Abstract
Background
While it is established that cirrhosis results in a decrease in liver volume (LV), whether LV itself predicts patient survival is unknown. We hypothesize that estimated LV is an important prognostic indicator in patients with cirrhosis.
Methods
Data was gathered retrospectively from consecutive patients evaluated for a liver transplant from January 2001 to June 2006. Of 500 patients identified, 323 patients met both inclusion and exclusion criteria. LV per ideal body weight (IBW) was used to correct for body size, and LV/IBW was stratified by median split for survival analyses. Patients were classified into one of three clinical groups: hepatocellular disease (n = 229), cholestatic disease (n = 56), and miscellaneous (n = 38). One of three possible clinical outcomes (survival, liver transplantation, or death) was recorded during the 5-year follow-up, the latter two grouped together as “transplant/death.”
Results
Transplant/death occurred in 283 (88 %) subjects. Overall, there was a significant increase in transplant/death in those with lower LV/IBW (χ2 = 5.27, p = 0.022). When considering the subset with hepatocellular disease, lower LV/IBW was a robust predictor of transplant/death (χ2 = 9.62, p = 0.002). In multivariate analyses, the LV/IBW trended toward predicting transplant/death (ExpB = 0.943, p = 0.053) independent of Model for End stage Liver Disease (MELD) (ExpB = 1.13, p = 0.001).
Discussion
LV has important predictive value in patients with cirrhosis from hepatocellular disease. This observation appears to be independent of MELD, suggesting LV may impart important prognostic information that is not captured by the MELD score alone. Thus, LV may serve as an important adjunct to the MELD score in patients with hepatocellular disease.
Keywords: Cirrhosis, Liver volume, Liver transplant, MELD
Introduction
As cirrhosis progresses, liver volume (LV) generally decreases [1, 2]. A smaller LV by radiologic estimation has been shown to correlate with increased mortality and necessity for transplantation in patients with acute liver failure [3]. Additionally, smaller LV has been shown to be associated with a higher Child-Pugh score in patients with chronic liver disease [1, 2]. Surgeons commonly use LV as a surrogate of functional hepatic reserve when evaluating patients undergoing resection of hepatic tumors [4–6]. However, to date no large study has directly correlated LV with clinical outcomes such as survival or mortality in patients with cirrhosis. Furthermore, to our knowledge, no large study has explored the difference in LV and its significance among different etiologies of liver disease.
Liver transplantation is a life-saving option for patients with end stage liver disease (ESLD) and selected hepatic malignancies. The Model for End stage Liver Disease (MELD) score is the primary determinant of liver allocation and the gold standard as a prognostic indicator over 90 days in patients with cirrhosis. The MELD score, however, has its limitations. Based on serum bilirubin, serum creatinine, and INR, the MELD score can fluctuate significantly on a daily to weekly basis. Furthermore, it predicts survival for 90 days, with limited value beyond [7, 8]. Refinements to the MELD score continue to be proposed in an effort to improve the efficiency of liver allocation [9–14].
We hypothesize LV may serve as an important prognostic indicator of disease severity and outcome in patients with cirrhosis. Furthermore, LV may even serve as a useful adjunct to the MELD score. The primary aim of this study was to test the hypothesis that patient outcome (survival, transplant, or death) is directly related to LV.
Methods
Subjects and Baseline Clinical Characteristics
Subjects were identified retrospectively from consecutive patients referred for liver transplantation evaluation at Barnes-Jewish Hospital from January 1, 2001 to May 31, 2006. During this time period, 500 patients presented for initial evaluation. To meet the inclusion criteria, patients had to be at least 18 years of age, have a recorded LV by CT or MRI, and a MELD score within 4 weeks of LV measurement. Patients were excluded if they had acute liver failure, a liver mass (hepatocellular cancer or meta-static disease), were evaluated for re-transplantation, or were lost to follow up within 5 years of initial presentation. Inclusion and exclusion criteria were met by 323 patients. Not having a recorded LV by either CT or MRI was the most common reason for patients to be excluded from the study, followed by liver mass, lack of a recent MELD score, and acute liver failure. For purposes of our analysis, patients were classified into one of three clinical groups based on the etiology of cirrhosis: hepatocellular disease (n = 229, 71 %), obstructive/cholestatic disease (n = 56, 17 %), and miscellaneous (n = 38, 12 %) (Table 1). For each patient, the first recorded LV was documented as was the closest MELD score and its individual components (bilirubin, creatinine, and INR), age, weight, height, and gender.
Table 1.
Three clinical groups of study patients
| Group 1: Hepatocellular damage (n = 229) | Group 2: Obstructive/cholestatic (n = 56) | Group 3: Miscellaneous (n = 38) |
|---|---|---|
| Chronic hepatitis C (n = 117) | Primary biliary cirrhosis (n = 24) | Cryptogenic (n = 29) |
| Alcoholic (n = 49) | Primary sclerosing cholangitis (n = 29) | Alpha-1-antitrypsin deficiency (n = 6) |
| Chronic hepatitis C plus alcoholic (n = 28) | Biliary atresia (n = 2) | Alagille’s syndrome(n = 1) |
| Chronic hepatitis B (n = 8) | Sarcoidosis (n = 1) | Polycystic liver disease (n = 1) |
| Autoimmune hepatitis (n = 12) | Cystic fibrosis (n = 2) | Hereditary hemorrhagic telangiectasia (n = 1) |
| Hemochromatosis (n = 3) | ||
| Wilson’s disease (n = 2) | ||
| Non-alcoholic steatohepatitis (n = 10) |
Liver Volume Determination
To determine the LV from images obtained from MRI, single shot turbo spin echo sequences were acquired via cross-sectional imaging with a slice thickness of 8 mm and gap of 2 mm. The liver contour is outlined on each slice and the areas for each transverse and coronal slice are added to generate an overall volume. This is carried out by a trained radiation technologist on the scanner console and reviewed by the radiologists. The accuracy of this method has been previously verified by Caldwell and colleagues [15]. The images obtained by CT scan are sent to a separate 3D workstation and the volumes are calculated by Vital Imaging (Minnetonka, MN) and Pathfinder Therapeutics (Nashville, TN) software.
LV per ideal body weight (IBW) was used to correct for body size. IBW was calculated using the Devine formula: 50 + 2.3 kg/in over 5 feet for men and 45.5 + 2.3 kg/in over 5 feet for women. Patients were stratified to one of two classes over LV using median split. Patients whose LV/IBW was above the median were assigned as “large LV/IBW” and patients whose LV/IBW was below the median were assigned as “small LV/IBW.”
Clinical Outcomes
Patient outcomes were recorded retrospectively over a 5-year period from the initial recorded LV. One of two possible clinical outcomes was recorded: (1) “Survival,” which was assigned when patients survived transplant free for 5 years after the initial recorded LV, and (2) “transplant/death,” which was assigned when patients were either transplanted or died within 5 years of the initial recorded LV.
Statistical Analyses
Between group differences in baseline clinical characteristics were determined using Student’s t tests for continuous variables, and either chi square tests or Fisher’s exact tests, as appropriate. Survival analyses were performed to assess the impact of LV/IBW on transplant/death. Statistical differences between the large- and small-LV/IBW groups were determined using log rank tests, and Kaplan–Meier plots were generated. A Cox proportional hazard model was employed to determine the predictive value of LV/IBW for transplant/death, independent of other established predictors of death and or transplant in cirrhotic patients, and included age, gender, and MELD score in the models. The decision to include these specific variables was based on the desire to control for demographics, and also to determine the predictive value of LV:IBW as a potential adjunct to the MELD score, a well-established predictor of ESLD events. All statistical analyses were conducted using IBM SPSS Statistics 20 (IBMCorp, Armonk, NY).
Results
Baseline characteristics of the study subjects are listed in Table 2. Notably, a higher male to female ratio was present in the hepatocellular disease group compared to the other groups. There was a statistically significant age difference between the three different groups, with the cholestatic patients slightly younger, and the miscellaneous liver disease group slightly older than the hepatocellular disease patients. The cholestatic group had lower BMIs compared to the other groups. There was no difference in the average MELD score, the percentage of transplant/death, or the time to transplant/death between the three groups. Between transplant and death, the most common outcome was transplantation in all three groups.
Table 2.
Baseline characteristics
| Characteristic | All subjects (n = 323) | Hepatocellular (n = 229) | Cholestatic (n = 56) | Miscellaneous (n = 38) | p value |
|---|---|---|---|---|---|
| Male gender | 223 (69 %) | 172 (75.1 %) | 29 (51.8 %) | 22 (57.9 %) | 0.001 |
| Age | 51.6 | 51.6 | 49.7 | 54.6 | 0.041 |
| BMI | 28.0 ± 6.1 | 28.7 ± 6.0 | 25.0 ± 5.1 | 28.6 ± 7.1 | < 0.001 |
| LV/IBW (cm3/kg) | 24.27 ± 9.67 | 22.75 ± 7.08 | 30.43 ± 12.06 | 24.37 ± 14.72 | < 0.001 |
| MELD | 14.4 ± 6.6 | 14.9 ± 6.5 | 12.9 ± 7.5 | 14.1 ± 6.2 | 0.14 |
| Terminal outcome, n (%) | 283 (88 %) | 200 (87 %) | 48 (86 %) | 35 (92 %) | 0.39 |
| Transplanted, n (%) | 222 (68.7 %) | 152 (66.4 %) | 39 (69.6 %) | 31 (81.6 %) | |
| Died, n (%) | 61 (18.9 %) | 48 (21.0 %) | 9 (16.1 %) | 4 (10.5 %) | |
| Time to terminal outcome (days) | 561.1 ± 600.6 | 543.1 ± 599.1 | 619.5 ± 641.0 | 583.5 ± 555.3 | 0.68 |
BMI body mass index, LV liver volume, IBW ideal body weight
For all 323 patients with cirrhosis, the mean LV was 1,640 cm3, and the mean LV/IBW was 24.3 cm3/kg. Overall, there was no significant difference in mean LV between subjects surviving without liver transplant or death and the transplant/death cases (1,679.9 ± 579.3 vs. 1,632.4 ± 652.3 cc, p = 0.68), nor was there a significant difference in LV/IBW (25.6. ± 7.9 vs. 24.0 ± 9.8, p = 0.37). However, within the hepatocellular disease group, a lower mean LV was seen in those experiencing transplant/death (1,546.3 ± 504.4 vs. 1,749.6 ± 515.2 cc, p = 0.059) and a significantly lower LV/IBW was observed (22.3 ± 7.0 vs. 26.4 ± 7.0, p = 0.006).
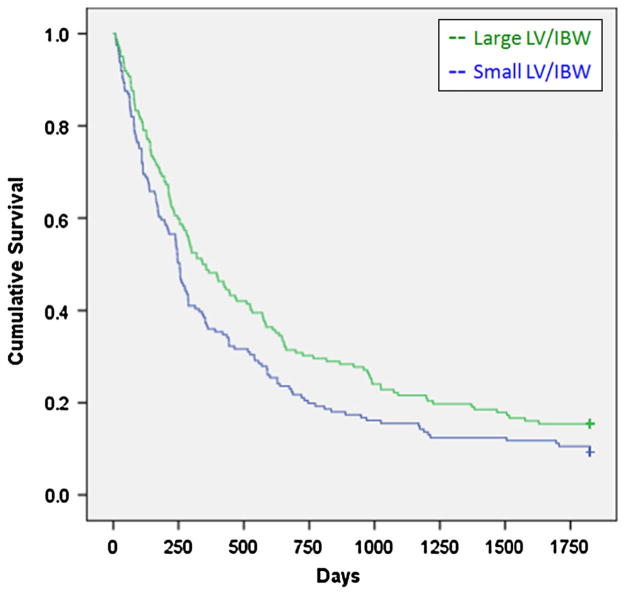
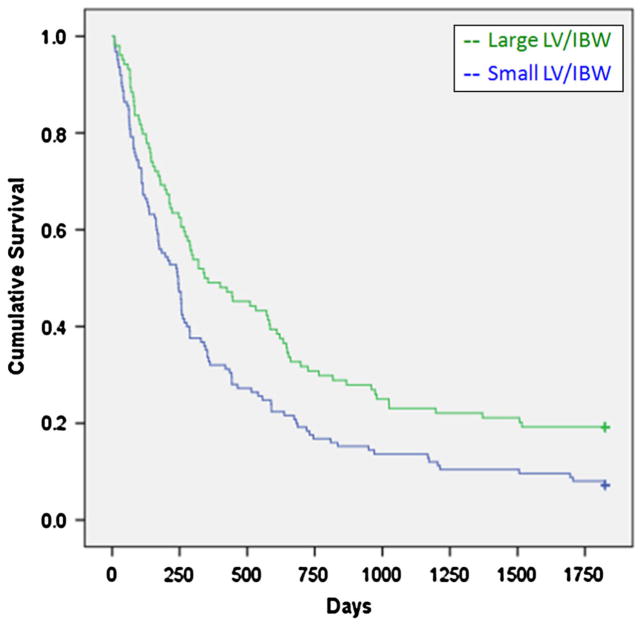
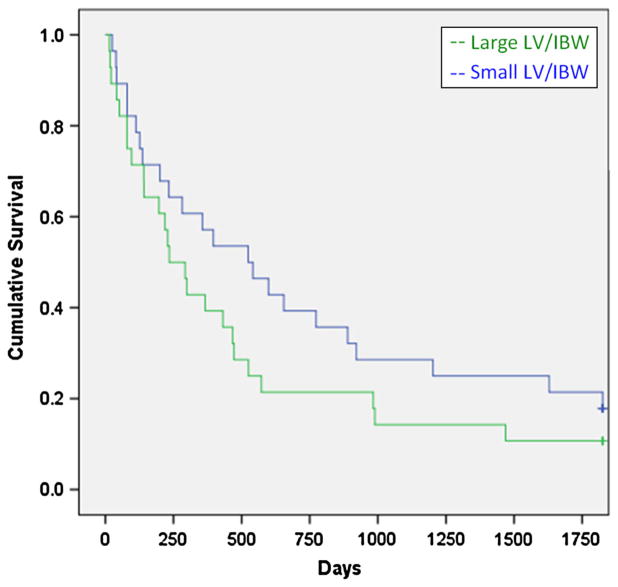
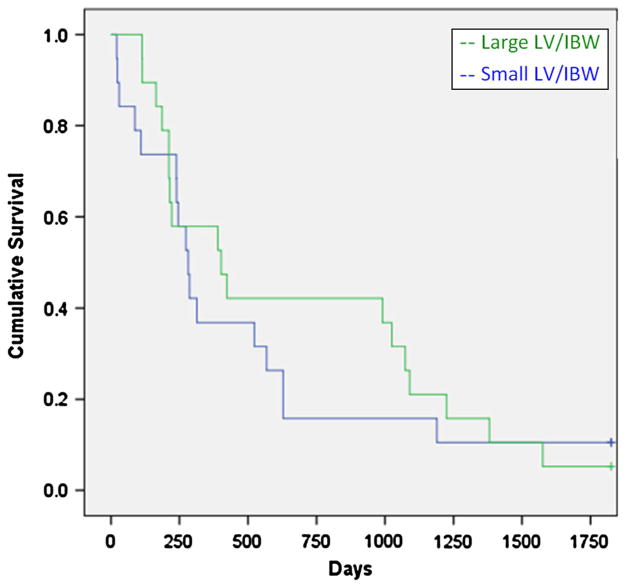
The mean LV/IBW was 31.2 cm3/kg for the large LV/IBW group, and 17.3 cm3/kg for the small LV/IBW group. LVs were determined by MRI in 297 patients and 26 were determined by CT. The cholestatic group had significantly larger livers compared to the other two groups. There was a statistically significant survival advantage for the large LV/IBW group (X2 = 5.27, p = 0.022) (Fig. 1). For the hepatocellular disease group, there was an even greater survival advantage for the large LV/IBW group (X2 = 9.62, p = 0.002) (Fig. 2). For the cholestatic disease group, there was a weak trend favoring a survival advantage for the small LV/IBW group. However, this was not statistically significant (X2 = 1.74, p = 0.18) (Fig. 3). For the miscellaneous group, there was a slight trend favoring a survival advantage for the large LV/IBW group, however, this was not statistically significant (X2 = 0.479, p = 0.48) (Fig. 4).
Fig. 1.
Survival over time in all patients with end stage liver disease (ESLD). There was a survival advantage for the large LV/IBW group in all 323 patients with ESLD (X2 = 5.27, p = 0.022)
Fig. 2.
Survival over time in end stage liver disease (ESLD) patients with hepatocellular disease. There was a distinct survival advantage for the large LV/IBW group in group 1—patients with hepatocellular injury (X2 = 9.62, p = 0.002)
Fig. 3.
Survival over time in end stage liver disease (ESLD) patients with cholestasis. There was a trend favoring a survival advantage for the small LV/IBW group in group 2—patients with cholestasis—which was not statistically significant (X2 = 1.74, p = 0.18). This is opposite from the trend we saw for group 1
Fig. 4.
Survival over time in end stage liver disease (ESLD) patients with miscellaneous causes. There may have been a slight trend favoring a survival advantage for the large LV/IBW group in group 3—patients with miscellaneous causes of ESLD; however this was not statistically significant (X2 = 0.479, p = 0.48)
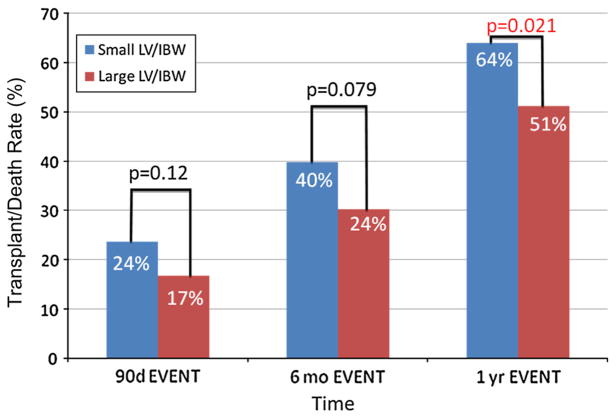
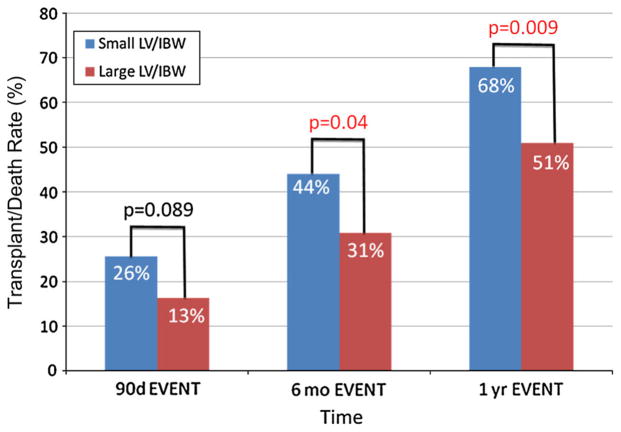
For all patients, the transplant/death rate was higher in the small LV/IBW group at 3, 6 months, and 1 year. This difference in the transplant/death rate reached statistical significance at 1 year, with a 64 % transplant rate in the small LV/IBW group compared to a 51 % transplant/death rate in the large LV/IBW group (p = 0.021) (Fig. 5). In the hepatocellular disease group, there was a higher transplant/death rate in the small LV/IBW group at all three time points. The difference in transplant/death rate reached statistical significance by 6 months. At 1 year, there was a 68 % transplant/death rate in the small LV/IBW group compared to a 51 % transplant/death rate in the large LV/IBW group (p = 0.009) (Fig. 6).
Fig. 5.
Transplant/death rate over time for all end stage liver disease (ESLD) patients. When looking at all 323 patients in the study, the transplant/death rate was higher in the small LV/IBW at 3, 6 months, and 1 year. This difference in transplant/death rate reached statistical significance at 1 year, with a 64 % transplant/death rate in the small LV/IBW group compared to a 51 % transplant/death rate in the large LV/IBW group (p = 0.021)
Fig. 6.
Transplant/death rate over time for end stage liver disease (ESLD) patients with hepatocellular disease. When looking at group 1—the patients with hepatocellular injury—again there was a higher transplant/death rate at all three time points. The difference in transplant/death rate reached statistical significance by the 6-month time period, and at 1 year there was a 68 % transplant/death rate in the small LV/IBW group compared to a 51 % transplant/death rate in the large LV/IBW group (p = 0.009)
As statistically significant differences in patient survival among those with large LV/IBW were seen in only the hepatocellular disease group, multivariate Cox proportional hazard analysis to determine the independent effect of this variable was conducted only in the hepatocellular group. Here, the Exp(B) for LV/IBW as a continuous variable was 0.943 (p = 0.053) (Table 3). In clinical terms, an increase of 1 cm3/kg increase in the LV/IBW would translate into a 5.7 % decrease in transplant/deaths observed over the 5-year period.
Table 3.
Cox proportional hazards model predicting transplant/death using MELD and LV/IBW in patients with hepatocellular injury disease pattern
| Characteristic | Exp (B) | 95 % CI for exp (B) | p |
|---|---|---|---|
| Age | 1.055 | 1.018–1.094 | 0.003 |
| Male sex | 1.094 | 0.520–2.300 | 0.814 |
| MELD | 1.128 | 1.053–1.209 | 0.001 |
| LV/IBW | 0.943 | 0.889–1.001 | 0.053 |
CI confidence interval, MELD model for end stage liver disease, LV liver volume, IBW ideal body weight
Discussion
Anectodally, LV often is regarded as a potential predictor of survival in patients with cirrhosis. Liver transplant teams worldwide are familiar with the discussion of patients with a “liver the size of my fist,” and the inherent concern regarding wait list survival, even in the absence of a high MELD score. Despite these convictions, no systematic analysis has been performed to either confirm or refute this widespread belief. In this study, LV estimation by cross-sectional imaging overall was indeed found to predict survival in patients with cirrhosis. Patients with a smaller LV by either MRI or CT had a statistically significant increase in transplant/deaths. Conversely, patients with larger LVs had a statistically significant survival advantage. These results were driven nearly entirely by the hepatocellular disease group. The cholestatic disease group did not follow this pattern. One possible, albeit simplistic, explanation is that cirrhosis secondary to hepatocellular disease may result in functional and physical loss of hepatocytes (the bulk of the liver parenchyma) at an earlier stage and with a more predictable pattern compared to patients with cholestatic disease [16, 17]. Cholestatic liver disease may result in injury focused in the portal areas for a significant period of time, with hepatocellular injury occurring later and less predictably [18]. Thus, LV may reflect the timing of hepatocellular injury with a lower LV in patients with earlier hepatocellular injury and necrosis. Whether this correlates with functional hepatic reserve needs to be clarified in larger studies.
Importantly, within the hepatocellular disease group, LV trended towards predicting transplant/deaths independent of the MELD score. Thus, LV may impart important prognostic information not captured by the MELD score. LV is less likely to vary over short periods of time, unlike the MELD score, which may vary significantly on a daily or weekly basis. This relative stability of LV as a predictive tool may explain its ability to predict transplant/deaths independent of an established prediction model in the MELD. Furthermore, the ability of LV to predict transplant/deaths up to 1 year after its measurement, may allow the use of LV as an adjunct to the MELD score.
Limitations of this study include those intrinsic to any retrospective design. LV was not determined by a single operator, potentially leading to operator variability. However, the data presented approximately a “real world” experience, and were LV to be adopted as a prognostic tool on a wider scale, multiple operators would be the usual practice. Other potentially important variables were not examined in this particular study, including medication use and the presence of a transjugular intrahepatic portosystemic shunt. In addition, for the purpose of this analysis, the most common outcome (transplant) was deemed a “transplant/death” equivalent to death. This approach was premised on the assumption that the risk of mortality was exceedingly high if a transplant was not undertaken at the time of the procedure. Although this assumption was likely an accurate one for the vast majority of patients, some patients may not have died imminently and survived without a transplant for an extended time.
In conclusion, this study is the first large study to show LV has important prognostic utility in patients with cirrhosis due to hepatocellular disease. Additionally, LV may impart important additional prognostic information that is not captured in the MELD score. Further studies are needed to determine if the use of LV as a prognostic tool may ultimately improve wait list outcomes and help optimize the liver allocation process.
Acknowledgments
Research support was provided from the Foundation for the Barnes-Jewish Hospital, Fund 4359-30 (JSC), and NIH K23 DK084113-03 (GSS).
Footnotes
Conflict of interest None.
Contributor Information
Michael T. Hagan, Division of Gastroenterology, Department of Internal Medicine, Washington University School of Medicine, 660 South Euclid, Campus Box 8124, St. Louis, MO, USA
Gregory S. Sayuk, Division of Gastroenterology, Department of Internal Medicine, Washington University School of Medicine, 660 South Euclid, Campus Box 8124, St. Louis, MO, USA
Mauricio Lisker-Melman, Division of Gastroenterology, Department of Internal Medicine, Washington University School of Medicine, 660 South Euclid, Campus Box 8124, St. Louis, MO, USA.
Kevin M. Korenblat, Division of Gastroenterology, Department of Internal Medicine, Washington University School of Medicine, 660 South Euclid, Campus Box 8124, St. Louis, MO, USA
Thomas A. Kerr, Division of Gastroenterology, Department of Internal Medicine, Washington University School of Medicine, 660 South Euclid, Campus Box 8124, St. Louis, MO, USA
William C. Chapman, Department of General Surgery, Section of Abdominal Transplantation, Washington University School of Medicine, St. Louis, MO 63110, USA
Jeffrey S. Crippin, Email: jcrippin@dom.wustl.edu, Division of Gastroenterology, Department of Internal Medicine, Washington University School of Medicine, 660 South Euclid, Campus Box 8124, St. Louis, MO, USA
References
- 1.Lin XZ, Sun YN, et al. Liver volume in patients with or without chronic liver diseases. Hepatogastroenterology. 1998;45:1069–1074. [PubMed] [Google Scholar]
- 2.Zhu JY, Leng XS, et al. Measurement of liver volume and its clinical significance in cirrhotic portal hypertensive patients. World J Gastroenterol. 1999;5:525–526. doi: 10.3748/wjg.v5.i6.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamagishi Y, Saito H, et al. A new prognostic formula for adult acute liver failure using computer tomography-derived hepatic volumetric analysis. J Gastroenterol. 2009;44:615–623. doi: 10.1007/s00535-009-0045-7. [DOI] [PubMed] [Google Scholar]
- 4.Kubota K, Makuuchi M, Kusaka K, et al. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology. 1997;26:1176–1181. doi: 10.1053/jhep.1997.v26.pm0009362359. [DOI] [PubMed] [Google Scholar]
- 5.Yanaga K, Honda H, Ikeda Y, Nishizaki AT, Yamamoto K, Sugimachi K. Significance of liver size in hepatic surgery. HPB Surg. 1997;10:195–200. doi: 10.1155/1997/34842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tu R, Xia LP, Yu AL, Wu L. Assessment of hepatic functional reserve by cirrhosis grading and liver volume measurement using CT. World J Gastroenterol. 2007;13:3956–3961. doi: 10.3748/wjg.v13.i29.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamath PS, Wiesner RH, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 8.Wiesner, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91–96. doi: 10.1053/gast.2003.50016. [DOI] [PubMed] [Google Scholar]
- 9.Luca A, Angermayr B, Bertolini G, et al. An integrated MELD model including serum sodium and age improves the prediction of early mortality in patients with cirrhosis. Liver Transpl. 2007;13:1174. doi: 10.1002/lt.21197. [DOI] [PubMed] [Google Scholar]
- 10.Biggins SW, Kim WR, Terrault NA, et al. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology. 2006;130:1652. doi: 10.1053/j.gastro.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018. doi: 10.1056/NEJMoa0801209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma P, Schaubel DE, Sima CS, Merion RM, Lok AS. Re-weighting the model for end-stage liver disease score components. Gastroenterology. 2008;135:1575. doi: 10.1053/j.gastro.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Leise MD, Kim WR, Kremers WK, Larson JJ, Benson JT, Therneau TM. A revised model for end-stage liver disease optimizes prediction of mortality among patients awaiting liver transplantation. Gastroenterology. 2011;140:1952. doi: 10.1053/j.gastro.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luca A, Angermayr B, Bertolini G, et al. An integrated MELD model including serum sodium and age improves the prediction of early mortality in patients with cirrhosis. Liver Transpl. 2007;13:1174. doi: 10.1002/lt.21197. [DOI] [PubMed] [Google Scholar]
- 15.Caldwell SH, deLange EE, Gaffey MJ, et al. Accuracy and significance of pretransplant liver volume measured by magnetic resonance imaging. Liver Transpl Surg. 1996;2:438–442. doi: 10.1002/lt.500020606. [DOI] [PubMed] [Google Scholar]
- 16.MacSween RNM. Pathology of the Liver. Edinburgh: Churchill Livingstone; 1994. Alcoholic liver disease; pp. 317–348. [Google Scholar]
- 17.MacSween RNM. Pathology of the Liver. Edinburgh: Churchill Livingstone; 1994. Chronic hepatitis; pp. 349–395. [Google Scholar]
- 18.MacSween RNM. Pathology of the Liver. Edinburgh: Churchill Livingstone; 1994. Diseases of the intrahepatic bile ducts; pp. 477–512. [Google Scholar]