Abstract
Background:
Randomized clinical trials showed that laparoscopic colectomy (LC) is superior to open colectomy (OC) in short-term surgical outcomes; however, the generalizability among real-world patients is not clear.
Methods:
The National Cancer Data Base was used to identify stage I-III colon cancer patients age 18 to 84 years in 2010 and 2011. A propensity score analysis with 1:1 matching (PS) was used to avoid the effect of treatment selection bias. Patients were clustered at the hospital level for multilevel regression analyses. The main outcomes measured were 30-day mortality, unplanned readmissions, length of stay (LOS), and initiation of adjuvant chemotherapy among stage III patients. All statistical tests were two-sided.
Results:
A total of 45 876 patients were analyzed, 18 717 (41%) LC and 27 159 (59%) OC. After PS matching, there were 18 230 patients in both groups and they were well balanced on their covariables. Compared with OC, LC showed consistent benefits in 30-day mortality (1.3% vs 2.3 %, odds ratio [OR] = 0.59, 95% confidence interval [CI] = 0.49 to 0.69, P < .001) and LOS (median 5 vs 6 days, incident rate ratio = 0.83, 95% CI = 0.8 to 0.84, P < .001). LC was also associated with a higher rate of adjuvant chemotherapy use in stage III patients (72.3% vs 67.0%, P < .001). LC was more likely to be performed by high-volume surgeons in high-volume hospitals, but there was no significant effect of the hospital/surgeon volume on short-term outcomes.
Conclusion:
In routine clinical practice, laparoscopic colectomy is associated with lower 30-day mortality, shorter length of stay, and greater likelihood of adjuvant chemotherapy initiation among stage III colon cancer patients when compared with open colectomy.
Over the past decade, laparoscopic colectomy (LC) has gained wide acceptance as a curative surgical procedure for nonmetastatic colon cancer (1–3). Randomized clinical trials have shown that when compared with open colectomies, LC has better short-term surgical outcomes and comparable long-term oncological outcomes (4–12). In addition, meta-analysis studies confirmed these findings (8,13–15). However, surgeon credentialing was an important component of some randomized trials, leading to questioning the external validity of the findings. Moreover, there is limited data on the comparative effectiveness of LC vs open colectomy (OC) in routine clinical practice where surgeon skills, treatment facility characteristics, and patient characteristics may be very different than existed during the randomized trials. Therefore, the generalizability of the benefits of LC demonstrated by the randomized clinical trials is subject to further examination of evidence from real-world colon cancer patients.
High hospital volume has been associated with increased likelihood of performing LC and favorable short-term outcomes for both OC and LC (16–18). However, whether LC is safe in a diverse population across various sources of payers, providers, and types of facilities is still not clear. More importantly, to the best of our knowledge, surgeon volume has not been discussed extensively and the relationship between hospital volume and surgeon volume in achieving the incremental benefits of LC vs OC is less clear to patients, providers, and policy makers.
Therefore, the purpose of this study is to investigate the short-term comparative effectiveness of LC vs OC and the impact of hospital/surgeon volume on surgical outcomes using a contemporary cohort of stage I-III colon cancer patients who underwent curative surgical resection identified from the National Cancer Data Base (NCDB).
Methods
Study Population
The NCDB, jointly sponsored by the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society, is a nationwide oncology outcomes database based on more than 1400 Commission-accredited cancer programs, covering approximately 70% of new cancer cases in the United States (19). Our cohort consisted of patients diagnosed with stage I-III colon cancer in 2010 and 2011, using the International Classification of Diseases for Oncology, 3rd Edition for sites (C18.0, C18.2–7) and histology codes (8140,8141-45, 8147, 8210, 8211, 8220, 8221, 8260–63, 8470, 8480, 8481, 8490). Patients were limited to those who had adenocarcinoma and underwent either curative LC or OC as their first course of treatment within 90 days after diagnoses. Patients were excluded if the primary site was appendix or missing, the primary surgery was for local tumor excision (eg, polypectomy), or contiguous organ resection was involved during colectomy (eg, small bowel, bladder, etc.) We also excluded patients who were either younger than age 18 years or older than 84 years at the time of diagnoses (Figure 1).
Figure 1.
A flow chart of stage I-III colon cancer patients who received either laparoscopic colectomy or open colectomy as their primary treatment. LC = laparoscopic colectomy; OC = open colectomy.
Variable Selection
Patients’ demographic variables included age (categorized 18–49, 50–64, 65–74, and 75–84 years), race/ethnicity, and sex. Several other patient-level variables included: insurance type, facility type (community cancer program, comprehensive community cancer program, teaching/research center, and National Cancer Institute [NCI]–designated cancer center), deidentified treating surgeon, and hospital information. Hospital/surgeon volumes were defined as the total number of LC and OC colectomies performed per hospital/surgeon within a particular year. The cutoff points for high volume were defined as the 90th percentiles for both surgeons and hospitals. Important clinical variables included Charlson Comorbidity Index (CCI), stage at diagnosis (American Joint Committee on Cancer staging, 7th edition), tumor grade, lymphovascular invasion, tumor size (categories based on quartiles), extent of resection, and margin status. Colon cancer sites were categorized into three groups: right colon (cecum, ascending colon, and hepatic flexure of colon), transverse colon (transverse colon and splenic flexure of colon), and left colon (descending colon and sigmoid colon). Other variables that were controlled for in the analysis but not reported were urban/rural living status, region, proxies for household income level (zip code based US 2000 census tract median household income), year of colectomy, adequate nodal retrieval (≥12 lymph nodes resected during colectomy), and an indicator for stage IIc T4 colon cancer.
Statistical Methods
Descriptive statistics were used to compare the distributions of patient-, surgeon-, and hospital-level characteristics between LC and OC groups using the whole sample. Multilevel regressions with clustering at the hospital-level were performed to investigate the short-term benefits associated with LC (ie, 30-day mortality, 30-day unplanned readmission, and length of stay [LOS]). In order to reduce the potential selection bias, a propensity score (PS) with 1:1 matching method was used to balance patient-level characteristics to create comparable LC and OC groups (20,21). The PS matching included all the variables of interest: colon cancer site, adequacy of nodal retrieval, tumor grade, margin status, lymphovascular invasion, extent of resection, tumor size, stage IIc T4 status, stage at diagnosis, CCI, year of colectomy, age, race/ethnicity, sex, urban living status, insurance type, facility type, high surgeon/hospital volume status, proxies for household income level, and region. The matched sample was generated using a matching algorithm with a caliper of 0.0001. Because multilevel analysis requires at least two observations per hospital, we excluded hospitals that performed only one colectomy during a particular year from our analysis.
Multilevel logistic regression was conducted for 30-day mortality and 30-day unplanned readmission, and multilevel Poisson regression was conducted for LOS. Both matched and unmatched analyses were multivariablely adjusted. Add itional analyses were conducted by stratifying the sample into four subsamples according to the volume measurement: low-volume surgeon low-volume hospital, high-volume surgeon low-volume hospital, low-volume surgeon high-volume hospital, and high-volume surgeon high-volume hospital. The rates of adjuvant chemotherapy initiation for stage III colon cancer patients within eight weeks after colectomy were compared between LC and OC groups. Sensitivity analyses were conducted to examine the robustness of our findings by excluding converted colectomies (LC completed only vs OC) and including the oldest age group (85 to 99 years). All analyses were intent-to-treat, ie, converted cases were included in the LC group for the primary analysis.
All multilevel regression analyses were performed using SAS version 9.3 (SAS Institute Inc, Cary, NC) Proc Glimmix, and propensity scores were constructed using STATA 13.1 (StataCorp LP, College Station, Texas) Command Teffects. Statistical comparisons were two-sided, and significance was defined as a P value of less than .05.
Results
Study Population
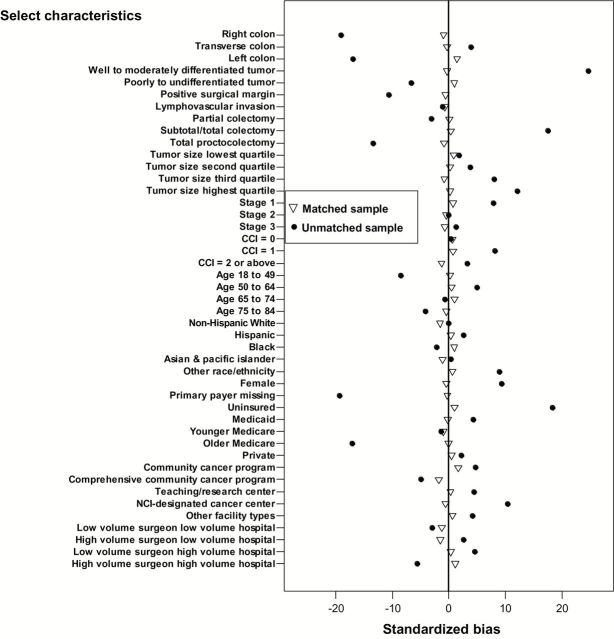
There were 45 876 stage I-III colon cancer patients; 18 717 (41%) received LC as their primary treatment, and 27 159 (59%) received OC. Overall, the percentage of laparoscopic converted to open colectomy was 5.7%. The distributions of various patient-, surgeon-, and hospital-level variables by surgical procedure type are shown in Table 1. Receipt of LC was associated with right-sided tumor location, small tumor size, early stage, nonextended resection, young age, female, low CCI, private insurance, and operation at academic and NCI-designated centers. Non-Hispanic Black patients were less likely to receive LC compared with non-Hispanic White patients. Patients undergoing LC were less likely to have lymphovascular invasion or a positive surgical margin (Figure 2; Supplementary Table 1, available online).
Table 1.
Distribution of select patient- and hospital-level variables for stage I-III colon cancer patients by surgical procedures (LC vs OC) before and after PS matching*
| Select variables | Unmatched | PS Matched | ||||
|---|---|---|---|---|---|---|
| LC | OC | P† | LC | OC | P | |
| n = 18 717 | n = 27 159 | n = 18 230 | n = 18 230 | |||
| No. (Col %) | No. (Col %) | No. (Col %) | No. (Col %) | |||
| Primary site‡ | ||||||
| Right colon | 10 049 (53.69) | 13 830 (50.92) | <.001 | 9756 (53.52) | 9860 (54.09) | .37 |
| Transverse colon | 2319 (12.39) | 3786 (13.94) | 2283 (12.52) | 2306 (12.65) | ||
| Left colon | 6349 (33.92) | 9543 (35.14) | 6191 (33.96) | 6064 (33.26) | ||
| Tumor grade | ||||||
| Well to moderately differentiated | 15 067 (80.50) | 21 536 (79.30) | <.001 | 14 663 (80.43) | 14 569 (79.92) | .26 |
| Poorly to undifferentiated | 3050 (16.30) | 4852 (17.87) | 2999 (16.45) | 3044 (16.70) | ||
| Missing | 600 (3.21) | 771 (2.84) | 568 (3.12) | 617 (3.38) | ||
| Positive surgical margin | ||||||
| No | 18 034 (96.35) | 25 558 (94.11) | <.001 | 17 554 (96.29) | 17 564 (96.35) | .96 |
| Yes | 628 (3.36) | 1493 (5.50) | 621 (3.41) | 612 (3.36) | ||
| Missing | 55 (0.29) | 108 (0.40) | 55 (0.30) | 54 (0.30) | ||
| Lymphovascular invasion | ||||||
| No | 13 165 (70.34) | 18 321 (67.46) | <.001 | 12 786 (70.14) | 12 741 (69.89) | .85 |
| Yes | 4046 (21.62) | 6380 (23.49) | 3972 (21.79) | 3994 (21.91) | ||
| Missing | 1506 (8.05) | 2458 (9.05) | 1472 (8.07) | 1495 (8.20) | ||
| Surgery of the primary site | ||||||
| Partial colectomy | 6935 (37.05) | 9414 (34.66) | <.001 | 6705 (36.78) | 6552 (35.94) | .23 |
| Subtotal/total colectomy | 11 720 (62.62) | 17 616 (64.86) | 11 463 (62.88) | 11 610 (63.69) | ||
| Total proctocolectomy | 62 (0.33) | 129 (0.47) | 62 (0.34) | 68 (0.37) | ||
| Tumor size | ||||||
| Lowest quartile (<30mm) | 5359 (28.63) | 5769 (21.24) | <.001 | 5084 (27.89) | 5082 (27.88) | .81 |
| Second quartile (30mm to 41mm) | 4572 (24.43) | 6474 (23.84) | 4507 (24.72) | 4428 (24.29) | ||
| Third quartile (42mm to 59mm) | 4089 (21.85) | 6422 (23.65) | 4045 (22.19) | 4035 (22.13) | ||
| Highest quartile (≥60mm) | 3646 (19.48) | 7379 (27.17) | 3625 (19.88) | 3695 (20.27) | ||
| Missing | 1051 (5.62) | 1115 (4.11) | 969 (5.32) | 990 (5.43) | ||
| Stage | ||||||
| Stage 1 | 6097 (32.57) | 6476 (23.84) | <.001 | 5731 (31.44) | 5710 (31.32) | .81 |
| Stage 2 | 6170 (32.96) | 10 154 (37.39) | 6104 (33.48) | 6066 (33.27) | ||
| Stage 3 | 6450 (34.46) | 10 529 (38.77) | 6395 (35.08) | 6454 (35.40) | ||
| Charlson Comorbidity Index | ||||||
| 0 | 12 574 (67.18) | 18 295 (67.36) | .005 | 12 245 (67.17) | 12 151 (66.65) | .57 |
| 1 | 4513 (24.11) | 6297 (23.19) | 4379 (24.02) | 4454 (24.43) | ||
| 2 or above | 1630 (8.71) | 2567 (9.45) | 1606 (8.81) | 1625 (8.91) | ||
| Age groups | ||||||
| 18 to 49 y | 1888 (10.09) | 2734 (10.07) | <.001 | 1833 (10.05) | 1749 (9.59) | .40 |
| 50 to 64 y | 6154 (32.88) | 8403 (30.94) | 5909 (32.41) | 5869 (32.19) | ||
| 65 to 74 y | 5512 (29.45) | 7914 (29.14) | 5400 (29.62) | 5486 (30.09) | ||
| 75 to 84 y | 5163 (27.58) | 8108 (29.85) | 5088 (27.91) | 5126 (28.12) | ||
| Race/ethnicity | ||||||
| Non-Hispanic White | 14 061 (75.12) | 19 374 (71.34) | <.001 | 13 651 (74.88) | 13 669 (74.98) | .62 |
| Hispanic | 868 (4.64) | 1454 (5.35) | 852 (4.67) | 802 (4.40) | ||
| Black | 2135 (11.41) | 3832 (14.11) | 2103 (11.54) | 2145 (11.77) | ||
| Asian & PI | 553 (2.95) | 817 (3.01) | 546 (3.00) | 565 (3.10) | ||
| Other | 1100 (5.88) | 1682 (6.19) | 1078 (5.91) | 1049 (5.75) | ||
| Female | ||||||
| No | 9333 (49.86) | 13 550 (49.89) | .95 | 9074 (49.78) | 9113 (49.99) | .68 |
| Yes | 9384 (50.14) | 13 609 (50.11) | 9156 (50.22) | 9117 (50.01) | ||
| Primary payer (insurance type) | ||||||
| Missing | 156 (0.83) | 465 (1.71) | <.001 | 153 (0.84) | 166 (0.91) | .87 |
| Uninsured | 466 (2.49) | 1290 (4.75) | 458 (2.51) | 464 (2.55) | ||
| Medicaid | 721 (3.85) | 1502 (5.53) | 716 (3.93) | 689 (3.78) | ||
| Younger Medicare | 716 (3.83) | 1246 (4.59) | 710 (3.89) | 717 (3.93) | ||
| Older Medicare | 8996 (48.06) | 13 291 (48.94) | 8836 (48.47) | 8912 (48.89) | ||
| Private | 7662 (40.94) | 9365 (34.48) | 7357 (40.36) | 7282 (39.95) | ||
| Facility type | ||||||
| Community cancer program | 2169 (11.59) | 4821 (17.75) | <.001 | 2157 (11.83) | 2175 (11.93) | .82 |
| Comprehensive community cancer program | 9827 (52.50) | 13 837 (50.95) | 9680 (53.10) | 9685 (53.13) | ||
| Teaching/research center | 3701 (19.77) | 5246 (19.32) | 3608 (19.79) | 3557 (19.51) | ||
| NCI-designated cancer center | 1081 (5.78) | 962 (3.54) | 923 (5.06) | 898 (4.93) | ||
| Other | 1939 (10.36) | 2293 (8.44) | 1862 (10.21) | 1915 (10.50) | ||
| Volume measurement§ | ||||||
| Low-volume surgeon low-volume hospital | 7868 (42.04) | 14 744 (54.29) | <.001 | 7832 (42.97) | 7802 (42.80) | .53 |
| High-volume surgeon low-volume hospital | 4476 (23.91) | 4636 (17.07) | 4285 (23.50) | 4397 (24.12) | ||
| Low-volume surgeon high-volume hospital | 2845 (15.20) | 4517 (16.63) | 2833 (15.54) | 2815 (15.44) | ||
| High-volume surgeon high- volume hospital | 3528 (18.85) | 3262 (12.01) | 3280 (17.99) | 3216 (17.64) | ||
* All statistical tests were two-sided. LC = laparoscopic colectomy; NCI = National Cancer Institute; OC = open colectomy; PI = Pacific Islander; PS = propensity score.
† P value calculated using Pearson’s chi-squared test.
‡ Right colon includes cecum, ascending colon, and hepatic flexure of colon, transverse colon includes transverse colon and splenic flexure of colon, and left colon includes descending colon and sigmoid colon.
§ High-volume surgeon is defined as more than six colectomies within a particular year; high-volume hospital is defined as more than 36 colectomies within a particular year, both of which are the top 10th percentiles.
Figure 2.
Standardized bias plot for select patient-, surgeon-, and hospital-level characteristics before and after propensity score matching. Open triangles = matched samples; closed circles = unmatched samples. CCI = Charlson Comorbidity Index; PS = propensity score.
Propensity Score Analysis
We anticipated that comparison of the two treatment groups would demonstrate statistical differences by factors (eg, age, comorbidity status, and payer type) that were likely to be associated with both the treatment assignment and our outcomes of interest. We therefore performed a PS analysis based on all potential predictor variables for LC vs OC and identified two PS matched (1:1) cohorts for the primary comparisons. Following PS matching, the distribution of the covariables was fully balanced with 18 230 patients in each group, LC and OC (Figure 2 and Table 1).
Clinical Outcomes Before and After Propensity Score Matching
In the unmatched analyses, multilevel logistic regression results demonstrated that when compared with OC, LC was associated with statistically significant reduction in 30-day mortality (1.3% vs 2.8%, odds ratio [OR] = 0.54, 95% confidence interval [CI] = 0.47 to 0.63, P < .001), rate of 30-day unplanned readmission (4.8% vs 5.5%, OR = 0.87, 95% CI = 0.79 to 0.96, P = .003), and LOS (median 5 vs 6 days, incident rate ratio [IRR] = 0.82, 95% CI = 0.8 to 0.83, P < .001) (Table 2). Following PS matching, LC remained associated with a lower rate of 30-day mortality (1.3% vs 2.3%, OR = 0.59, 95% CI = 0.49 to 0.69, P < .001) and shorter LOS (median 5 vs 6 days, IRR = 0.83, 95% CI = 0.8 to 0.84, P < .001), but had only a modest association with rate of 30-day unplanned readmission (4.8% vs 5.1%, OR = 0.90, 95% CI = 0.81 to 1.0, P = .052) (Table 2). Not surprisingly, factors independently associated with increased 30-day mortality also included older age, greater comorbidity, lower socioeconomic status, larger and more advanced tumors, and positive surgical margin (Tables 3 and 4).
Table 2.
Unmatched and PS matched multilevel regression adjusted outcomes between LC and OC*
| Outcome | Unmatched | PS Matched | ||||||
|---|---|---|---|---|---|---|---|---|
| LC (n = 18 717) | OC (n = 27 159) | OR/IRR§ (95%CI) | P† | LC (n = 18 230) | OC (n = 18 230) | OR/IRR (95%CI) | P | |
| Stage I-III patients | ||||||||
| 30-day mortality No. (%) | 244 (1.30) | 766 (2.82) | 0.54 (0.47 to 0.63) | <.001 | 243 (1.33) | 420 (2.30) | 0.59 (0.49 to 0.69) | <.001 |
| 30-day readmission No. (%) | 899 (4.80) | 1498 (5.52) | 0.87 (0.79 to 0.96) | .003 | 881 (4.83) | 412 (5.11) | 0.90 (0.81 to 1.00) | .052 |
| LOS, median (IQR) | 5 (3 to 6) | 6 (4 to 8) | 0.82 (0.80 to 0.83) | <.001 | 5 (3 to 6) | 6 (4 to 8) | 0.83 (0.80 to 0.84) | <.001 |
| Stage III patients only | LC (n = 6450) | OC (n = 10 529) | --- | P | LC (n = 6395) | OC (n = 6454) | --- | P |
| Adjuvant chemother- apy initiation No. (%) | 4669 (72.39) | 6828 (64.85) | --- | <.001 | 4,622 (72.27) | 4324 (67.00) | --- | <.001 |
| Initiated within 8 wks No. (%) | 3661 (56.76) | 4937 (46.89) | --- | <.001 | 3,623 (56.65) | 3199 (49.57) | --- | <.001 |
* All statistical tests were two-sided. Full details of all other patient-level variables results from the multilevel regression are available in tables 3 and 4. CI = confidence interval; IQR = interquartile range; IRR = incident rate ratio; LC = laparoscopic colectomy; OC = open colectomy; OR = odds ratio; PS = propensity score.
† P values derived from adjusted multilevel regressions.
§ Referent = OC.
Table 3.
Multilevel regression results of associations between patient-, surgeon-, and hospital-level factors and short-term surgical outcomes of LC vs OC for stage I-III colon cancer patients using the unmatched sample*
| Select variables (n = 45 876) | 30-day mortality | 30-day readmission | Length of hospital stay | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P† | OR (95% CI) | P | IRR (95% CI) | P | |
| Primary site | ||||||
| Right colon | Ref | Ref | Ref | |||
| Transverse colon | 1.06 (0.88 to 1.27) | .539 | 1.10 (0.97 to 1.24) | .124 | 1.12 (1.09 to 1.15) | <.001 |
| Left colon | 1.01 (0.85 to 1.18) | .918 | 0.99 (0.89 to 1.10) | .893 | 1.10 (1.07 to 1.12) | <.001 |
| Tumor frade | ||||||
| Well to moderately differentiated | Ref | Ref | Ref | |||
| Poorly to undifferentiated | 1.12 (0.95 to 1.30) | .174 | 0.98 (0.87 to 1.10) | .755 | 1.01 (0.98 to 1.03) | .424 |
| Missing | 0.79 (0.50 to 1.22) | .294 | 0.73 (0.54 to 0.96) | .029 | 0.98 (0.92 to 1.02) | .340 |
| Positive surgical margin | ||||||
| No | Ref | Ref | Ref | |||
| Yes | 1.74 (1.39 to 2.16) | <.001 | 1.43 (1.20 to 1.70) | <.001 | 1.15 (1.10 to 1.19) | <.001 |
| Missing | 1.94 (0.95 to 3.94) | .068 | 1.60 (0.91 to 2.79) | .100 | 1.34 (1.16 to 1.53) | <.001 |
| Lymphovascular invasion | ||||||
| No | Ref | Ref | Ref | |||
| Yes | 1.03 (0.87 to 1.20) | .744 | 1.12 (1.00 to 1.24) | .046 | 1.03 (1.00 to 1.05) | .018 |
| Missing | 0.94 (0.74 to 1.18) | .600 | 1.04 (0.88 to 1.22) | .624 | 1.02 (0.98 to 1.05) | .262 |
| Surgery of the primary site | ||||||
| Partial colectomy | Ref | Ref | Ref | |||
| Subtotal/total colectomy | 1.03 (0.88 to 1.20) | .667 | 1.14 (1.02 to 1.26) | .018 | 1.06 (1.03 to 1.08) | <.001 |
| Total proctocolectomy | ---‡ | 2.35 (1.45 to 3.78) | .001 | 1.30 (1.14 to 1.47) | <.001 | |
| Tumor size | ||||||
| Lowest quartile (<30mm) | Ref | Ref | Ref | |||
| Second quartile (30mm to 41mm) | 1.26 (1.03 to 1.54) | .021 | 0.99 (0.87 to 1.12) | .925 | 1.04 (1.01 to 1.06) | .002 |
| Third quartile (42mm to 59mm) | 1.29 (1.05 to 1.58) | .015 | 0.97 (0.85 to 1.10) | .666 | 1.06 (1.03 to 1.08) | <.001 |
| Highest quartile (≥60mm) | 1.44 (1.17 to 1.77) | .000 | 1.01 (0.88 to 1.15) | .869 | 1.11 (1.08 to 1.14) | <.001 |
| Missing | 1.24 (0.86 to 1.78) | .249 | 1.08 (0.87 to 1.35) | .473 | 0.99 (0.94 to 1.03) | .741 |
| Stage | ||||||
| Stage 1 | Ref | Ref | Ref | |||
| Stage 2 | 1.25 (1.02 to 1.51) | .029 | 1.03 (0.90 to 1.15) | .694 | 1.03 (1.00 to 1.05) | .018 |
| Stage 3 | 1.60 (1.31 to 1.94) | <.001 | 1.04 (0.91 to 1.17) | .567 | 1.04 (1.01 to 1.07) | .001 |
| Charlson Comorbidity Index | ||||||
| 0 | Ref | Ref | Ref | |||
| 1 | 1.32 (1.14 to 1.52) | <.001 | 1.14 (1.02 to 1.25) | .012 | 1.05 (1.02 to 1.07) | <.001 |
| 2 or above | 2.02 (1.70 to 2.38) | <.001 | 1.41 (1.23 to 1.60) | <.001 | 1.18 (1.15 to 1.21) | <.001 |
| Age groups | ||||||
| 50 to 64 y | Ref | Ref | Ref | |||
| 18 to 49 y | 3.25 (1.87 to 5.63) | <.001 | 0.98 (0.84 to 1.14) | .804 | 1.05 (1.02 to 1.08) | .001 |
| 65 to 74 y | 7.87 (4.47 to 13.86) | <.001 | 0.89 (0.72 to 1.09) | .255 | 1.16 (1.11 to 1.21) | <.001 |
| 75 to 84 y | 17.05 (9.69 to 29.98) | <.001 | 0.95 (0.76 to 1.16) | .599 | 1.31 (1.25 to 1.36) | <.001 |
| Race/ethnicity | ||||||
| Non-Hispanic White | Ref | Ref | Ref | |||
| Hispanic | 0.69 (0.48 to 0.97) | .034 | 1.27 (1.03 to 1.54) | .019 | 1.04 (1.00 to 1.08) | .046 |
| Black | 0.96 (0.77 to 1.17) | .677 | 1.05 (0.91 to 1.20) | .470 | 1.10 (1.07 to 1.13) | <.001 |
| Asian & PI | 0.75 (0.47 to 1.17) | .207 | 1.03 (0.77 to 1.36) | .854 | 0.96 (0.91 to 1.01) | .131 |
| Other | 1.35 (1.07 to 1.71) | .012 | 1.19 (0.97 to 1.45) | .083 | 1.00 (0.96 to 1.04) | .932 |
| Female | ||||||
| No | Ref | Ref | Ref | |||
| Yes | 0.68 (0.59 to 0.76) | <.001 | 0.98 (0.90 to 1.06) | .706 | 0.91 (0.89 to 0.92) | <.001 |
| Primary payer (insurance type) | ||||||
| Private | Ref | Ref | Ref | |||
| Missing | 1.08 (0.60 to 1.93) | .794 | 1.28 (0.88 to 1.85) | .195 | 1.07 (1.03 to 1.09) | <.001 |
| Uninsured | 2.11 (1.41 to 3.13) | <.001 | 1.35 (1.08 to 1.67) | .006 | 1.25 (1.19 to 1.30) | <.001 |
| Medicaid | 2.24 (1.62 to 3.08) | <.001 | 1.48 (1.22 to 1.79) | <.001 | 1.25 (1.19 to 1.29) | <.001 |
| Younger Medicare | 2.90 (2.00 to 4.20) | <.001 | 1.24 (1.01 to 1.53) | .040 | 1.15 (1.09 to 1.20) | <.001 |
| Older Medicare | 1.23 (0.99 to 1.52) | .052 | 1.33 (1.12 to 1.55) | .001 | 1.00 (0.92 to 1.08) | .965 |
| Facility type | ||||||
| Community cancer program | Ref | Ref | Ref | |||
| Comprehensive community cancer program | 1.01 (0.83 to 1.20) | .944 | 1.12 (0.89 to 1.39) | .315 | 1.02 (0.98 to 1.05) | .296 |
| Teaching/research center | 1.16 (0.92 to 1.45) | .201 | 1.24 (0.93 to 1.64) | .139 | 1.06 (1.01 to 1.10) | .017 |
| NCI-designated cancer center | 0.87 (0.55 to 1.34) | .524 | 0.88 (0.51 to 1.51) | .642 | 1.09 (1.00 to 1.18) | .033 |
| Other | 1.12 (0.85 to 1.46) | .420 | 1.24 (0.88 to 1.74) | .210 | 1.07 (1.01 to 1.12) | .020 |
| Volume measurement | ||||||
| Low-volume surgeon low-volume hospital | Ref | Ref | Ref | |||
| High-volume surgeon low-volume hospital | 0.77 (0.64 to 0.91) | .004 | 0.96 (0.82 to 1.11) | .548 | 0.90 (0.87 to 0.92) | <.001 |
| Low-volume surgeon high-volume hospital | 0.86 (0.70 to 1.04) | .126 | 0.98 (0.84 to 1.13) | .765 | 1.00 (0.95 to 1.03) | .882 |
| High-volume surgeon high- volume hospital | 0.60 (0.47 to 0.76) | <.001 | 0.98 (0.84 to 1.14) | .826 | 0.88 (0.84 to 0.91) | <.001 |
* All statistical tests were two-sided. CI = confidence interval; IQR = interquartile range; IRR = incident rate ratio; LC = laparoscopic colectomy; OC = open colectomy; OR = odds ratio; PS = propensity score.
† P values derived from adjusted multilevel regressions.
‡ Results were not available because of nonconvergence of the model.
Table 4.
Multilevel regression results of associations between patient-, surgeon-, and hospital-level factors and short-term surgical outcomes of LC vs OC for stage I-III colon cancer patients using the PS matched sample*
| Select variables (n = 36 460) | 30-day mortality | 30-day readmission | Length of hospital stay | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P† | OR (95% CI) | P | IRR (95% CI) | P | |
| Primary site | ||||||
| Right colon | Ref | Ref | Ref | |||
| Transverse colon | 0.98 (0.77 to 1.24) | .870 | 1.06 (0.91 to 1.22) | .474 | 1.13 (1.09 to 1.10) | <.001 |
| Left colon | 0.83 (0.67 to 1.02) | .087 | 0.92 (0.81 to 1.04) | .213 | 1.12 (1.09 to 1.10) | <.001 |
| Tumor grade | ||||||
| Well to moderately differentiated | Ref | Ref | Ref | |||
| Poorly to undifferentiated | 1.10 (0.89 to 1.35) | .351 | 0.95 (0.83 to 1.08) | .482 | 1.01 (0.98 to 1.00) | .431 |
| Missing | 0.49 (0.26 to 0.92) | .028 | 0.81 (0.60 to 1.09) | .166 | 0.97 (0.91 to 1.00) | .275 |
| Positive surgical margin | ||||||
| No | Ref | Ref | Ref | |||
| Yes | 1.47 (1.04 to 2.06) | .028 | 1.43 (1.12 to 1.80) | .003 | 1.11 (1.05 to 1.10) | <.001 |
| Missing | 1.19 (0.38 to 3.72) | .763 | 2.32 (1.25 to 4.30) | .008 | 1.24 (1.05 to 1.40) | .010 |
| Lymphovascular invasion | ||||||
| No | Ref | Ref | Ref | |||
| Yes | 1.06 (0.86 to 1.30) | .583 | 1.16 (1.02 to 1.31) | .022 | 1.01 (0.98 to 1.00) | .288 |
| Missing | 1.28 (0.96 to 1.70) | .083 | 0.96 (0.78 to 1.16) | .676 | 1.02 (0.98 to 1.00) | .258 |
| Surgery of the primary site | ||||||
| Partial colectomy | Ref | Ref | Ref | |||
| Subtotal/total colectomy | 0.87 (0.71 to 1.05) | .162 | 1.15 (1.01 to 1.30) | .023 | 1.07 (1.04 to 1.00) | <.001 |
| Total proctocolectomy | ---‡ | 2.54 (1.40 to 4.60) | .002 | 1.40 (1.19 to 1.60) | <.001 | |
| Tumor size | ||||||
| Lowest quartile (<30mm) | Ref | Ref | Ref | |||
| Second quartile (30mm to 41mm) | 1.16 (0.92 to 1.45) | .201 | 1.00 (0.87 to 1.15) | .964 | 1.05 (1.02 to 1.00) | <.001 |
| Third quartile (42mm to 59mm) | 1.16 (0.91 to 1.46) | .228 | 0.93 (0.80 to 1.07) | .333 | 1.08 (1.05 to 1.10) | <.001 |
| Highest quartile (≥60mm) | 1.11 (0.85 to 1.42) | .432 | 1.09 (0.93 to 1.26) | .284 | 1.11 (1.07 to 1.10) | <.001 |
| Missing | 1.25 (0.83 to 1.87) | .278 | 1.24 (0.98 to 1.55) | .071 | 0.97 (0.92 to 1.00) | .267 |
| Stage | ||||||
| Stage 1 | Ref | Ref | Ref | |||
| Stage 2 | 1.24 (0.98 to 1.56) | .062 | 0.97 (0.85 to 1.11) | .682 | 1.01 (0.98 to 1.00) | .278 |
| Stage 3 | 1.59 (1.26 to 1.99) | <.001 | 0.99 (0.86 to 1.14) | .915 | 1.02 (0.99 to 1.00) | .114 |
| Charlson Comorbidity Index (CCI) | ||||||
| CCI = 0 | Ref | Ref | Ref | |||
| CCI = 1 | 1.62 (1.35 to 1.92) | <.001 | 1.05 (0.93 to 1.17) | .420 | 1.04 (1.01 to 1.00) | .001 |
| CCI = 2 or above | 2.43 (1.96 to 3.00) | <.001 | 1.43 (1.22 to 1.66) | <.001 | 1.15 (1.11 to 1.10) | <.001 |
| Age groups | ||||||
| 50 to 64 y | Ref | Ref | Ref | |||
| 18 to 49 y | 4.10 (1.83 to 9.16) | .001 | 1.07 (0.89 to 1.28) | .456 | 1.04 (1.00 to 1.07) | .028 |
| 65 to 74 y | 10.55 (4.64 to 23.95) | <.001 | 0.77 (0.60 to 0.99) | .048 | 1.14 (1.09 to 1.10) | <.001 |
| 75 to 84 y | 23.70 (10.44 to 53.77) | <.001 | 0.86 (0.66 to 1.11) | .259 | 1.30 (1.23 to 1.30) | <.001 |
| Race/ethnicity | ||||||
| Non-Hispanic White | Ref | Ref | Ref | |||
| Hispanic | 0.63 (0.38 to 1.02) | .063 | 1.17 (0.91 to 1.49) | .225 | 1.08 (1.02 to 1.10) | .002 |
| Black | 1.27 (0.98 to 1.64) | .067 | 1.03 (0.87 to 1.21) | .689 | 1.11 (1.07 to 1.10) | <.001 |
| Asian & PI | 0.59 (0.30 to 1.14) | .119 | 0.92 (0.65 to 1.28) | .619 | 0.95 (0.89 to 1.00) | .077 |
| Other | 1.56 (1.13 to 2.13) | .007 | 1.25 (0.99 to 1.57) | .052 | 1.04 (0.99 to 1.00) | .063 |
| Female | ||||||
| No | Ref | Ref | Ref | |||
| Yes | 0.63 (0.53 to 0.73) | <.001 | 0.98 (0.89 to 1.07) | .673 | 0.91 (0.89 to 0.90) | <.001 |
| Primary payer (insurance type) | ||||||
| Private | Ref | Ref | Ref | |||
| Missing | 0.93 (0.34 to 2.47) | .881 | 1.29 (0.76 to 2.17) | .346 | 1.08 (0.97 to 1.10) | .143 |
| Uninsured | 2.21 (1.25 to 3.87) | .006 | 1.28 (0.94 to 1.73) | .116 | 1.17 (1.10 to 1.24) | <.001 |
| Medicaid | 1.79 (1.13 to 2.84) | .013 | 1.59 (1.25 to 2.00) | .000 | 1.24 (1.17 to 1.30) | <.001 |
| Younger Medicare | 2.35 (1.45 to 3.79) | .001 | 1.30 (1.02 to 1.65) | .033 | 1.32 (1.25 to 1.30) | <.001 |
| Older Medicare | 1.01 (0.77 to 1.29) | .967 | 1.66 (1.35 to 2.02) | <.001 | 1.08 (1.03 to 1.10) | <.001 |
| Facility type | ||||||
| Community cancer program | Ref | Ref | Ref | |||
| Comprehensive community cancer program | 0.99 (0.74 to 1.32) | .944 | 1.00 (0.76 to 1.29) | .969 | 1.01 (0.96 to 1.00) | .680 |
| Teaching/research center | 1.17 (0.81 to 1.66) | .407 | 1.10 (0.78 to 1.53) | .589 | 1.04 (0.98 to 1.10) | .138 |
| NCI-designated cancer center | 0.87 (0.46 to 1.64) | .673 | 0.68 (0.36 to 1.26) | .221 | 1.08 (0.98 to 1.10) | .097 |
| Other | 1.01 (0.66 to 1.53) | .974 | 1.01 (0.67 to 1.49) | .981 | 1.08 (1.01 to 1.10) | .017 |
| Volume measurement | ||||||
| Low-volume surgeon low-volume hospital | Ref | Ref | Ref | |||
| High-volume surgeon low-volume hospital | 0.64 (0.50 to 0.79) | <.001 | 1.16 (1.00 to 1.33) | .040 | 0.88 (0.86 to 0.90) | <.001 |
| Low-volume surgeon high-volume hospital | 0.87 (0.64 to 1.17) | .372 | 1.09 (0.81 to 1.46) | .557 | 0.98 (0.93 to 1.00) | .411 |
| High-volume surgeon high-volume hospital | 0.55 (0.40 to 0.76) | <.001 | 0.77 (0.57 to 1.04) | .093 | 0.86 (0.82 to 0.90) | <.001 |
* All statistical tests were two-sided. CI = confidence interval; IQR = interquartile range; IRR = incident rate ratio; LC = laparoscopic colectomy; NCI = National Cancer Institute; OC = open colectomy; OR = odds ratio; PS = propensity score.
† P values derived from adjusted multilevel regressions.
‡ Results were not available because of nonconvergence of the model.
Impact of Facility and Surgeon Volume
Because of the learning curve associated with LC and as prior randomized trials have included experienced surgical investigators and major academic/research centers, we sought to further evaluate the impact of facility type, annual hospital volume, and annual surgeon volume on the short-term benefits associated with LC. In our sample, the 90th percentile cutoff points were 6 colectomies for high surgeon volume and 36 colectomies for high hospital volume annually. Among 8414 individual surgeons that were identified in our sample, 44.4% performed OC only. Moreover, among 954 high-volume surgeons, 51.5% performed OC more frequently than LC. Prior to PS matching, LC was more likely to be performed by high-volume surgeons in high-volume hospitals, and high surgeon volume was associated with lower rate of 30-day mortality and shorter LOS (Tables 3 and 4; Supplementary Table 1, available online).
Despite the hospital and surgeon volume effects on the likelihood of performing LC vs OC, the benefits of LC in terms of 30-day mortality and LOS were observed across all hospital and surgeon volume strata before and after PS matching (Table 5). There was no statistically significant effect of the type of reporting facility on 30-day mortality, 30-day unplanned readmission, or LOS.
Table 5.
Sensitivity analyses stratified by volume measurement at both surgeon- and hospital-level*
| Sample | 30-day mortality | 30-day readmission | Length of hospital stay | |||
|---|---|---|---|---|---|---|
| OR† (95% CI) | P‡ | OR (95% CI) | P | IRR (95% CI) | P | |
| Unmatched sample (total n = 45 876) | ||||||
| Low-volume surgeon low-volume hospital (n = 22 612) |
0.54 (0.44 to 0.66) | <.001 | 0.90 (0.79 to 1.02) | .095 | 0.84 (0.82 to 0.86) | <.001 |
| High-volume surgeon low-volume hospital (n = 9112) |
0.52 (0.37 to 0.73) | <.001 | 0.85 (0.71 to 1.03) | .095 | 0.82 (0.80 to 0.85) | <.001 |
| Low-volume surgeon high-volume hospital (n = 7362) |
0.59 (0.40 to 0.86) | <.001 | 0.82 (0.66 to 1.03) | .087 | 0.80 (0.78 to 0.83) | <.001 |
| High-volume surgeon high-volume hospital (n = 6790) |
0.53 (0.33 to 0.85) | <.001 | 0.91 (0.62 to 1.34) | .429 | 0.81 (0.79 to 0.84) | <.001 |
| PS matched sample (total n = 36 460) | ||||||
| Low-volume surgeon low-volume hospital (n = 15 634) |
0.54 (0.43 to 0.67) | <.001 | 0.93 (0.80 to 1.07) | .299 | 0.84 (0.82 to 0.85) | <.001 |
| High-volume surgeon low-volume hospital (n = 8682) |
0.63 (0.43 to 0.90) | .012 | 0.84 (0.70 to 1.02) | .075 | 0.85 (0.83 to 0.88) | <.001 |
| Low-volume surgeon high-volume hospital (n = 5648) |
0.54 (0.36 to 0.83) | .004 | 0.91 (0.71 to 1.16) | .434 | 0.81 (0.78 to 0.84) | <.001 |
| High-volume surgeon high-volume hospital (n = 6496) |
0.60 (0.36 to 0.98) | .040 | 1.27 (0.98 to 1.64) | .069 | 0.83 (0.81 to 0.86) | <.001 |
* All statistical tests were two-sided. CI = confidence interval; IRR = incident rate ratio; OR = odds ratio; PS = propensity score.
† Laparoscopic colectomy vs open colectomy. Referent = open colectomy.
‡ P values derived from adjusted multilevel regressions.
Time to Initiation of Adjuvant Chemotherapy
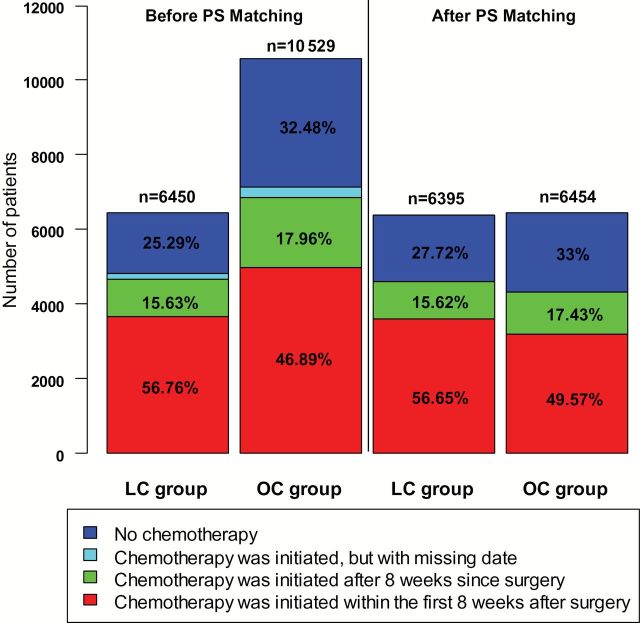
We further evaluated the impact of surgical approach on the ability to initiate adjuvant chemotherapy within the recommended eight-week window among patients with stage III colon cancer. After PS matching, the overall rate of adjuvant chemotherapy was higher among patients undergoing LC than OC (72.3% vs 67.0%, P < .001). Moreover, among patients who did receive adjuvant chemotherapy, LC patients were more likely to receive chemotherapy within eight weeks than OC patients (56.56% vs 49.57%, P < .001) (Figure 3).
Figure 3.
Initiation and timing of chemotherapy after receipt of colectomy among stage III colon cancer patients. Before propensity score matching, 150 patients (2.33%) of the laparoscopic colectomy group and 281 patients (2.67%) of the open colectomy group received chemotherapy; however, their timing information was missing. LC = laparoscopic colectomy; OC = open colectomy; PS = Propensity Score.
Discussion
In this study, we investigated the short-term comparative effectiveness of LC vs OC among stage I-III colon cancer patients in 2010 and 2011 using propensity score matching. Our results showed that in routine clinical practice LC is associated with a lower 30-day mortality rate, a shorter LOS, and a modest reduction in the rate of unplanned readmissions. Importantly, among patients with stage III colon cancer, those who underwent LC were associated with a higher likelihood of receiving adjuvant chemotherapy and receiving it without delay than those who underwent OC.
The short-term benefits and the oncologic efficacy of LC for cancer have been described in previous studies. However, these data have primarily come from high-volume institutions and from clinical trials where the participating surgeons have demonstrated expertise in LC for cancer (4–7). Therefore, the generalizability of the conclusions from randomized clinical trials to routine clinical practice in a diverse patient population with various types of payers and providers has not been established. We analyzed data from the NCDB, which is a hospital-based cancer registry data (22). Our study included more than 8400 surgeons and 1300 hospitals, which is highly representative of colon cancer care in the United States. We noted that the short-term benefits associated with the receipt of LC could be achieved in both low- and high-volume hospitals and by low- and high-volume surgeons. By utilizing the method of PS matching to minimize the effect of treatment selection bias, we demonstrate the generalizability of our findings to the broad community of colon cancer patients and providers and also highlight a potential priority area for improving treatment outcomes and reducing variation.
Prior evaluation of LC within the NCDB examined surgery performed prior to the publication of the US intergroup randomized trial (23). As a result, the laparoscopic approach was utilized in less than 5% of the cohort, suggesting substantial susceptibility to the influence of institutional and surgeon experience. Moreover, the corresponding analysis could not account for treatment selection (23,24). Our analysis showed that the contemporary utilization of LC for cancer was over 41% in 2010 and 2011, which is the highest US rate documented in the literature but still with room for improvement (23,25,26). Currently, the laparoscopic approach is available to just under one in two eligible patients with nonmetastatic colon cancer.
The present study also shows that LC is performed at a wide variety of facility types and among both low- and high-volume hospitals and surgeons with more than 64% of LC cases performed in community hospitals and more than 70% performed by low-volume surgeons, demonstrating the widespread availability of the laparoscopic approach. Stratified and propensity score matched analysis demonstrated that the benefits of LC are realized across the diverse spectrum of cancer programs and surgeons. However, close to half of all surgeons still performed only OC, and more than half of high-volume surgeons performed OC more frequently than LC in their routine clinical practice. While clearly not all patients with colon cancer will be candidates for a laparoscopic approach, the use of LC vs OC represents considerable practice variation in the surgical treatment of colon cancer. Moreover, as utilization of hospital days is one of the key cost-drivers of a hospitalization episode, improving LC utilization has the potential to markedly reduce the overall costs of treating patients with colon cancer. Our results indicate that in order for more patients to realize benefits associated with LC at the national level, policies should create incentives within the healthcare system to expand of the capability to perform LC through education and training.
Our findings of shorter length of stay and reduced mortality are consistent with a recent study that examined the perioperative outcomes between LC and OC using the US National Inpatient Sample database (27). However, by using the NCDB, we were additionally able to account for tumor characteristics and evaluate oncologic treatment outcomes. Although the duration of follow-up in this study was too short to address long-term effects on survival, it is highly notable that among stage III patients, LC was associated with improved odds of receiving adjuvant chemotherapy compared with OC. Moreover among stage III patients who did receive adjuvant chemotherapy, LC patients were more likely to initiate chemotherapy within eight weeks, an important quality-of-care endpoint as delays beyond this have been associated with decreased survival (28). These findings were observed after balancing all covariates through PS matching.
Recent implementation of enhanced recovery-after-surgery (ERAS) pathways and modern approaches to perioperative management have been shown to have the potential to reduce LOS and perioperative complications following OC (29,30). The combination of LC and ERAS may have the potential to yield further benefits and is now the subject of ongoing randomized studies (31–33).
Our study has a number of strengths. Conventional regression modeling for our primary comparison does not address threats to internal validity because of treatment selection bias when comparing LC to OC, and statistically significant differences exist between the groups among the covariables. PS matching methods are widely recognized to be an important strategy for reducing confounding because of selection bias by balancing on the background covariables (34,35). Second, we utilized multilevel regression analysis to reduce clustering effects at the hospital-level. Intrahospital variations exist when patients who received colectomies at one hospital on average have better or worse outcomes compared with those who received colectomies at another hospital (36,37). This could be because of differences in clinical pathways between hospitals.
Despite these strengths, there are also several limitations. At the time of the analysis, there was no information about the long-term oncologic outcomes. Moreover, we were unable to distinguish between emergent vs elective indications for colectomy, although our findings were robust across a variety of practice environments. In our analysis of surgeon and hospital volume, it should be acknowledged that even the “high” volume surgeons were only performing six or more colectomies each year; however, this suggests greater generalizability of our findings to the average surgeon’s practice. While the propensity score matching is an important tool for accounting for selection bias as evidenced by the balanced covariables, the analysis may still be at risk for hidden biases. Some important risk factors that could adversely impact the likelihood of receiving LC, such as surgery’s emergency status, existence of extensive adhesions/contraindications to pneumoperitoneum, and anatomical selection of patients, were not available in the NCDB. Patients requiring urgent colectomy may require OC, which may enlarge the differences in short-term outcomes between LC and OC groups. Additional sensitivity analyses have been conducted to account for urgent cases by using the timing of colectomy from diagnosis as a proxy measurement, but the findings remained similar to our main analyses. Instrumental variable analysis could be another potential approach to address the selection bias; however, there are challenges to identifying a valid instrument that is itself not subject to limitations. The large number of colectomies included in the analysis and the robustness of our findings through alternative analyses provide important information to clinicians, policy makers, and the general public about the comparative effectiveness of LC vs OC in routine clinical practice.
Compared with open colectomy, laparoscopic colectomy is associated with a lower 30-day mortality rate, shorter length of hospital stay, and moderate improvement in unplanned readmissions in routine clinical practice. Moreover, laparoscopic colectomy is associated with improved rates of adjuvant chemotherapy administration for patients with stage III colon cancer. We have demonstrated the generalizability of the benefits of laparoscopic colectomy found in randomized clinical trials among real-world patients where the majority of colectomies were performed in community cancer programs. However, more than half of patients still undergo open resection. Wider diffusion of laparoscopy at the population level with incentives to improve laparoscopic skills acquisition through training and monitoring of outcomes, particularly among current high volume surgeons who perform only open colectomy, may benefit patients and improve the efficiency of colon cancer care throughout the healthcare system.
Funding
This project was supported by American Cancer Society Intramural Research Funding, the National Institutes of Health (NIH)/National Cancer Institute (NCI; grant K07-CA133187 to GJC, and the NIH/NCI CA16672 (The University of Texas MD Anderson Cancer Center Support Grant, GJC).
Supplementary Material
This study used the National Cancer Data Base (NCDB). The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the American College of Surgeons and the Commission on Cancer in the creation of the National Cancer Data Base. The data used in the study are derived from a deidentified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed or the conclusions drawn from these data by the authors.
References
- 1. Sticca RP, Alberts SR, Mahoney MR, et al. Current Use and Surgical Efficacy of Laparoscopic Colectomy in Colon Cancer. J Am Coll Surg. 2013;217(1):56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kwon S, Billingham R, Farrokhi E, et al. Adoption of Laparoscopy for Elective Colorectal Resection: A Report from the Surgical Care and Outcomes Assessment Program. J Am Coll Surg. 2012;214(6):909–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kang CY, Halabi WJ, Luo R, Pigazzi A, Nguyen NT, Stamos MJ. Laparoscopic colorectal surgery: A better look into the latest trends. Arch Surg. 2012;147(8):724–731. [DOI] [PubMed] [Google Scholar]
- 4. Lacy AM, García-Valdecasas JC, Delgado S, et al. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet. 2002;359(9325):2224–2229. [DOI] [PubMed] [Google Scholar]
- 5. Group CS. A Comparison of Laparoscopically Assisted and Open Colectomy for Colon Cancer. New Engl J Med. 2004;350(20):2050–2059. [DOI] [PubMed] [Google Scholar]
- 6. Group CS. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. 2005;6(7):477–484. [DOI] [PubMed] [Google Scholar]
- 7. Guillou PJ, Quirke P, Thorpe H, et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365(9472):1718–1726. [DOI] [PubMed] [Google Scholar]
- 8. Reza MM, Blasco JA, Andradas E, Cantero R, Mayol J. Systematic review of laparoscopic versus open surgery for colorectal cancer. Br J Surg. 2006;93(8):921–928. [DOI] [PubMed] [Google Scholar]
- 9. Fleshman J, Sargent DJ, Green E, et al. Laparoscopic Colectomy for Cancer Is Not Inferior to Open Surgery Based on 5-Year Data From the COST Study Group Trial. Ann Surg. 2007;246(4):655–664. [DOI] [PubMed] [Google Scholar]
- 10. Colon Cancer Laparoscopic or Open Resection Study Group BM, Veldkamp R, Hop WC, Kuhry E, Jeekel J, Haglind E, Påhlman L, Cuesta MA, Msika S, Morino M, Lacy A, Bonjer HJ. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. 2009;10(1):44–52. [DOI] [PubMed] [Google Scholar]
- 11. Jayne DG, Thorpe HC, Copeland J, Quirke P, Brown JM, Guillou PJ. Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg. 2010;97(11):1638–1645. [DOI] [PubMed] [Google Scholar]
- 12. Weeks JC, Nelson H, Gelber S, Sargent D, Schroeder G, for the Clinical Outcomes of Surgical Therapy Study G. Short-term quality-of-life outcomes following laparoscopic-assisted colectomy vs open colectomy for colon cancer: A randomized trial. JAMA. 2002;287(3):321–328. [DOI] [PubMed] [Google Scholar]
- 13. Ohtani H, Tamamori Y, Arimoto Y, Nishiguchi Y, Maeda K, Hirakawa K. A meta-analysis of the short- and long-term results of randomized controlled trials that compared laparoscopy-assisted and open colectomy for colon cancer. J Cancer. 2012;3:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abraham NS, Young JM, Solomon MJ. Meta-analysis of short-term outcomes after laparoscopic resection for colorectal cancer. Br J Surg. 2004;91(9):1111–1124. [DOI] [PubMed] [Google Scholar]
- 15. Transatlantic Laparoscopically Assisted vs Open Colectomy Trials Study GHJB, Hop Wim C. J., Nelson Heidi, Sargent Daniel J., Lacy Antonio M, Castells Antoni, Guillou Pierre J., Thorpe Helen, Brown Julia, Delgado Salvadora, Kuhrij Esther, Haglind Eva, Påhlman Lars, Laparoscopically assisted vs open colectomy for colon cancer: A meta-analysis. Arch Surg. 2007;142(3):298–303. [DOI] [PubMed] [Google Scholar]
- 16. Group CS. Impact of hospital case volume on short-term outcome after laparoscopic operation for colonic cancer. Surg Endosc. 2005;19(5):687–692. [DOI] [PubMed] [Google Scholar]
- 17. Singla A, Simons J, Carroll J, et al. Hospital volume as a surrogate for laparoscopically assisted colectomy. Surg Endosc. 2010;24(3):662–669. [DOI] [PubMed] [Google Scholar]
- 18. Kuwabara K, Matsuda S, Fushimi K, Ishikawa K, Horiguchi H, Fujimori K. Impact of Hospital Case Volume on the Quality of Laparoscopic Colectomy in Japan. J Gastrointest Surg. 2009;13(9):1619–1626. [DOI] [PubMed] [Google Scholar]
- 19. Lerro C, Robbins A, Phillips J, Stewart A. Comparison of Cases Captured in the National Cancer Data Base with Those in Population-based Central Cancer Registries. Ann Surg Oncol. 2013;20(6):1759–1765. [DOI] [PubMed] [Google Scholar]
- 20. Hemmila MR, Birkmeyer NJ, Arbabi S, Osborne NH, Wahl WL, Dimick JB. Introduction to propensity scores: A case study on the comparative effectiveness of laparoscopic vs open appendectomy. Arch Surg. 2010;145(10):939–945. [DOI] [PubMed] [Google Scholar]
- 21. Wright JD, Ananth CV, Lewin SN, et al. RObotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. JAMA. 2013;309(7):689–698. [DOI] [PubMed] [Google Scholar]
- 22. Bilmoria KY SA, Winchester DP, Ko CY. The National Cancer Data Base: A powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bilimoria KY, Bentrem DJ, Nelson H, et al. Use and outcomes of laparoscopic-assisted colectomy for cancer in the united states. Arch Surg. 2008;143(9):832–840. [DOI] [PubMed] [Google Scholar]
- 24. Attiyeh FF. Is laparoscopic colectomy a good operation for colon cancer? Arch Surg. 2009;144(3):289–292. [DOI] [PubMed] [Google Scholar]
- 25. Robinson C, Chen GJ, Balentine C, et al. Minimally Invasive Surgery Is Underutilized for Colon Cancer. Ann Surg Oncol. 2011;18(5):1412–1418. [DOI] [PubMed] [Google Scholar]
- 26. Carmichael JC, Masoomi H, Mills S, Stamos MJ, Nguyen NT. Utilization of Laparoscopy in Colorectal Surgery for Cancer at Academic Medical Centers: Does Site of Surgery Affect Rate of Laparoscopy? Am Surg. 2011;77(10):1300–1304. [PubMed] [Google Scholar]
- 27. Juo Y, Hyder O, Haider AH, Camp M, Lidor A, Ahuja N. Is minimally invasive colon resection better than traditional approaches?: First comprehensive national examination with propensity score matching. JAMA Surg. 2014;149(2):177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Biagi JJ, Raphael MJ, Mackillop WJ, Kong W, King WD, Booth CM. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: A systematic review and meta-analysis. JAMA. 2011;305(22):2335–2342. [DOI] [PubMed] [Google Scholar]
- 29. Rawlinson A, Kang P, Evans J, Khanna A. A systematic review of enhanced recovery protocols in colorectal surgery. Ann Royal Coll Surg Engl. 2011;93(8):583–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Spanjersberg WR RJ, Keus F, van Laarhoven CJ. Fast track surgery versus conventional recovery strategies for colorectal surgery. Cochrane Database Syst Rev. Feb 16 2011;16(2):CD007635. [DOI] [PubMed] [Google Scholar]
- 31. Aarts M-A, Okrainec A, Glicksman A, Pearsall E, Charles Victor J, McLeod R. Adoption of enhanced recovery after surgery (ERAS) strategies for colorectal surgery at academic teaching hospitals and impact on total length of hospital stay. Surg Endosc. 2012;26(2):442–450. [DOI] [PubMed] [Google Scholar]
- 32. Vlug MS, Wind J, Hollmann MW, et al. Laparoscopy in Combination with Fast Track Multimodal Management is the Best Perioperative Strategy in Patients Undergoing Colonic Surgery: A Randomized Clinical Trial (LAFA-study). Ann Surg. 2011;254(6):868–875. [DOI] [PubMed] [Google Scholar]
- 33. Kennedy RH, Francis A, Dutton S, et al. EnROL: a multicentre randomised trial of conventional versus laparoscopic surgery for colorectal cancer within an enhanced recovery programme. BMC Cancer. 2012;12:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sox HC, Goodman SN. The Methods of Comparative Effectiveness Research. Ann Rev Public Health. 2012;33(1):425–445. [DOI] [PubMed] [Google Scholar]
- 35. ROSENBAUM PR, RUBIN DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 36. Osler M, Iversen LH, Borglykke A, et al. Hospital Variation in 30-Day Mortality After Colorectal Cancer Surgery in Denmark: The Contribution of Hospital Volume and Patient Characteristics. Ann Surg. 2011;253(4):733–738. [DOI] [PubMed] [Google Scholar]
- 37. Zheng Z, Hanna N, Onukwugha E, Bikov KA, Mullins CD. Hospital Center Effect for Laparoscopic Colectomy Among Elderly Stage I-III Colon Cancer Patients. Ann Surg. 2014;259(5):924–929. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.