed] [Google Scholar]