Abstract
Background
Obesity and fat distribution patterns [subcutaneous vs. visceral adipose tissue (VAT)] are important predictors of future cardiometabolic risk. As accurate VAT measurement entails imaging, surrogate anthropometric measurements that would be cheaper and quicker to obtain would be highly desirable. Sagittal abdominal diameter (SAD) may be better than other VAT surrogate measures in adults, but the value of SAD to predict magnetic resonance imaging (MRI)-determined VAT in adolescents of different races, sexes, and pubertal stages has not been determined.
Aim
To test the hypothesis that SAD correlates more strongly with volumetric VAT than other anthropometric measurements, independent of age, sex, race, and Tanner stage.
Subjects and methods
Twenty-eight normal-weight and 44 obese adolescents underwent Tanner staging, anthropometric examinations, and abdominal MRI for volumetric partitioned fat calculation.
Results
VAT increased exponentially in the body mass index (BMI) > 97th percentile range. SAD, waist circumference (WC), BMI, and BMI Z-score correlated strongly with VAT (correlation coefficients of 0.85–0.86, all p-values < 0.0005); waist–hip ratio was less predictive of VAT (r = 0.68, p < 0.0005). On hierarchical regression, the strongest predictors of VAT in obese subjects were BMI Z-score and SAD (R2 = 0.34 vs. 0.31, respectively, p < 0.0005); in normal-weight subjects, most anthropometric measures predicted VAT equally (R2 = 0.16–0.18, p-values = 0.018–0.026).
Conclusions
Unlike adults, in obese adolescents, SAD is not the strongest predictor of visceral adiposity. BMI Z-score is equivalently predictive and, together with BMI, provides sufficient information to assess visceral adiposity; more specialized anthropometric measurements (e.g., SAD and WC) do not add additional predictive value.
Keywords: anthropometric measures, children, obesity, visceral adiposity
The prevalence of pediatric obesity, defined as body mass index (BMI) ≥ 95th percentile for age and sex (1), has greatly increased and currently affects 16.0% of US children (2). Pediatric obesity increases the risk of diseases such as the metabolic syndrome (3) and type 2 diabetes (4). The challenge for treating physicians is identifying those at higher risk of obesity-related complications to appropriately target interventions. One complicating factor is that adipose tissue domains carry varying risk levels. Visceral (intra-abdominal) fat deposition is an important risk factor for cardiometabolic complications (5) and all-cause mortality in adult men (6) and for insulin resistance in children (7). Visceral fat measurement entails imaging, via dual-energy X-ray absorptiometry (not validated in children), computerized tomography, or magnetic resonance imaging (MRI) (8); however, this is clinically impractical and expensive. Accurate surrogate anthropometric measurements of visceral vs. subcutaneous adipose tissue (VAT vs. SAT), which can easily be performed in physicians’ offices without extensive training or time, are needed to identify those at highest risk of visceral adiposity and its potential sequelae.
Various anthropometric measurements have been proposed as screens for visceral adiposity in both adults (9) and children (10, 11), including BMI, waist circumference (WC), waist–hip ratio (WHR), and more specialized measurements requiring equipment and training (e.g., skinfold thicknesses) (12). Less widely used is the sagittal abdominal diameter (SAD), which measures abdominal thickness at waist level in the supine position. In adults, SAD is associated with dyslipidemia, insulin resistance, hypertension (13), and cardiovascular disease risk (14), and it may be a better predictor of the metabolic syndrome (15) and hyperglycemia (16) than other anthropometric measures such as WC. In adults, SAD correlates highly with VAT mass (17); it has been proposed to be a better measure of VAT than WC or WHR (18), deriving from the theory that gravity moves the subcutaneous fat to the sides while visceral fat projects the abdomen vertically (18–20). However, pediatric data regarding predictive capacity of SAD for visceral adiposity are limited. Adult findings may not be generalizable to children: growth and body composition changes are expected in children, and BMI changes in early adolescence may relate more to lean mass than fat mass acquisition (21). To our knowledge, SAD has only been directly correlated with MRI-measured visceral fat in adolescents in one small study, which included both preadolescents and adolescents and did not adjust for pubertal stage, race, or sex (22). Assessing the utility of SAD in children while specifically accounting for these factors is crucial, given normal puberty-and sex-related changes in body composition (23) and known differences in VAT between populations (24). We hypothesized that, in both normal-weight and obese adolescents, SAD would better correlate with MRI-measured VAT volume than other surrogate anthropometric measurements of adiposity, independent of age, sex, race, and Tanner stage.
Methods
This study, part of a larger study evaluating pulmonary complications of obesity, was an observational, cross-sectional study of two groups of adolescents: obese (BMI ≥ 95th percentile) and normal-weight (BMI < 85th percentile).
Exclusion criteria included obesity secondary to genetic syndromes (e.g., Prader–Willi), pregnancy, and medical conditions requiring chronic medications (e.g., polycystic ovary syndrome requiring oral contraceptives; well-controlled asthma not requiring chronic glucocorticoids was the only exception). Subjects were recruited from the Children’s Hospital of Philadelphia (CHOP) Sleep Center and Healthy Weight Program and from the general population via advertisements. The study was approved by the CHOP Institutional Review Board. Written informed consent and age-appropriate assent were obtained from all parents/guardians and subjects before participation.
Tanner staging of breast/genitalia and pubic hair development was based upon a self-assessment form (25). All girls underwent urine pregnancy tests. With subjects wearing hospital gowns, standing height (to 0.1 cm) was measured using a stadiometer (Holtain, Crymych, UK) and weight (to 0.1 kg) was measured on a digital electronic scale (Scaletronix, White Plains, NY, USA). BMI Z-scores were assessed using age-and gender-specific reference data (26). Waist and hip measurements (to 0.1 cm) were performed in triplicate using non-stretchable fiberglass tape and averaged. WC was measured per National Heart, Lung and Blood Institute (NHLBI) Guidelines (27), hip circumference was measured at the greater trochanter with subjects standing, and WHR was calculated. SAD was measured with supine subjects, legs flat, and hips extended as the distance at end-expiration between abdominal caliper blades (Seritex, East Rutherford, NJ, USA) placed below the back and above the abdomen, slightly proximal to the iliac crest (13).
Subjects underwent abdominal spin-echo MRI using a 3-T scanner (Sonata, Siemens, Malvern, PA, USA) with a body coil for partitioning of abdominal fat into subcutaneous and visceral deposits. Axial images were acquired with an MR gradient echo pulse sequence and an axial T1-weighted sequence [repetition time (TR): 600/echo time (TE): 10]. The abdominal compartment was defined as extending from the inferior aspect of the xiphoid process to the most superior slice depicting the iliac crest. Each MRI slice was manually examined to segment fat using image and volumetric analysis (Amira 4.1.2., Visage Imaging, San Diego, CA, USA) (28). Automated thresholding and manual segmentation by the region growing tool were used to segment SAT and VAT volumes by pixel valuation.
Statistical analyses were performed using SPSS v.16 software (SPSS, Chicago, IL, USA). The p-values ≤0.05 were considered statistically significant. Histograms and Kolmogorov–Smirnov one-sample tests examined normality of distribution. We generated descriptive statistics, including mean and standard deviation (SD), and natural log-transformed significantly skewed variables. Frequency counts and percentages were used for categorical variables (e.g., gender). We used Student’s t-test or Wilcoxon rank-sum tests to compare means of continuous variables between groups, and chi-squared testing for ordinal or nominal variables. Given the differences in body composition between normal-weight and obese (29), African-Americans and Caucasians (30), and males vs. females (31), we compared the relationships between anthropometric measurements and VAT volume in the whole sample and in the above-mentioned subgroups. Associations between anthropometric variables and fat distribution measured by imaging [VAT vs. SAT and total adipose tissue (TAT)] were explored via Pearson’s or Spearman’s correlations. Fisher’s Z transformation assessed differences between correlation coefficients. A race-by-TAT interaction term was used in the regression analysis to confirm that African-Americans have less visceral adiposity than Caucasians [as observed by others (32)] and to include this in our models as an important consideration in examining relationships between anthropometric measures and VAT. Hierarchical linear regression models further examined associations between anthropometric and imaging outcomes. First, demographic variables (age, race, sex, and Tanner stage) were entered en block. Second, either WC, SAD, BMI, or BMI Z-score was added in a stepwise fashion. Finally, the remaining anthropometric variables (e.g., SAD, WHR, BMI, and BMI Z-score in the WC model) were entered serially to assess their impact upon the model.
Results
Study group
Ninety-eight adolescents (41 normal-weight and 57 obese) aged 12–16 yr enrolled in the study. Of these, 12 normal-weight and 8 obese subjects refused to undergo MRI because of claustrophobia, 3 obese subjects had metallic artifacts (braces and hip screw), and 1 obese subject had significant motion artifact. Two outliers were excluded: one ‘normal-weight’ subject whose BMI Z-score (−3.45) was >2 SDs lower than the next BMI Z-score (−1.37) and was below the first percentile, and one obese subject whose VAT volume (2885 cm3) was 44% higher than the second-highest VAT volume (2005 cm3). Finally, given the differences in body composition between different population ancestries (races) (32), subjects who were neither African-American nor Caucasian (n = 3: 1 Asian and 2 more than one race) were excluded from analysis. Thus, 69 adolescents (28 normal-weight and 41 obese) completed the study.
Descriptive characteristics and comparison of normal-weight vs. obese subjects are presented in Table 1. There were no significant differences in age, sex, Tanner stages, race, or ethnicity between the two groups. As expected, all anthropometric and imaging measures differed significantly between normal-weight and obese groups.
Table 1.
Comparison of normal-weight and obese subjects
| Normal-weight | Obese | p-Value | |
|---|---|---|---|
| N | 28 | 41 | |
| Age (yr) | 14.7 ± 1.5 (12.5–16.8) | 14.5 ± 1.4 (12.2–16.8) | 0.57 |
| Sex: male, N (%) | 16 (57%) | 23 (56%) | >0.999* |
| Tanner stages | |||
| 1 | 0 (0%) | 1 (2%) | 0.45* |
| 2 | 2 (7%) | 1 (2%) | |
| 3 | 6 (21%) | 3 (7%) | |
| 4 | 14 (50%) | 17 (41%) | |
| 5 | 6 (21%) | 11 (27%)‡ | |
| Race | |||
| African-American | 20 (71%) | 33 (80%) | 0.40* |
| Caucasian | 8 (29%) | 8 (20%) | |
| Ethnicity | |||
| Hispanic | 5 (18%) | 3 (7%) | 0.26* |
| Non-Hispanic | 23 (82%) | 38 (93%) | |
| Height (cm)s | 162.9 ± 10.7 (145.9–186.2) | 164.2 ± 9.0 (143.0–185.3) | 0.57 |
| Weight (kg) | 55.2 ± 11.8 (36.8–79.8) | 92.3 ± 18.1 (61–129) | <0.0005 |
| BMI (kg/m2) | 20.6 ± 2.5 (16.5–24.6) | 34.7 ± 6.6 (25.7–57.9) | <0.0005 |
| BMI Z-score | 0.24 ± 0.78 (−1.37 to 1.52) | 2.27 ± 0.37 (1.64–3.02) | <0.0005 |
| Waist circumference (cm) | 70.6 ± 6.0 (61.0–82.1) | 101.4 ± 12.0 (79–124) | <0.0005 |
| Hip circumference (cm) | 89.9 ± 8.1 (75–103) | 115.0 ± 9.5 (99–136) | <0.0005 |
| Waist–hip ratio | 0.79 ± 0.04 (0.69–0.88) | 0.88 ± 0.06 (0.71–1.03) | <0.0005 |
| Sagittal abdominal diameter (cm) | 16.5 ± 1.6 (14.0–20.6) | 24.0 ± 2.8 (19.1–29.5) | <0.0005 |
| Volumetric subcutaneous adipose tissue (SAT) volume (cm3) | 771 ± 608 (110–2736) | 6493 ± 2864 (2192–11 916) | <0.0005 |
| Volumetric visceral adipose tissue (VAT) volume (cm3) | 90 ± 75 (14–341)† | 664 ± 489 (71–2005)† | <0.0005† |
| Volumetric total adipose tissue (TAT) volume (cm3) | 861 ± 658 (135–2994) | 7157 ± 3199 (2298–13 425) | <0.0005 |
| VAT/TAT ratio | 0.12 ± 0.06 (0.03–0.33) | 0.09 ± 0.04 (0.02–0.19) | 0.134 |
BMI, body mass index.
Values represent number (%) or mean ± SD (range). Numbers may total >100% because of rounding.
Chi-square or Fisher’s exact test.
Skewed variable (Mann–Whitney used).
Tanner data not available for nine obese subjects; therefore, percentages do not add up to 100.
Sex differences in adiposity
Height was significantly lower (159.7 vs. 167.8 cm, p = 0.003) and BMI significantly higher (37.0 vs. 32.9 kg/m2, p = 0.04) in obese females than males. SAD trended lower in normal-weight females than males (15.8 vs. 16.9 cm, p = 0.059). VAT volumes were similar between the sexes, but SAT and TAT volumes were significantly greater in females vs. males in all groups:
Obese group (mean ± SD, females vs. males):
SAT: 7721 ± 2865 vs. 5531 ± 2525 cm3, p = 0.013;
TAT: 8473 ± 3221 vs. 6126 ± 2839 cm3, p = 0.018.
Normal-weight group (mean ± SD, females vs. males):
SAT: 1164 ± 710 vs. 468 ± 258 cm3, p = 0.002;
TAT: 1280 ± 770 vs. 547 ± 315 cm3, p = 0.004.
Effect of puberty
Partitioned abdominal fat did not differ significantly between subjects of different pubertal stages. No anthropometric differences by puberty stage were seen in obese subjects at all, nor in the entire study population except height in the latter. However, in the normal-weight group, later puberty stages were associated with increasing height, weight, and hip circumference [F-statistic (p-value): 4.30 (0.01), 3.60 (0.03), and 3.71 (0.03), respectively].
Effect of population ancestry
Anthropometric and imaging measures (unadjusted) did not differ significantly between African-Americans and Caucasians in either the entire population nor in the normal-weight or obese subgroups (data not shown).
Relationship between anthropometric measures and partitioned abdominal fat
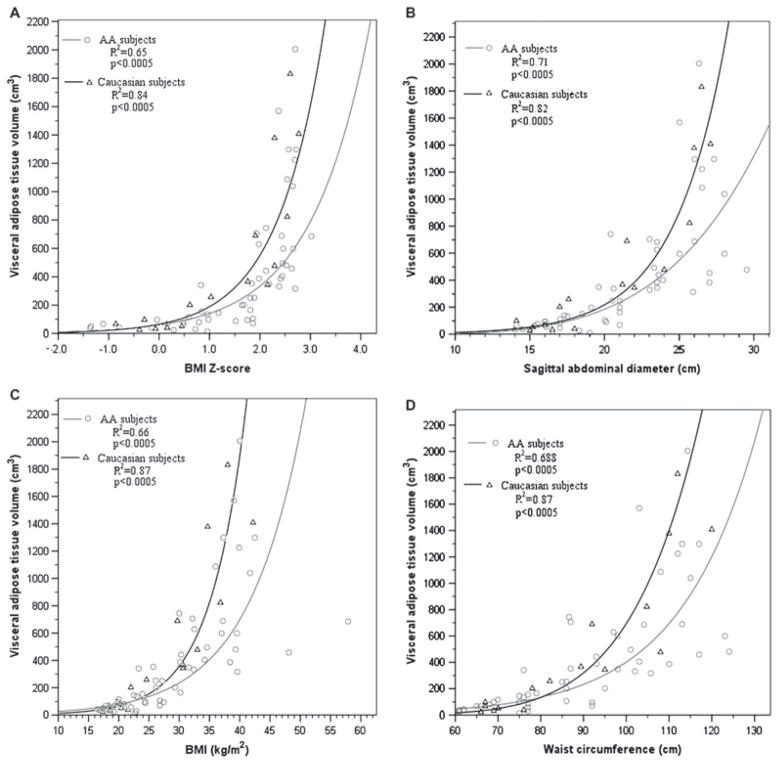
Figure 1 depicts the relationships between VAT and several anthropometric measures (WC, SAD, BMI, and BMI Z-score). VAT volume increased exponentially in subjects with BMI Z-scores above 1.9 (Fig. 1A). Correlations between anthropometric measures and partitioned abdominal fat are shown in Table 2. SAD, WC, BMI, and BMI Z-score all correlated strongly to SAT and TAT and less strongly to VAT. WHR had the weakest associations with abdominal fat partitions.
Fig. 1.
Relationships between anthropometric measures [(A) body mass index (BMI) Z-score, (B) BMI, (C) sagittal abdominal diameter (SAD), and (D) waist circumference (WC) on x-axis] and visceral adipose tissue (VAT) volume (y-axis) for African-Americans (AA) and Caucasians.
Table 2.
Correlations between anthropometric measurements and partitioned abdominal fat—all subjects
| Age | Waist circumference* | Waist–hip ratio* | Sagittal abdominal diameter* | BMI* | BMI Z-score* | |
|---|---|---|---|---|---|---|
| Subcutaneous adipose tissue | −0.07 | 0.94 | 0.75 | 0.93 | 0.89 | 0.82 |
| Visceral adipose tissue† | 0.03 | 0.85 | 0.64 | 0.85 | 0.83 | 0.83 |
| Total adipose tissue | −0.07 | 0.94 | 0.75 | 0.93 | 0.89 | 0.82 |
BMI, body mass index.
Two-tailed significance. Numbers in bold indicate significant association.
p < 0.0005.
Natural log-transformed for non-normal distribution.
All anthropometric measurements correlated more strongly with VAT volume in Caucasians than African-Americans, with WC, BMI, BMI Z-score, and SAD being equivalent (correlation coefficients 0.805–0.839 vs. 0.903–0.933 within African-American vs. Caucasian groups, all p-values < 0.0005); the WHR correlations were equivalent but lower [correlation coefficients 0.636 (p < 0.005) vs. 0.71 (p = 0.006)] in African-Americans vs. Caucasians. Fisher’s Z transformation, which is used to assess significance of the difference between correlation coefficients in two independent samples, found no significant differences between the correlation coefficients between anthropometric predictors and VAT volume in African-Americans vs. Caucasians (p-values 0.10–0.67). Linear regression demonstrated that at a given TAT, VAT was lower in African-Americans vs. Caucasians: at the mean TAT volume (4601.9 cm3), African-Americans’ VAT volume (385.3 vs. 553.8 cm3) and VAT/TAT slope (0.09 vs. 0.139) were significantly lower. The VAT–TAT relationship differed significantly between races (interaction p = 0.013), i.e., for a given total abdominal adiposity, African-Americans had significantly less VAT than Caucasians. Figure 2 illustrates differences in fat partitioning in two age-, pubertal stage-, and BMI Z-score-matched females.
Fig. 2.
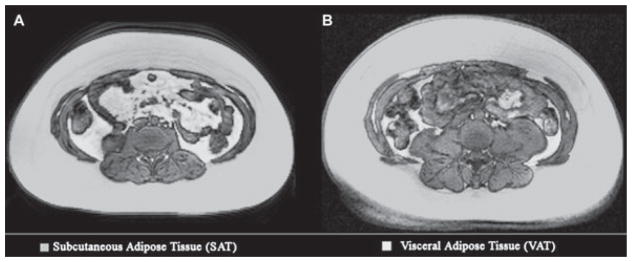
Fat partitioning patterns [transverse magnetic resonance imaging (MRI) slices (L2–L3)] in obese Caucasian (A) and African-American (B) females. Demographics: (A) 14.3-yr old, Tanner 4, body mass index (BMI) 34.7, and BMI Z-score 2.29. (B) 14.8-yr old, Tanner 4, BMI 37.2, and BMI Z-score 2.43.
Hierarchical regression analysis was used to determine whether relationships between anthropometric predictors and VAT volume differed among normal-weight and obese adolescents, controlling for gender, race, sex, and Tanner stage. All anthropometric measures predicted VAT better in obese than normal-weight adolescents (Table 3). Among normal-weight adolescents, all anthropometric measures except WHR were equally predictive of VAT (R2 values: 0.16–0.18), WHR did not significantly predict VAT, and no variable significantly entered any other’s regression models except that of WHR. By contrast, in obese adolescents, BMI Z-score was the strongest predictor of VAT volume, somewhat more than SAD (R2 = 0.39 vs. 0.31) and considerably more than WC, BMI, or WHR. Furthermore, WC and WHR failed to improve the predictive capacity of either SAD or BMI Z-score models, whereas both SAD and BMI Z-score significantly enhanced all other models in subsequent steps, with BMI Z-score contributing substantially more than SAD. Also, BMI Z-score significantly enhanced the SAD model but not the converse. Thus, all anthropometric measurements (except WHR) were equivalently predictive of VAT volume in normal-weight adolescents, while BMI Z-score was the strongest predictor of volumetric VAT in obese adolescents. Of note, BMI and BMI Z-score were not interchangeable; both added significantly to each other’s predictive capacity, suggesting that the two measures provide independent, important information about adiposity in obese adolescents.
Table 3.
Regression analysis—predictors of volumetric visceral fat
| Normal weight (n = 28)
|
Obese (n= 41)
|
|||
|---|---|---|---|---|
| R2 | p-Value | R2 | p-Value | |
| Base model (controlling for age, sex, race, and Tanner) | 0.28 | 0.28 | 0.23 | 0.14 |
| Sagittal abdominal diameter (SAD)* | 0.173 | 0.021 | 0.311 | < 0.0005 |
| After SAD | ||||
| WC adds | 0.028 | 0.33 | 0.006 | 0.50 |
| WHR adds | 0.000 | 0.92 | 0.012 | 0.36 |
| BMI adds | 0.031 | 0.30 | 0.017 | 0.26 |
| BMI Z-score adds | 0.025 | 0.36 | 0.062 | 0.028 |
| Waist circumference (WC)* | 0.181 | 0.018 | 0.261 | < 0.0005 |
| After WC | ||||
| SAD adds | 0.019 | 0.41 | 0.076 | 0.024 |
| WHR adds | 0.005 | 0.68 | 0.034 | 0.14 |
| BMI adds | 0.014 | 0.49 | 0.036 | 0.13 |
| BMI Z-score adds | 0.009 | 0.57 | 0.127 | 0.002 |
| Waist–hip ratio (WHR)* | 0.012 | 0.58 | 0.074 | 0.069 |
| After WHR | ||||
| SAD adds | 0.161 | 0.029 | 0.268 | < 0.0005 |
| WC adds | 0.175 | 0.022 | 0.221 | < 0.0005 |
| BMI adds | 0.181 | 0.020 | 0.185 | 0.002 |
| BMI Z-score adds | 0.167 | 0.026 | 0.320 | < 0.0005 |
| Body mass index (BMI)* | 0.179 | 0.019 | 0.259 | < 0.0005 |
| After BMI | ||||
| SAD adds | 0.025 | 0.35 | 0.089 | 0.014 |
| WC adds | 0.016 | 0.45 | 0.038 | 0.12 |
| WHR adds | 0.014 | 0.49 | < 0.0005 | 0.89 |
| BMI Z-score adds | 0.002 | 0.80 | 0.190 | < 0.0005 |
| BMI Z-score* | 0.162 | 0.026 | 0.388 | < 0.0005 |
| After BMI Z-score | ||||
| SAD adds | 0.035 | 0.27 | 0.005 | 0.54 |
| WC adds | 0.028 | 0.33 | 0.001 | 0.79 |
| WHR adds | 0.016 | 0.46 | 0.006 | 0.47 |
| BMI adds | 0.018 | 0.44 | 0.061 | 0.019 |
Regression model adjusted for age, sex, race, and Tanner stage.
Discussion
This study showed that in obese adolescents, BMI Z-score is a better predictor of VAT volume than SAD, and both measures outperformed BMI, WC, and WHR. This contrasts with findings in adults.
Identification of good anthropometric predictors of visceral adiposity is crucial, as visceral adiposity is associated with cardiometabolic risk (5) and all-cause mortality (6) in adults, and with insulin resistance and hypertriglyceridemia in children (7). The identification in this study that BMI Z-score was somewhat superior to SAD and substantially superior to WC as a predictor of VAT is particularly helpful, as both WC and SAD have practical disadvantages – WC is less standardized (at least three different methods of WC measurement are used in the literature) (33–35), while SAD measurement requires expensive equipment.
Our study had several other significant findings. First, VAT increased exponentially as BMI exceeded the 97th percentile for age [equivalent to BMI Z-score of 1.88 (26)]. Second, WHR was the weakest predictor of VAT. Third, all non-WHR anthropometric measures were less predictive of VAT in normal-weight than obese adolescents. Fourth, there were no significant differences in VAT volumes in our population relating to pubertal stage. Finally, African-American and Caucasian adolescents’ fat deposition patterns differed significantly, with VAT comprising a lower proportion of TAT among African-American adolescents. Anthropometric measures trended toward being stronger predictors of VAT volume in Caucasian than in African-American adolescents, albeit with differences not reaching statistical significance.
Inherent predispositions influencing fat partitioning patterns (36) may play a larger role in determining VAT when total adipose volume is smaller, rendering the relationships between surrogate markers and VAT more variable in lean than in obese adolescents. In obese teens, heredity-, race-, and sex-related contributions to fat deposition patterns may be minimal compared with the more predictable effects of the greater amount of adipose tissue. Additionally, lean mass comprises a greater proportion of total body mass in normal-weight than obese subjects (37); this may also render surrogate markers of visceral adiposity less useful in non-obese than obese adolescents.
The non-linear relationship between VAT and BMI Z-score is an important finding, which lends more evidence to the biological significance of the 2000 Centers for Disease Control (CDC) growth curves’ highest BMI percentile cutoff. Others have shown curvilinear relationships between BMI and adiposity measures [e.g., fat mass (21)] at higher BMI percentiles (38); however, to our knowledge, the exponential relationship with VAT has not been previously shown in adolescents.
In normal-weight subjects, all non-WHR anthropometric measures performed similarly in predicting VAT, whereas in obese subjects, BMI Z-score modestly outperformed SAD; both measures were modestly better than BMI and significantly better than WC in predicting VAT. This is in marked contrast to adults, in whom SAD is more highly correlated with VAT (18) than WC or BMI (39). One study even found a stronger association between VAT and SAD in normal-weight and moderately overweight than in obese adults (17), opposite of our findings. Also unlike adult findings, we found that WHR was the poorest predictor of VAT in our subjects. Finally, our subjects’ total VAT volume and VAT/TAT ratios were lower than the values reported in adults (40), underscoring the fact that adult findings cannot necessarily be applied to children.
Prior studies have examined body composition (29, 37, 38) and visceral adiposity (7) in children. However, most examined VAT mass rather than volume – an important methodological difference, as single-slice images are less precise than volumes calculated from multiple slices (41). To our knowledge, only one previous pediatric study has examined the association between anthropometric predictors and partitioned volumetric intra-abdominal fat (22). That study found marginally higher correlations between WC and VAT than either SAD or BMI and VAT. However, their sample size was limited (n = 28), and they did not adjust for race, sex, or puberty. These are important limitations, as this and prior studies have found that VAT mass is lower in African-American than Caucasian adolescents (30) and adults (24) despite a higher overall obesity prevalence.
An interesting finding from the hierarchical regression analysis was that BMI contributed significantly along with BMI Z-score in predicting VAT in obese subjects, suggesting that BMI and BMI Z-score are not interchangeable, although the latter derives from the former. There is a known limitation of BMI Z-score relating to skewness in BMI percentiles: differences between percentiles are significantly smaller below than above the 85th percentile. Thus, a wide range of BMI in the upper ranges (>40 kg/m2) becomes compressed into a very narrow range of BMI Z-scores (42). Absolute BMI values had additional predictive value in this select population.
Although adulthood rather than childhood obesity has been found to be the primary determinant of metabolic sequelae such as type 2 diabetes, childhood obesity is a strong risk factor for adulthood obesity (43) and is independently associated with increased risk of adulthood hypertension independently of adulthood obesity (43). Adolescence may be an especially crucial time, as SAT and VAT growth appears to occur at different rates in children (44), and adolescent BMI percentile changes reflect changes in lean as well as fat mass (21). That said, a significant BMI increase in adolescence predicts adult visceral fat accumulation (45). Thus, useful indicators of VAT are needed in adolescents.
This study had a number of strengths: a good sample size, robust imaging measures of VAT, comparison with a range of surrogate anthropometric measures, and adjustment for sex, race, and pubertal stage in the analyses. There were also some limitations. First and most important, there were fewer Caucasians than African-Americans, so power to detect associations in that subgroup (and possibly in-between groups) was more limited. Second, pubertal staging was based upon self-assessment, not examination by medical professionals, potentially limiting accuracy. Finally, abdominal skinfold thickness measurements were not performed, so we cannot compare their potential predictive capacity for volumetric visceral fat from abdominal skinfold measurements to the anthropometric measurements we examined. Future studies incorporating larger numbers of normal-weight subjects, Caucasians, and subjects from other ethnic and racial groups are indicated to expand our findings.
Given the metabolic importance of visceral adiposity, having good surrogate measures to predict VAT is crucial. In our study population, the risk of visceral adiposity increased exponentially in adolescents above the 97th BMI-for-age percentile. As neither SAD nor WC predicted visceral adiposity more strongly than the more easily obtained BMI and BMI Z-score, these results support using BMI Z-scores (or, in the very obese, BMI) over other technically more difficult anthropometric measures to predict who is at greatest risk of visceral adiposity.
Acknowledgments
The authors would like to thank Dr Lorraine E. Levitt Katz for her insightful comments. NHLBI (grant number R01HL058585) and NCRR (grant number UL1RR024134) provided support for carrying out this study.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Ogden CL, Flegal KM. Changes in terminology for childhood overweight and obesity. Natl Health Stat Report. 2010;25:1–5. [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307:483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss R, Dziura J, Burgert TS, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362–2374. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 4.Rosenbloom AL, Joe JR, Young RS, Winter WE. Emerging epidemic of type 2 diabetes in youth. Diabetes Care. 1999;22:345–354. doi: 10.2337/diacare.22.2.345. [DOI] [PubMed] [Google Scholar]
- 5.Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–1013. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 6.Kuk JL, Katzmarzyk PT, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral fat is an independent predictor of all-cause mortality in men. Obesity (Silver Spring) 2006;14:336–341. doi: 10.1038/oby.2006.43. [DOI] [PubMed] [Google Scholar]
- 7.Gower BA, Nagy TR, Goran MI. Visceral fat, insulin sensitivity, and lipids in prepubertal children. Diabetes. 1999;48:1515–1521. doi: 10.2337/diabetes.48.8.1515. [DOI] [PubMed] [Google Scholar]
- 8.Gomi T, Kawawa Y, Nagamoto M, Terada H, Kohda E. Measurement of visceral fat/subcutaneous fat ratio by 0. 3 tesla. MRI Radiat Med. 2005;23:584–587. [PubMed] [Google Scholar]
- 9.Pouliot MC, Despres JP, Lemieux S, et al. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994;73:460–468. doi: 10.1016/0002-9149(94)90676-9. [DOI] [PubMed] [Google Scholar]
- 10.de Ridder CM, de Boer RW, Seidell JC, et al. Body fat distribution in pubertal girls quantified by magnetic resonance imaging. Int J Obes Relat Metab Disord. 1992;16:443–449. [PubMed] [Google Scholar]
- 11.Fox K, Peters D, Armstrong N, Sharpe P, Bell M. Abdominal fat deposition in 11-year-old children. Int J Obes Relat Metab Disord. 1993;17:11–16. [PubMed] [Google Scholar]
- 12.Goran MI, Gower BA, Treuth M, Nagy TR. Prediction of intra-abdominal and subcutaneous abdominal adipose tissue in healthy pre-pubertal children. Int J Obes Relat Metab Disord. 1998;22:549–558. doi: 10.1038/sj.ijo.0800624. [DOI] [PubMed] [Google Scholar]
- 13.Gustat J, Elkasabany A, Srinivasan S, Berenson GS. Relation of abdominal height to cardiovascular risk factors in young adults: the Bogalusa heart study. Am J Epidemiol. 2000;151:885–891. doi: 10.1093/oxfordjournals.aje.a010292. [DOI] [PubMed] [Google Scholar]
- 14.Iribarren C, Darbinian JA, Lo JC, Fireman BH, Go AS. Value of the sagittal abdominal diameter in coronary heart disease risk assessment: cohort study in a large, multiethnic population. Am J Epidemiol. 2006;164:1150–1159. doi: 10.1093/aje/kwj341. [DOI] [PubMed] [Google Scholar]
- 15.Hoenig MR. MRI sagittal abdominal diameter is a stronger predictor of metabolic syndrome than visceral fat area or waist circumference in a high-risk vascular cohort. Vasc Health Risk Manag. 2010;6:629–633. doi: 10.2147/vhrm.s10787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pimentel GD, Moreto F, Takahashi MM, Portero-McLellan KC, Burini RC. Sagittal abdominal diameter, but not waist circumference is strongly associated with glycemia, triacylglycerols and HDL-C levels in overweight adults. Nutr Hosp. 2011;26:1125–1129. doi: 10.1590/S0212-16112011000500031. [DOI] [PubMed] [Google Scholar]
- 17.Zamboni M, Turcato E, Armellini F, et al. Sagittal abdominal diameter as a practical predictor of visceral fat. Int J Obes Relat Metab Disord. 1998;22:655–660. doi: 10.1038/sj.ijo.0800643. [DOI] [PubMed] [Google Scholar]
- 18.Sampaio LR, Simoes EJ, Assis AM, Ramos LR. Validity and reliability of the sagittal abdominal diameter as a predictor of visceral abdominal fat. Arq Bras Endocrinol Metabol. 2007;51:980–986. doi: 10.1590/s0004-27302007000600013. [DOI] [PubMed] [Google Scholar]
- 19.Kvist H, Chowdhury B, Grangard U, Tylen U, Sjostrom L. Total and visceral adipose-tissue volumes derived from measurements with computed tomography in adult men and women: predictive equations. Am J Clin Nutr. 1988;48:1351–1361. doi: 10.1093/ajcn/48.6.1351. [DOI] [PubMed] [Google Scholar]
- 20.van der Kooy K, Leenen R, Seidell JC, Deurenberg P, Visser M. Abdominal diameters as indicators of visceral fat: comparison between magnetic resonance imaging and anthropometry. Br J Nutr. 1993;70:47–58. doi: 10.1079/bjn19930104. [DOI] [PubMed] [Google Scholar]
- 21.Demerath EW, Schubert CM, Maynard LM, et al. Do changes in body mass index percentile reflect changes in body composition in children? Data from the Fels Longitudinal Study. Pediatrics. 2006;117:e487–e495. doi: 10.1542/peds.2005-0572. [DOI] [PubMed] [Google Scholar]
- 22.Siegel MJ, Hildebolt CF, Bae KT, Hong C, White NH. Total and intraabdominal fat distribution in preadolescents and adolescents: measurement with MR imaging. Radiology. 2007;242:846–856. doi: 10.1148/radiol.2423060111. [DOI] [PubMed] [Google Scholar]
- 23.Mihalopoulos NL, Holubkov R, Young P, Dai S, Labarthe DR. Expected changes in clinical measures of adiposity during puberty. J Adolesc Health. 2010;47:360–366. doi: 10.1016/j.jadohealth.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katzmarzyk PT, Bray GA, Greenway FL, et al. Racial differences in abdominal depot-specific adiposity in white and African American adults. Am J Clin Nutr. 2010;91:7–15. doi: 10.3945/ajcn.2009.28136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duke PM, Litt IF, Gross RT. Adolescents’ self-assessment of sexual maturation. Pediatrics. 1980;66:918–920. [PubMed] [Google Scholar]
- 26.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002;314:1–190. [PubMed] [Google Scholar]
- 27.Klein S, Allison DB, Heymsfield SB, et al. Waist circumference and cardiometabolic risk: a consensus statement from shaping America’s health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Diabetes Care. 2007;30:1647–1652. doi: 10.2337/dc07-9921. [DOI] [PubMed] [Google Scholar]
- 28.Sutherland K, Lee RW, Phillips CL, et al. Effect of weight loss on upper airway size and facial fat in men with obstructive sleep apnoea. Thorax. 2011;66:797–803. doi: 10.1136/thx.2010.151613. [DOI] [PubMed] [Google Scholar]
- 29.Wells JC, Fewtrell MS, Williams JE, Haroun D, Lawson MS, Cole TJ. Body composition in normal weight, overweight and obese children: matched case-control analyses of total and regional tissue masses, and body composition trends in relation to relative weight. Int J Obes (Lond) 2006;30:1506–1513. doi: 10.1038/sj.ijo.0803402. [DOI] [PubMed] [Google Scholar]
- 30.Bacha F, Saad R, Gungor N, Janosky J, Arslanian SA. Obesity, regional fat distribution, and syndrome X in obese black versus white adolescents: race differential in diabetogenic and atherogenic risk factors. J Clin Endocrinol Metab. 2003;88:2534–2540. doi: 10.1210/jc.2002-021267. [DOI] [PubMed] [Google Scholar]
- 31.Liu J, Fox CS, Hickson DA, et al. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: the Jackson Heart Study. J Clin Endocrinol Metab. 2010;95:5419–5426. doi: 10.1210/jc.2010-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goran MI, Nagy TR, Treuth MS, et al. Visceral fat in white and African American prepubertal children. Am J Clin Nutr. 1997;65:1703–1708. doi: 10.1093/ajcn/65.6.1703. [DOI] [PubMed] [Google Scholar]
- 33.McCarthy HD, Jarrett KV, Crawley HF. The development of waist circumference percentiles in British children aged 5.0–16. 9 y. Eur J Clin Nutr. 2001;55:902–907. doi: 10.1038/sj.ejcn.1601240. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr. 2004;145:439–444. doi: 10.1016/j.jpeds.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 35.Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch Pediatr Adolesc Med. 2003;157:821–827. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- 36.Rolland-Cachera MF, Bellisle F, Deheeger M, Pequignot F, Sempe M. Influence of body fat distribution during childhood on body fat distribution in adulthood: a two-decade follow-up study. Int J Obes. 1990;14:473–481. [PubMed] [Google Scholar]
- 37.Freedman DS, Wang J, Maynard LM, et al. Relation of BMI to fat and fat-free mass among children and adolescents. Int J Obes (Lond) 2005;29:1–8. doi: 10.1038/sj.ijo.0802735. [DOI] [PubMed] [Google Scholar]
- 38.Flegal KM, Ogden CL, Yanovski JA, et al. High adiposity and high body mass index-for-age in US children and adolescents overall and by race-ethnic group. Am J Clin Nutr. 2010;91:1020–1026. doi: 10.3945/ajcn.2009.28589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yim JY, Kim D, Lim SH, et al. Sagittal abdominal diameter is a strong anthropometric measure of visceral adipose tissue in the Asian general population. Diabetes Care. 2010;33:2665–2670. doi: 10.2337/dc10-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maislin G, Ahmed MM, Gooneratne N, et al. Single slice vs. volumetric MR assessment of visceral adipose tissue: reliability and validity among the overweight and obese. Obesity (Silver Spring) 2012;20:2124–2132. doi: 10.1038/oby.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas EL, Bell JD. Influence of undersampling on magnetic resonance imaging measurements of intra-abdominal adipose tissue. Int J Obes Relat Metab Disord. 2003;27:211–218. doi: 10.1038/sj.ijo.802229. [DOI] [PubMed] [Google Scholar]
- 42.Woo JG. Using body mass index Z-score among severely obese adolescents: a cautionary note. Int J Pediatr Obes. 2009;4:405–410. doi: 10.3109/17477160902957133. [DOI] [PubMed] [Google Scholar]
- 43.Juonala M, Magnussen CG, Berenson GS, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011;365:1876–1885. doi: 10.1056/NEJMoa1010112. [DOI] [PubMed] [Google Scholar]
- 44.Huang TT, Johnson MS, Figueroa-Colon R, Dwyer JH, Goran MI. Growth of visceral fat, subcutaneous abdominal fat, and total body fat in children. Obes Res. 2001;9:283–289. doi: 10.1038/oby.2001.35. [DOI] [PubMed] [Google Scholar]
- 45.Kindblom JM, Lorentzon M, Hellqvist A, et al. BMI changes during childhood and adolescence as predictors of amount of adult subcutaneous and visceral adipose tissue in men: the GOOD Study. Diabetes. 2009;58:867–874. doi: 10.2337/db08-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]