Abstract
Purpose of review
Genome wide associations studies (GWAS) have been used as an unbiased tool to identify novel genes that contribute to variations in LDL-C levels in the hopes of uncovering new biology and new therapeutic targets for the treatment of atherosclerotic cardiovascular disease (ASCVD). The locus identified by GWAS with the strongest association with LDL-C and ASCVD is the 1p13 SORT1 locus. Here we review the identification and characterization of this locus, the initial physiological studies describing the role of SORT1 in lipoprotein metabolism, and recent work that has begun to sort out the complexity of this role.
Recent findings
Studies by several groups support an important role for sortilin in lipoprotein metabolism; however, the directionality of the effect of sortilin on plasma cholesterol and its role in the secretion of hepatic lipoproteins remains controversial. Studies by several groups support a role for sortilin in inhibiting lipoprotein export whereas other studies suggest that sortilin facilitates lipoprotein export.
Summary
Understanding the mechanism by which sortilin affects LDL-C will increase our understanding of the regulation of lipoprotein metabolism and hepatic lipoprotein export and may also allow us to harness the power of the 1p13 SORT1 locus for the treatment of ASCVD.
Keywords: Sort1, genome wide association study, atherosclerotic cardiovascular disease, low-density lipoprotein cholesterol, lipid metabolism
Introduction
Atherosclerotic cardiovascular disease (ASCVD) and its sequela of myocardial infarction (MI) is the leading cause of morbidity and mortality in the developed world[1]. The lifetime risk of developing ASCVD is strongly influenced by environmental factors including smoking, alcohol consumption, obesity, and sedentary lifestyle and also by medical risk factors such as diabetes, hypertension, decreased high density lipoprotein cholesterol (HDL-C) and increased levels of low-density lipoprotein cholesterol (LDL-C). LDL-C is intimately involved in each stage of atherosclerotic lesion development; LDL in plasma can be modified and taken up by macrophages, leading to macrophage engorgement and foam cell formation, cytokine release, vascular smooth muscle cell proliferation, and atherosclerotic plaque formation[2].
ASCVD is highly heritable due to the strong influence of genetics on LDL-C and other cardiovascular risk factors as well as directly on the atherosclerotic process. Some of the most dramatic examples of the heritability of LDL-C levels and ASCVD risk come from the study of rare Mendelian disorders of hypercholesterolemia including autosomal dominant hypercholesterolemia, which is caused by mutations in LDLR, PCSK9, and APOB, autosomal recessive hypercholesterolemia, which is caused by loss of function mutations in LDLRAP1, and sitosterolemia, which is caused by mutations in ABCG5 or ABCG8. All of these disorders are characterized by markedly increased levels of LDL-C and premature ASCVD development[3].
Though Mendelian disorders provide a dramatic example of the influence of genetics on ASCVD development, they do not explain most of the variability in LDL-C levels and ASCVD risk in the population. Genome wide association studies (GWAS) have identified novel loci associated with LDL-C and ASCVD at the population level[4–9]. One of the most compelling novel loci in the human genome associated with both LDL-C and ASCVD is a locus on chromosome 1p13. The study of this locus has served as a paradigm for moving from locus identification by GWAS to biological mechanism through functional genomics. Here we review the early work of identifying the causal gene at this locus and then discuss the complexity of the physiology that has arisen as a result of these studies.
Identification of the causal gene at 1p13 as SORT1
Determination of the causal gene at the 1p13 LDL/MI locus was complicated by the locus structure; seven genes map to the associated region, SARS, CELSR2, PSRC1, MYBPHL, SORT1, PSMA5, and SYPL2, the associated SNPs lie in a noncoding region between CELSR2 and PSRC1, and none of the seven genes have a clear role in lipoprotein metabolism[10]. Using expression quantitative trait loci (eQTL) data in human liver, Musunuru et al demonstrated an association between the minor allele haplotype and elevated expression of three genes at the locus, PSRC1, CELSR2 and SORT1, with SORT1 and PSRC1 expression most strongly affected, increasing 5-6 fold with each copy of the minor allele. Importantly, expression of these genes in omental and subcutaneous adipose was not influenced by genotype, suggesting a liver-specific phenomenon. Linsel-Nitschke et al replicated the association between minor allele homozygosity at 1p13 and reduced LDL-C and protection from ASCVD in an independent population and further found that homozygosity for the minor allele haplotype correlated with increased expression of SORT1 in peripheral blood with no change in expression of CELSR2 or PSRC1 [11].
With the eQTL data most strongly implicating PSRC1 or SORT1 as the causal gene and the liver as a relevant organ, efforts began to study the effects of overexpression and deficiency of these genes in liver. Adeno-associated viruses (AAV) were used to overexpress Psrc1 and Sort1 in hyperlipidemic Ldlr−/− mice, and while Psrc1 overexpression had no effect on plasma cholesterol, Sort1 overexpression led to a 40% reduction in plasma cholesterol [10, 12](Table One). As a complementary approach, careful phenotyping of Psrc1+/− and Psrc1−/− mice did not reveal any differences in plasma cholesterol, whereas Sort1 knockdown in mouse liver using siRNAs was associated with a 20-40% increase in plasma cholesterol [10, 12]. These results strongly implicated SORT1 as the causal gene at the 1p13 locus whose elevated expression confers reduction in LDL-C.
Table One.
Summary Of The Effects Of Sort1 Manipulation On Lipoprotein Metabolism In Cells And Mice
| Directionality | Model System |
Mechanism Of Manipulation |
Plasma Cholesterol |
ApoB Secretion |
LDL Clearance |
Reference |
|---|---|---|---|---|---|---|
| Overexpression | Apobec1−/−; Ldlr−/− Mice | AAV | Decreased | ----- | ----- | Musunuru et al[10] |
| Apobec1−/−; hAPOBTg Mice | AAV | Decreased | Decreased | ----- | Musunuru et al [10] | |
| Apobec1−/−; Ldlr+/−; hAPOBTg Mice | AAV | Decreased | Decreased | ----- | Musunuru et al [10] and Strong et al [13] | |
| Apobec1−/−; Ldlr−/−; hAPOBTg Mice | AAV | Decreased | ----- | ----- | Musunuru et al [10] | |
| Ob/ob Mice | AAV | ----- | Decreased | ----- | Ai et al [14] | |
| DIO Mice | AAV | ----- | Decreased | ----- | Ai et al [14] | |
| Wild-type Mice | AAV | Decreased | Decreased | Increased | Strong et al [13] | |
| Ldlr−/− Mice | AAV | Decreased | Decreased | Increased | Strong et al [13] | |
| Wild Type Mice | Adeno | Decreased | ----- | ----- | Bi et al [15] | |
| Ob/ob Mice | Adeno | Decreased | ----- | ----- | Bi et al [15] | |
| HEK293 Cells | Transfection | ----- | ----- | Increased | Linsel-Nitschke et al [11] and Strong et al [13] | |
| HuH7 Cells | Transduction | ----- | Decreased | Increased | Strong et al [13] | |
| McA Cells | Transduction | ----- | Decreased | ----- | Strong et al [13] | |
| CHO Cells | Transfection | ----- | ----- | Increased | Strong et al [13] | |
| ldlD Cells | Transfection | ----- | ----- | Increased | Strong et al [13] | |
| HELA-TREx Cells | Transfection | ----- | ----- | Increased | Tveten et al [16] | |
| Primary Mouse Hepatocytes | Transduction | ----- | Decreased | ----- | Bi et al [15] | |
| HepG2 Cells | Transduction | ----- | Decreased | ----- | Bi et al [15] | |
| Knockdown | HUES Cells | TALEN mediated Gene Deletion | Increased apoB | ----- | ----- | Ding et al [17] |
| Apobec1−/−; Ldlr−/− Mice | siRNA | Increased | ----- | ----- | Musunuru et al [10] | |
| Apobec1−/−; hAPOBTg Mice | siRNA | Increased | ----- | ----- | Musunuru et al [10] | |
| Apobec1−/−; Ldlr+/−; hAPOBTg Mice | siRNA | Increased | ----- | ----- | Musunuru et al [10] | |
| Sort1−/− Mouse | Knockout | Decreased | Decreased | No Difference | Kjolby et al [18] | |
| Sort1−/−; Ldlr−/− Mouse | Knockout | Decreased | ----- | ----- | Kjolby et al [18] | |
| Sort1−/− Mouse | Knockout | No Difference | Decreased | Decreased | Strong et al [13] | |
| Sort1−/−; Ldlr−/− Mouse | Knockout | Decreased | ----- | Decreased | Strong et al [13] | |
| Apobec1−/−; Sort1−/−; hAPOBTg Mouse | Knockout | No Difference | Decreased | ----- | Strong et al [13] |
Early studies of the effect of sortilin on lipoprotein uptake
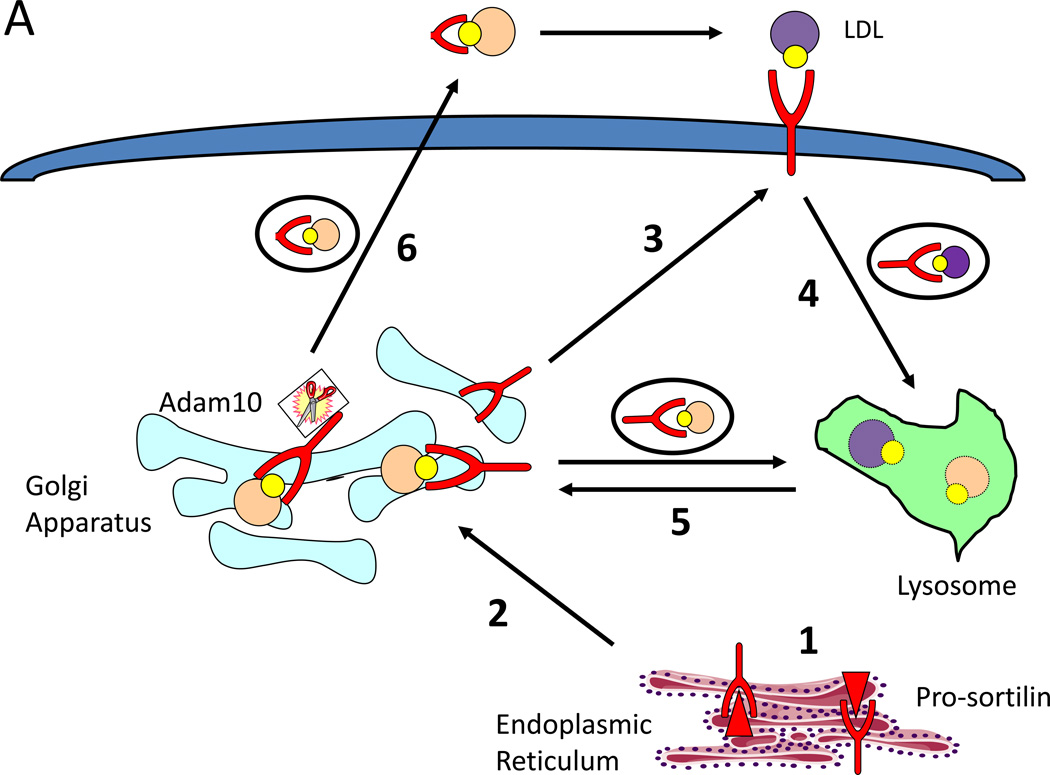
SORT1 encodes the protein sortilin, a VPS10 multi-ligand sorting receptor involved in Golgi to lysosome trafficking. Like other VPS10 proteins, sortilin consists of an N-terminal propeptide with a furin cleavage site, an extracellular VPS10 domain for ligand binding, a transmembrane domain and a cytoplasmic tail harboring two lysosomal sorting motifs[19, 20]. Sortilin localizes primarily to the Golgi apparatus [20, 21] where it serves as a trafficking receptor to sort lysosomal hydrolases to the lysosome [19, 22, 23], and a small fraction also traffics to the cell surface where it can act as a signaling receptor for pro-neurotrophins [24, 25], serve as an uptake receptor [26–28], or be cleaved by the alpha secretase A Disintegrin And Metalloproteinase domain-containing protein 10 (Adam10) to generate a soluble extracellular domain of yet poorly characterized function [29]. Sortilin has many ligands; however, at the time of its discovery as a GWAS hit for LDL-C, the only known lipid related ligand was lipoprotein lipase (LPL), which is involved principally in triglyceride metabolism, failing to explain the strong association between elevated SORT1 expression and reduced LDL-C [30].
With the identification of SORT1 as the causal gene, efforts began to determine the mechanism by which increased hepatic expression of SORT1 reduced plasma LDL-C. Though initially characterized as an intracellular sorting receptor, a cell surface role for sortilin in ligand uptake is becoming increasingly appreciated; recent studies have found that sortilin acts as a cell surface internalization receptor for progranulin [26], apolipoprotein E [27], and alpha-galactosidase A [28]. Such observations raised the question of whether LDL itself might be a ligand for sortilin. Through a series of in vitro LDL uptake studies and in vivo kinetic studies, Strong et al showed that increased Sort1 expression in mouse liver and in multiple different cell lines increased LDL clearance [13]. Conversely, Sort1 deficiency was found to impair LDL clearance in vivo. Importantly, absence of the LDL receptor did not reduce the effect of Sort1 overexpression or deficiency on LDL uptake, suggesting an LDL receptor independent mechanism. Strong et al further demonstrated that sortilin serves as a bona fide cell surface receptor for LDL and facilitates its cellular uptake and lysosomal degradation. These findings are supported by studies from other laboratories. Linsel-Nitschke et al reported that SORT1 overexpression in HEK293 cells increased LDL uptake and that this increase is abrogated by co-incubation with known sortilin ligands including RAP and LPL [11]. Tveten et al showed that sortilin overexpression in HeLa-T-REx cells increases LDL surface binding and uptake, and a sortilin trafficking mutant that localizes to the plasma membrane and is deficient in its ability to traffic to the endolysosomal system increases LDL cell surface binding, supporting a direct cell surface interaction between sortilin and LDL [16]. An additional study also demonstrated impaired clearance of VLDL and chylomicrons in the context of reduced Sort1 expression, also consistent with a role for sortilin in the clearance of apoB-containing lipoproteins [31]. These studies suggest that hepatic sortilin influences LDL-C levels at least in part through promoting its uptake and degradation (Table One).
Sortilin and hepatic lipoprotein export
In addition to a role in the clearance of LDL particles, Musunuru et al also found that AAV-mediated hepatic expression of Sort1 in the livers of mice reduced the VLDL secretion rate contributing to the reduced plasma cholesterol levels seen in Sort1-overexpressing mice [10], consistent with the directionality predicted by the human data. In contrast, Kjolby et al reported that Sort1 deficient mice also had a reduced VLDL secretion rate with a concomitant reduction in plasma cholesterol levels [18]. These seemingly conflicting reports called into question the physiological role of hepatic sortilin in regulating VLDL secretion and plasma cholesterol levels and at the most basic level left unanswered the fundamental question of the directionality by which hepatic sortilin modulates LDL metabolism (Table One).
Kjolby et al used surface plasmon reasonance (SPR) studies, cell fractionation studies, and pulse chase experiments in loss-of-function models to convincingly suggest a role for sortilin in facilitating VLDL export. Strong et al and used surface plasmon reasonance, sortilin trafficking mutants, and lysosome inhibition to demonstrate that sortilin inhibited VLDL secretion by directly binding apoB-containing lipoproteins in the Golgi apparatus and promoting their presecretory lysosomal degradation [13]. The general finding that increased hepatic Sort1 expression reduces VLDL secretion through a lysosomal pathway was supported by other laboratories, as well. Ai et al reported that pharmacological and genetic manipulations that increase Sort1 expression are associated with reductions in VLDL secretion, whereas pharmacological and genetic manipulations that reduce Sort1 expression increase VLDL secretion. Ai et al further demonstrated that Sort1-specific siRNAs in systems with genetically increased Sort1 expression restore VLDL secretion, whereas reconstitution of Sort1 in models with reduced Sort1 expression normalizes VLDL secretion [14]. Bi et al also reported reductions in VLDL secretion in hepatocytes overexpressing Sort1 [15]. Chamberlain et al showed that insulin, a known reducer of VLDL secretion, promotes the association of sortilin and apoB-containing lipoproteins and increases their lysosomal localization [32] (Table One). A role for sortilin in the lysosomal degradation of its ligands has become increasingly recognized, and sortilin has been reported to traffic adiponectin [33], TGF-B [34], alpha-1 antitrypsin [35] and the sodium chloride cotransporter [36] to the lysosome for degradation.
A second wave of literature emerged, which used mouse and cell models with reduced hepatic Sort1 expression to study the role of Sort1 in cholesterol metabolism. Jun et al reported that Ins2+/Akita; Apoe−/− mice that spontaneously develop type I diabetes and hypoleptinemia have reduced hepatic Sort1 expression accompanied by hypercholesterolemia and increased atherosclerosis [37]. Leptin replacement restored hepatic Sort1 expression and reduced plasma cholesterol and atherosclerotic disease burden. Klingenberg et al found that depletion of regulatory T-cells reduced hepatic Sort1 expression and increased plasma cholesterol and atherosclerotic disease burden [31]. Bi et al found that mouse models and humans with reduced hepatic Sort1 expression have increased plasma cholesterol and reconstitution of Sort1 expression using adenovirus rescues the hypercholesterolemia [15]. As a more direct approach, Ding et al used TALENs to functionally delete the SORT1 locus in HUES cells and reported that HUES cells deficient in SORT1 expression have increased apoB mass in the media when differentiated into hepatocytes [17]. All of these studies are consistent with the directionality of the human genetic data and suggest that reduced hepatic SORT1 increases plasma cholesterol (Table One).
A number of reviews were published [12, 38–41] proposing a variety of explanations for the discrepant findings: total body Sort1 deficiency versus liver-specific manipulations, the genetic background used in each study, adenovirus versus adeno-associated virus, western-type diet versus chow diet, and the nature of the knockout mouse model itself, though no conclusions were reached. Strong et al, in confirmation of Kjolby et al, reported that an independently generated Sort1−/− mouse with no residual Sort1 expression also had reduced VLDL secretion on both a wild-type and Apobec1−/−; hAPOB Tg background [13]. Thus the fundamental question remained: why do both sortilin deficiency and overexpression reduce VLDL secretion?
Discussion: Resolving the controversy
One hypothesis put forth to explain the contradictory findings was that the Sort1 deficiency studies were done in a total body knockout mouse, whereas the GWAS, overexpression and knockdown studies all involved liver-specific manipulations exclusively, so the knockout phenotype could be driven by sortilin deficiency in extra-hepatic tissues. Inconsistent with this hypothesis is the finding reported both by Kjolby et al and Strong et al that primary hepatocytes isolated from Sort1−/− mice have reduced VLDL secretion. Studies in a liver specific Sort1 conditional knockout mouse should definitively address this issue.
Another hypothesis is that there is a fundamental difference between partial reduction and total deficiency of Sort1 expression. The most likely model to explain this is that sortilin serves as both a chaperone and degrader of apoB-containing lipoproteins in a concentration dependent manner. Specifically, low levels of sortilin may be required for efficient VLDL export; however, at higher sortilin levels, such as those seen in individuals homozygous for the minor allele haplotype at 1p13, sortilin may promote the degradation of pre-secretory VLDL. Consistent with the hypothesis that sortilin can serve as both a chaperone and degrader of its ligands, Evans et al reported that sortilin facilitates the secretion as well as the lysosomal targeting and degradation of proBDNF. Specifically, Evans et al identified Adam10 as the metallopeptidase that cleaves sortilin at the juxtamembrane stalk both intracellularly and at the plasma membrane, separating sortilin’s ligand binding domain from its lysosomal sorting motifs. They further suggested that proBDNF bound to full length sortilin that is not cleaved by Adam10 is trafficked with sortilin to the lysosome for degradation, whereas proBDNF bound to cleaved sortilin is secreted from cells [29]. One can envision a similar paradigm for apoB-containing lipoproteins: under physiological conditions the majority of sortilin is cleaved by Adam10 and facilitates VLDL secretion, so loss of sortilin reduces VLDL secretion, whereas in the context of increased Sort1 expression, Adam10 is limiting, and most sortilin remains full length and facilitates the endolysosomal degradation of VLDL (Figure 1A). This cleavage pathway may explain the reduction in VLDL secretion seen with both Sort1 overexpression and deficiency.
Figure One.
Potential models to reconcile the overexpression and deficiency data
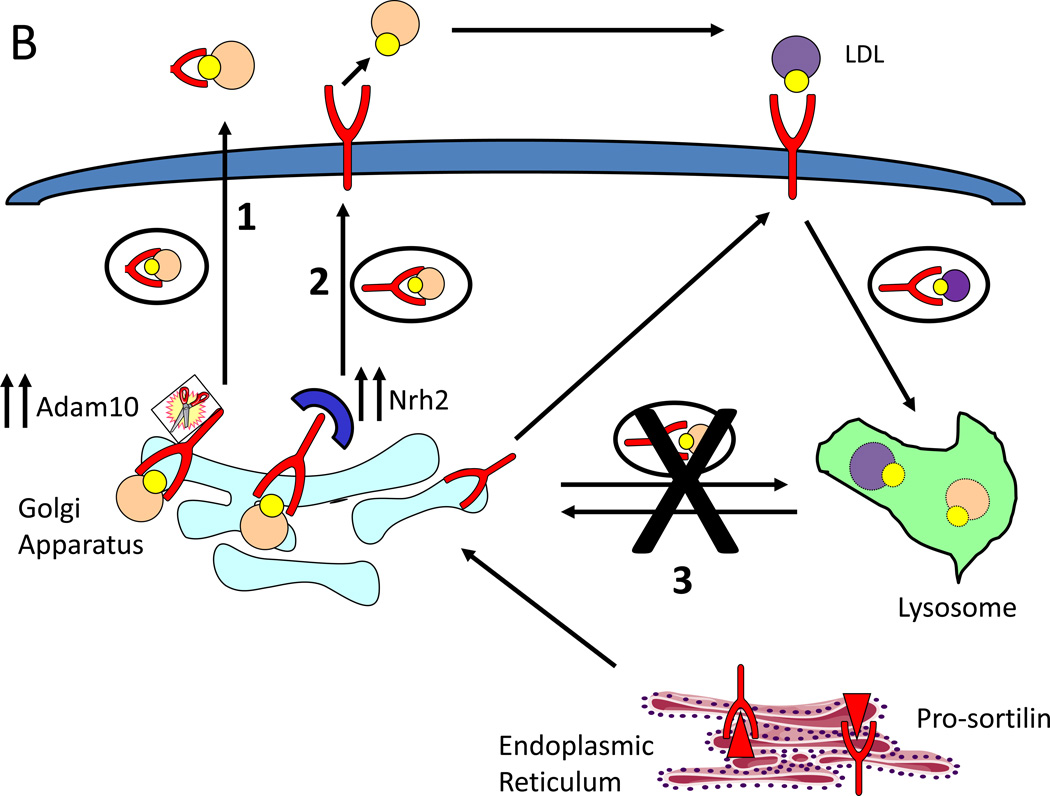
Sortilin cleavage alone cannot explain why reductions in Sort1 expression in different genetic systems can lead to both increased and decreased VLDL secretion. The answer may lie in recent advances in understanding sortilin trafficking and regulation. Generally accepted dogma is that 10% of sortilin localizes to the plasma membrane while 90% is intracellular; however, Kim et al found that this ratio can be altered and that neurotrophin receptor homolog 2 (Nrh2) is a molecular switch that promotes the trafficking of sortilin to the cell surface [42]. This begs the question, what factors affect sortilin trafficking and what effect does sortilin redistribution have on VLDL secretion?
ER stress is emerging as an important modulator atherosclerosis and VLDL secretion [43]. Importantly, the studies by Ai et al, Jun et al and Klingenberg et al which found an association between reductions in Sort1 expression and increased VLDL secretion were all done in mouse models of ER stress. Nrh2 and Adam10 are known ER stress responsive genes [44, 45]. It is possible that on the genetic backgrounds of ER stress used by Ai et al, Jun et al and Klingenberg et al there is increased Nrh2 and Adam10 expression, increasing the ratio of cleaved to full length sortilin and diverting sortilin away from the endolysosomal system, thereby driving the sortilin chaperone function (Figure 1B). Conversely, in the absence of ER stress such as the models used by Strong et al and Kjolby et al Adam10 and Nrh2 are not upregulated, there is a reduction in cleaved sortilin due to the reduction in total Sort1 expression, and VLDL secretion becomes compromised. Further studies will have to be done to address these hypotheses.
Perspectives And Future Directions
Clearly, the role of sortilin in cholesterol metabolism is multi-faceted and complex and much work is needed to elucidate the intricacies of the biology (Table One). Recently, a number of new sortilin-interacting partners have been described, most notably PCSK9. Gustafsen et al reported a high affinity interaction between sortilin and PCSK9 and proposed a model in which sortilin binds PCSK9 and facilitates its secretion. Gustafsen et al found that Sort1−/− mice have increased intracellular levels of PCSK9, reduced plasma PCSK9 and increased LDL receptor levels [46].
The role of sortilin in atherosclerotic disease is a subject of intense investigation. Tveten et al sequenced the SORT1 gene in more than 800 hypercholesterolemic individuals and found no pathological mutations and concluded that SORT1 mutations are unlikely to cause autosomal dominant hypercholesterolemia [16]. Interestingly, Mendoza-Barberá identified three novel mutations in ApoAV in three individuals with unexplained hypertriglyceridemia, and in vitro functional studies show that these mutants are impaired in their ability to interact with sortilin [47]. Finally, the role of sortilin in the blood vessel wall is emerging as an important factor in atherosclerotic disease. Campagnolo et al reported that sortilin is expressed in atherosclerotic lesions and plays a role in vascular smooth muscle cell remodeling and apoptosis [48], Jones et al reported a genetic association between the SORT1 locus and abdominal aortic aneurysm independent of the lipid and atherosclerotic association [49, 50], and Aikawa et al has recently described a role for sortilin in vascular calcification. Beyond these novel avenues, further work is still needed to elucidate the precise mechanistic relationship between sortilin and VLDL secretion and the basis of the association between elevated SORT1 expression and small dense LDL subspecies.
Conclusion
The SORT1 locus represents a promising target for the treatment of ASCVD; however the disparate findings in Sort1 overexpression versus deficiency systems calls into question the therapeutic strategy that should be pursued – while overexpression studies suggest that SORT1 overexpression will reduce LDL-C and ASCVD risk, deficiency studies suggest that SORT1 inhibition will be of therapeutic benefit. The sortilin field is at its infancy, and hopefully we can look forward to many new discoveries in this intriguing and novel pathway of lipoprotein metabolism.
Key Points.
The locus identified by GWAS with the strongest association with LDL-C and ASCVD is the novel 1p13 SORT1 locus
Sortilin reduces LDL-C by serving as an LDL receptor independent pathway for LDL clearance and by promoting the presecretory lysosomal degradation of VLDL
The Sort1−/− mouse has a paradoxical reduction in VLDL secretion
The effect of reductions in Sort1 expression on VLDL secretion remains controversial with disparate findings in different animal models
Acknowledgements
The work in the authors’ laboratory is supported by grants from the National Institute of Health and from the Foundation Leducq.
Footnotes
There are no conflicts of interest relevant to this article to report.
References
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• outstanding interest
- 1.Lloyd-Jones D, et al. Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121(7):948–954. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 2.Lusis AJ. Atherosclerosis. Nature. 2000;407(6801):233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hobbs H, Rader DJ. In: Lipoprotein Metabolism, in Harrison's Principles of Internal Medicine. Kasper D, Braunwald E, Fauci AS, editors. New York: McGraw Hill Publishers; 2007. pp. 2416–2429. [Google Scholar]
- 4.Kathiresan S, et al. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat Genet. 2008;40(2):189–197. doi: 10.1038/ng.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willer CJ, et al. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet. 2008;40(2):161–169. doi: 10.1038/ng.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kathiresan S, et al. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat Genet. 2009;41(1):56–65. doi: 10.1038/ng.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teslovich TM, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466(7307):707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samani NJ, et al. Genomewide association analysis of coronary artery disease. N Engl J Med. 2007;357(5):443–453. doi: 10.1056/NEJMoa072366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schunkert H, et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. 2011;43(4):333–338. doi: 10.1038/ng.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Musunuru K, et al. From noncoding variant to phenotype via SORT1 at the 1p13 cholesterol locus. Nature. 2010;466(7307):714–719. doi: 10.1038/nature09266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linsel-Nitschke P, et al. Genetic variation at chromosome 1p13.3 affects sortilin mRNA expression, cellular LDL-uptake and serum LDL levels which translates to the risk of coronary artery disease. Atherosclerosis. 2010;208(1):183–189. doi: 10.1016/j.atherosclerosis.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 12.Strong A. Sortilin as a novel regulator of plasma cholesterol, very-low density lipoprotein secretion and LDL catabolism, in Cellular and Molecular Biology. Philadelphia: University of Pennsylvania; 2012. [Google Scholar]
- 13.Strong A, et al. Hepatic sortilin regulates both apolipoprotein B secretion and LDL catabolism. J Clin Invest. 2012;122(8):2807–2816. doi: 10.1172/JCI63563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ai D, et al. Activation of ER stress and mTORC1 suppresses hepatic sortilin-1 levels in obese mice. J Clin Invest. 2012;122(5):1677–1687. doi: 10.1172/JCI61248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bi L, et al. Saturated fatty acids activate ERK signaling to downregulate hepatic sortilin 1 in obese and diabetic mice. J Lipid Res. 2013;54(10):2754–2762. doi: 10.1194/jlr.M039347. This study found that mouse and human models with reduced Sort1 expression have increased plasma cholesterol and Sort1 overexpression reduces plasma cholesterol and apoB secretion.
- 16.Tveten K, et al. Mutations in the SORT1 gene are unlikely to cause autosomal dominant hypercholesterolemia. Atherosclerosis. 2012;225(2):370–375. doi: 10.1016/j.atherosclerosis.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 17. Ding Q, et al. A TALEN genome-editing system for generating human stem cell-based disease models. Cell Stem Cell. 2013;12(2):238–251. doi: 10.1016/j.stem.2012.11.011. This study used TALENs to genetically delete SORT1 from HUES cells and found that SORT1 deletion is associated with increased apoB mass.
- 18.Kjolby M, et al. Sort1, encoded by the cardiovascular risk locus 1p13.3, is a regulator of hepatic lipoprotein export. Cell Metab. 2010;12(3):213–223. doi: 10.1016/j.cmet.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen MS, et al. The sortilin cytoplasmic tail conveys Golgi-endosome transport and binds the VHS domain of the GGA2 sorting protein. EMBO J. 2001;20(9):2180–2190. doi: 10.1093/emboj/20.9.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen CM, et al. Molecular identification of a novel candidate sorting receptor purified from human brain by receptor-associated protein affinity chromatography. J Biol Chem. 1997;272(6):3599–3605. doi: 10.1074/jbc.272.6.3599. [DOI] [PubMed] [Google Scholar]
- 21.Morris NJ, et al. Sortilin is the major 110-kDa protein in GLUT4 vesicles from adipocytes. J Biol Chem. 1998;273(6):3582–3587. doi: 10.1074/jbc.273.6.3582. [DOI] [PubMed] [Google Scholar]
- 22.Canuel M, et al. Sortilin mediates the lysosomal targeting of cathepsins D and H. Biochem Biophys Res Commun. 2008;373(2):292–297. doi: 10.1016/j.bbrc.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 23.Lefrancois S, et al. The lysosomal trafficking of sphingolipid activator proteins (SAPs) is mediated by sortilin. EMBO J. 2003;22(24):6430–6437. doi: 10.1093/emboj/cdg629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nykjaer A, et al. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004;427(6977):843–848. doi: 10.1038/nature02319. [DOI] [PubMed] [Google Scholar]
- 25.Arnett MG, Ryals JM, Wright DE. Pro-NGF, sortilin, and p75NTR: potential mediators of injury-induced apoptosis in the mouse dorsal root ganglion. Brain Res. 2007;1183:32–42. doi: 10.1016/j.brainres.2007.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu F, et al. Sortilin-mediated endocytosis determines levels of the frontotemporal dementia protein, progranulin. Neuron. 2010;68(4):654–667. doi: 10.1016/j.neuron.2010.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carlo AS, et al. The pro-neurotrophin receptor sortilin is a major neuronal apolipoprotein E receptor for catabolism of amyloid-beta peptide in the brain. J Neurosci. 2013;33(1):358–370. doi: 10.1523/JNEUROSCI.2425-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prabakaran T, et al. Mannose 6-phosphate receptor and sortilin mediated endocytosis of alpha-galactosidase A in kidney endothelial cells. PLoS One. 2012;7(6):e39975. doi: 10.1371/journal.pone.0039975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evans SF, et al. Neuronal brain-derived neurotrophic factor is synthesized in excess, with levels regulated by sortilin-mediated trafficking and lysosomal degradation. J Biol Chem. 2011;286(34):29556–29567. doi: 10.1074/jbc.M111.219675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nielsen MS, et al. Sortilin/neurotensin receptor-3 binds and mediates degradation of lipoprotein lipase. J Biol Chem. 1999;274(13):8832–8836. doi: 10.1074/jbc.274.13.8832. [DOI] [PubMed] [Google Scholar]
- 31. Klingenberg R, et al. Depletion of FOXP3+ regulatory T cells promotes hypercholesterolemia and atherosclerosis. J Clin Invest. 2013;123(3):1323–1334. doi: 10.1172/JCI63891. This study finds that reductions in hepatic Sort1 expression as a result of FoxP3 cell depletion is associated with increased atherosclerosis and plasma cholesterol
- 32. Chamberlain JM, et al. Insulin suppression of apolipoprotein B in McArdle RH7777 cells involves increased sortilin 1 interaction and lysosomal targeting. Biochem Biophys Res Commun. 2013;430(1):66–71. doi: 10.1016/j.bbrc.2012.11.022. This study demonstrates that insulin treatment increases the colocalization between sortilin, apoB and the lysosome, suggesting that insulin reduces apoB secretion by promoting its interaction with sortilin and its subsequent lysosomal degradation.
- 33.Karki S, et al. The multi-level action of fatty acids on adiponectin production by fat cells. PLoS One. 2011;6(11):e28146. doi: 10.1371/journal.pone.0028146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwon S, Christian JL. Sortilin associates with transforming growth factor-beta family proteins to enhance lysosome-mediated degradation. J Biol Chem. 2011;286(24):21876–21885. doi: 10.1074/jbc.M111.228262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gelling CL, et al. The endosomal protein-sorting receptor sortilin has a role in trafficking alpha-1 antitrypsin. Genetics. 2012;192(3):889–903. doi: 10.1534/genetics.112.143487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou B, et al. WNK4 enhances the degradation of NCC through a sortilin-mediated lysosomal pathway. J Am Soc Nephrol. 2010;21(1):82–92. doi: 10.1681/ASN.2008121275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jun JY, et al. Leptin treatment inhibits the progression of atherosclerosis by attenuating hypercholesterolemia in type 1 diabetic Ins2(+/Akita):apoE(-/-) mice. Atherosclerosis. 2012;225(2):341–347. doi: 10.1016/j.atherosclerosis.2012.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dube JB, Johansen CT, Hegele RA. Sortilin: an unusual suspect in cholesterol metabolism: from GWAS identification to in vivo biochemical analyses, sortilin has been identified as a novel mediator of human lipoprotein metabolism. Bioessays. 2011;33(6):430–437. doi: 10.1002/bies.201100003. [DOI] [PubMed] [Google Scholar]
- 39.Willnow TE, Kjolby M, Nykjaer A. Sortilins: new players in lipoprotein metabolism. Curr Opin Lipidol. 2011;22(2):79–85. doi: 10.1097/MOL.0b013e3283416f2b. [DOI] [PubMed] [Google Scholar]
- 40.Tall AR, Ai D. Sorting out sortilin. Circ Res. 2011;108(2):158–160. doi: 10.1161/RES.0b013e31820d7daa. [DOI] [PubMed] [Google Scholar]
- 41.Coutinho MF, et al. Sortilin and the risk of cardiovascular disease. Rev Port Cardiol. 2013;32(10):793–799. doi: 10.1016/j.repc.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 42.Kim T, Hempstead BL. NRH2 is a trafficking switch to regulate sortilin localization and permit proneurotrophin-induced cell death. EMBO J. 2009;28(11):1612–1623. doi: 10.1038/emboj.2009.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ozcan L, Tabas I. Role of endoplasmic reticulum stress in metabolic disease and other disorders. Annu Rev Med. 2012;63:317–328. doi: 10.1146/annurev-med-043010-144749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X, et al. NRADD, a novel membrane protein with a death domain involved in mediating apoptosis in response to ER stress. Cell Death Differ. 2003;10(5):580–591. doi: 10.1038/sj.cdd.4401208. [DOI] [PubMed] [Google Scholar]
- 45.Reinhardt S, et al. Unfolded protein response signaling by transcription factor XBP-1 regulates ADAM10 and is affected in Alzheimer's disease. FASEB J. 2014;28(2):978–997. doi: 10.1096/fj.13-234864. [DOI] [PubMed] [Google Scholar]
- 46. Gustafsen C, et al. The hypercholesterolemia-risk gene SORT1 facilitates PCSK9 secretion. Cell Metab. 2014;19(2):310–318. doi: 10.1016/j.cmet.2013.12.006. This study finds that sortilin binds with high affinity to PCSK9 and facilitates its secretion.
- 47.Mendoza-Barbera E, et al. Structural and functional analysis of APOA5 mutations identified in patients with severe hypertriglyceridemia. J Lipid Res. 2013;54(3):649–661. doi: 10.1194/jlr.M031195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Campagnolo L, et al. Sortilin expression is essential for pro-nerve growth factor-induced apoptosis of rat vascular smooth muscle cells. PLoS One. 2014;9(1):e84969. doi: 10.1371/journal.pone.0084969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones GT, et al. A sequence variant associated with sortilin-1 (SORT1) on 1p13.3 is independently associated with abdominal aortic aneurysm. Hum Mol Genet. 2013;22(14):2941–2947. doi: 10.1093/hmg/ddt141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Claudia Goettsch MA, Singh Sasha, Shibasaki Manabu, Aikawa Elena. Sortilin 1 is a Novel Inducer of Vascular Calcification via a Phosphate-Dependent Mechanism in American Heart Association. Los Angeles, California: 2012. [Google Scholar]