Abstract
Introduction
Sleep related breathing disorders (SRBD) are associated with both obesity and systemic inflammation. While the relationship between obesity and SRBD is established, the causality between inflammation and SRBD remains unclear. In this study we investigated the relation between SRBD and C-reactive protein (CRP) as a parameter of inflammation and the influence of SRBD treatment on CRP with additional regard to changes in metabolic and cardiovascular parameters.
Methods
Polysomnography (PSG) and laboratory data of patients diagnosed with SRBD over a period of 5 years were prospectively collected in a database and retrospectively analysed regarding the association of SRBD (according to apnoea-hypopnoea- index (AHI), duration of events and extent of desaturation) to CRP, blood pressure, cholesterol, fasting plasma glucose, HbA1c, quality of life measured via a visual analogue scale (VAS 0–100%), and the effects of SRBD therapy on these parameters.
Results
716 patients were included in the study, 171 with mild SRBD (AHI ≥5 to <15/h), 209 with moderate SRBD (AHI 15 to <30/h), 336 with severe SRBD (AHI ≥30/h). Results according to severity of SRBD. Severe SRBD was significantly associated with elevated levels of CRP (3.7 [1.8–7.0] mg/l, vs. moderate (p = 0.001), and mild SRBD (p<0.001), and higher prevalence of hypertension as compared to moderate and mild SRBD (p<0.001, respectively). Results in highly successful treatment. If SRBD treatment was highly successful (AHI <5/h), CRP and quality of life improved significantly (p = 0.001 and p = 0.002), as did blood pressure (p<0.001 for systolic and diastolic values), although BMI increased (p<0.001). Results in partially successful treatment. If success was defined as reduction of AHI of ≥50%, CRP also decreased (p<0.001), as did blood pressure (p<0.001). Again, BMI increased (p<0.001).
Conclusion
This is the first study to show an association of SRBD and CRP independently of BMI in a large cohort. The SRBD therapy-induced CRP decrease was not associated with BMI changes or metabolic changes but rather with the magnitude of AHI improvement.
Introduction
Sleep related breathing disorders (SRBD) comprise various types. The most important and predominant ones are obstructive sleep apnoea (OSAS) and central sleep apnoea (CSAS), although with distinct differences in pathophysiology. In OSAS, repeated episodes of upper airway closure (leading to cessation of respiratory flow = apnoea, or diminished flow with hypoxia and/or arousal = hypopnoea []) during sleep lead to intermittent hypoxia, sympathetic activation, cytokine release and cardiovascular morbidity and mortality [1]. The estimated prevalence is approximately 20 to 30 percent in males and 10 to 15 percent in females when OSAS is defined as an AHI >5 events per hour as measured by a polysomnography [2].
In CSAS, episodes of apnoea result from temporary suspension of ventilatory effort. This is due to disturbances in ventilatory response which is dependent on metabolic control system (chemoreceptors, pCO2). This type includes idiopathic central sleep apnoea as well as secondary CSAS such as Cheyne-Stokes respiration, hypnotics- and high-altitude induced CSAS [3]. Effects on morbidity and mortality may be similar to OSAS, but fewer data exist according to apparently lower prevalence.
Sleep-related breathing disorders (SRBD) are associated with increased cardiovascular morbidity and mortality [4, 5, 6]. Cardiovascular disease is associated with systemic inflammation [7, 8, 9, 10, 11, 12], and this may be the link between SRBD and cardiovascular disease [13, 14, 15]. Accordingly, elevated C-reactive protein (CRP) plasma levels were found in SRBD [16, 17, 18, 19], and nasal continuous positive airway pressure (CPAP) not only improved SRBD but also CRP levels [20, 21, 22]. However, obesity which is strongly associated with SRBD, may also be associated with inflammation [23, 24, 25] posing the question if obesity is the true link between SRBD and inflammation. The aim of this study was to analyse CRP in association with SRBD in different levels of severity as well as changes of CRP, BMI, BP, and QoL with SRBD therapy.
Methods
We reviewed the data of all patients referred to the sleep laboratory of the Department of Internal Medicine/Division of Pulmonology at the Medical University of Graz, Austria, between 2007 and 2012. Data had been prospectively entered into a database. All patients diagnosed with SRBD and at least one polysomnographic control after established positive airway pressure or positional therapy (at least three months after initiation) were included. The study was approved by the local ethics committee at the Medical University of Graz. Patients´ written consent was obtained.
PSG examination was conducted using a standard montage according to AASM [26]. Polysomnograms were scored by a polysomnographic technologist and examined by a sleep specialist. Polysomnography was performed using a digital device (Schwarzer™, Domino™). Respiratory events and sleep staging were scored according to the rules of the AASM [26].
Treatment consisted of CPAP, AutoCPAP, or bilevel positive airway pressure (BPAP), depending on patient characteristics or patient needs. In case of central sleep apnoea or complex sleep apnoea, adaptive servoventilation (ASV) was applied if necessary. All currently available types of CPAP/AutoCPAP/BPAP/ASV devices by Philipps Respironics® Inc., ResMed® Inc., and Weinmann® were used. Only 18 patients used positional therapy as long-term treatment. Titration was performed by a nurse specialist in sleep medicine according to instructions by the sleep specialist physician.
The evaluated parameters were apnoea-hypopnoea index (AHI), longest duration of a respiratory event (longest event), minimum capillary oxygen saturation level during sleep (minSpO2), oxygen desaturation index (ODI), percent of total sleep time below 90% SpO2 (%TST<90). Additional measurements included weight, height, body mass index (BMI), and clinical chemistry from blood samples taken in the morning after the overnight polysomnography. The evaluated parameters were C-reactive protein (CRP), high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), total cholesterol (Chol), triglycerides (TG), fasting plasma glucose (FPG), and glycosylated haemoglobin (HbA1c). The following co-morbidities were evaluated: coronary artery disease (CAD), atrial fibrillation (AF), apoplexy (APO), arterial blood pressure (BP), renal insufficiency (GFR according to MDRD). All patients were asked to estimate their overall quality of life (Qol) on a visual analogue scale (VAS, 0% = worst possible, 100% = best possible) before and after treatment.
All data were stored and processed in a central database that automatically extracted data from the sleep laboratory system and was fed manually for the resting parameters. The patients were divided into SRBD patients (AASM category II, Sleep-Related Breathing Disorders) and others (AASM category I, III-VIII). Overall and in each subgroup changes in parameters from baseline to the final examination were evaluated by Wilcoxon- signed-rank test. For continuous variables differences between groups were analysed using Kruskal-Wallis Test. To analyse differences in categorical variables Chi square Test and, when appropriate, Fisher’s exact Test were used. To analyse associations between continuous variables Spearman’s rank correlation (ρ) was calculated. Statistical analyses were performed using SPSS 19.0 (SPSS, Chicago, IL, USA). Continuous data are presented as median and interquartile range. Categorical data are presented as absolute and relative frequencies.
Results
From 2007 to 2012, 716 patients who underwent diagnostic PSG revealing SRBD started SRBD therapy and had at least one PSG control within this period (m/f: 512/204, 71.5%/28.5%). An initial AHI of ≤5 to <15/h (mild SRBD), of 15 to <30/h (moderate SRBD), and of ≥30/h (severe SRBD) was found in 171 (m/f: 117/54, 68%/32%), 209 (m/f: 139/70, 67%/33%), and 336 (m/f: 256/80, 76%/24%) patients. Characteristics at the diagnostic and final PSG as well as changes are shown in Tables 1–3, results according to success in Tables 4 and 5, comparison between the severity groups in Table 6.
Table 1. Characteristics of patients with mild sleep apnoea syndrome; median (interquartile range).
| preAHI > = 5 bis <15 | initial PSG | final PSG | Change | sign. change | |
|---|---|---|---|---|---|
| Age | years | 58.1 (50.2–69.2) | 59.5 (51.1–70.1) | 1.1 (0.5–1.7) | <.001 |
| Height | cm | 171.0 (164.0–176.0) | 171.0 (164.0–176.0) | 0.0 (0.0–0.0) | n/a |
| Weight | kg | 86.0 (74.0–96.0) | 87.0 (77.0–98.0) | 0.0 (-1.0–3.0) | .035 |
| BMI | kg/m² | 29.3 (26.5–33.0) | 29.4 (26.8–33.8) | 0.0 (-0.4–1.1) | .035 |
| HDL | mg/dl | 53.0 (42.0–63.0) | 51.0 (41.0–64.0) | 0.0 (-6.0–5.0) | .803 |
| LDL | mg/dl | 111.0 (92.0–139.0) | 114.5 (85.5–135.0) | -1.0 (-12.0–10.0) | .564 |
| Chol | mg/dl | 189.0 (162.0–220.0) | 182.5 (156.0–216.0) | -5.0 (-21.0–12.0) | .044 |
| CRP | mg/l | 2.0 (1.0–4.4) | 1.9 (1.0–3.8) | 0.0 (-1.0–0.6) | .296 |
| TG | mg/dl | 114.0 (92.0–173.0) | 116.0 (88.0–163.0) | -3.0 (-27.0–16.5) | .105 |
| FPG | mg/dl | 97.0 (89.0–109.0) | 99.0 (91.0–112.0) | 2.0 (-5.0–11.0) | .013 |
| HbA1c | IFCC: mmol/mol | 37.7 (35.5–42.1) | 37.7 (35.5–42.1) | 0.9 (-1.1–2.2) | .026 |
| AHI | 10.7 (8.6–12.8) | 1.6 (0.4–4.0) | -8.5 (-10.7–-5.5) | <.001 | |
| Longest event | sec | 55.8 (43.0–80.5) | 31.0 (20.0–48.0) | -22.7 (-46.5–-4.2) | <.001 |
| Min O2 | % | 82.9 (78.9–86.9) | 89.0 (86.0–91.0) | 6.0 (2.1–9.1) | <.001 |
| ODI | 9.7 (7.3–12.4) | 1.5 (0.3–4.5) | -7.1 (-9.8–-4.3) | <.001 | |
| % TST<90 | 2.7 (0.6–13.3) | 0.0 (0.0–1.2) | -1.9 (-10.7–-0.3) | <.001 | |
| BP syst | mmHg | 137.5 (125.0–151.0) | 134.0 (123.0–146.0) | -2.0 (-15.5–8.0) | .010 |
| BP diast | mmHg | 89.0 (81.0–97.0) | 85.0 (77.0–92.0) | -3.0 (-11.0–3.0) | <.001 |
| QoL | 72.5 (53.8–80.0) | 80.0 (60.0–90.0) | 0.0 (-5.0–20.0) | <.001 |
Table 3. Characteristics of patients with severe sleep apnoea syndrome; median (interquartile range).
| preAHI > = 30 | initial PSG | final PSG | Change | sign. change | |
|---|---|---|---|---|---|
| Age | years | 61.8 (51.2–69.9) | 63.2 (51.7–71.5) | 1.1 (0.6–1.8) | <.001 |
| Height | cm | 170.0 (164.0–177.0) | 170.0 (164.0–177.0) | 0.0 (0.0–0.0) | n/a |
| Weight | kg | 98.0 (85.0–112.0) | 97.0 (85.0–113.0) | 0.0 (-2.0–2.5) | .402 |
| BMI | kg/m² | 34.6 (30.2–38.8) | 34.5 (30.1–38.4) | 0.0 (-0.7–0.9) | .391 |
| HDL | mg/dl | 48.0 (39.0–59.0) | 49.0 (40.0–58.0) | -1.0 (-5.0–5.0) | .418 |
| LDL | mg/dl | 112.0 (92.0–131.5) | 109.0 (89.0–132.0) | 0.0 (-17.0–14.0) | .566 |
| Chol | mg/dl | 187.0 (159.0–214.0) | 183.5 (156.0–213.0) | -1.0 (-18.0–15.0) | .372 |
| CRP | mg/l | 3.7 (1.8–7.0) | 3.0 (1.6–5.8) | -0.4 (-2.2–0.8) | <.001 |
| TG | mg/dl | 132.0 (98.0–179.0) | 131.5 (96.0–184.0) | 1.0 (-28.0–30.0) | .676 |
| FPG | mg/dl | 104.0 (96.0–120.0) | 105.0 (97.0–120.0) | 1.0 (-7.0–8.0) | .624 |
| HbA1c | IFCC: mmol/mol | 39.9 (36.6–45.4) | 40.0 (36.6–45.4) | 0.0 (-2.2–2.2) | .238 |
| AHI | 49.9 (38.4–64.3) | 2.9 (0.9–6.7) | -43.7 (-58.7–-33.2) | <.001 | |
| Longest event | sec | 68.0 (51.1–92.0) | 41.0 (25.8–70.0) | -24.1 (-51.1–0.8) | <.001 |
| Min O2 | % | 75.9 (66.0–80.0) | 88.0 (85.0–90.0) | 12.1 (7.0–21.0) | <.001 |
| ODI | 50.3 (37.2–63.8) | 3.0 (0.8–7.7) | -43.1 (-58.3–-32.9) | <.001 | |
| % TST<90 | 27.2 (11.5–52.3) | 0.2 (0.0–2.0) | -23.7 (-48.4–-10.1) | <.001 | |
| BP syst | mmHg | 143.0 (131.0–157.0) | 137.0 (125.0–152.5) | -5.0 (-18.5–7.0) | <.001 |
| BP diast | mmHg | 90.0 (81.5–99.0) | 85.0 (77.0–93.0) | -6.0 (-15.0–4.5) | <.001 |
| QoL | 75.0 (56.3–80.0) | 80.0 (60.0–90.0) | 0.0 (-10.0–20.0) | .004 |
Table 4. Characteristics of the success group; median (interquartile range).
| postAHI < 5/h | initial PSG | final PSG | Change | sign. change | |
|---|---|---|---|---|---|
| Age | years | 60.2 (51.7–69.6) | 61.7 (52.7–70.9) | 1.1 (0.6–1.8) | <.001 |
| Height | cm | 170.0 (164.0–176.0) | 170.0 (164.0–176.0) | 0.0 (0.0–0.0) | n/a |
| Weight | kg | 91.0 (80.0–106.0) | 92.0 (81.0–105.0) | 1.0 (-1.0–3.0) | <.001 |
| BMI | kg/m² | 31.6 (27.7–35.6) | 31.7 (28.0–36.3) | 0.3 (-0.5–1.1) | .000 |
| HDL | mg/dl | 50.0 (41.0–61.0) | 50.0 (41.0–61.0) | 0.0 (-5.0–5.0) | .716 |
| LDL | mg/dl | 114.0 (92.0–138.0) | 114.0 (90.0–137.0) | -1.0 (-15.0–15.0) | .806 |
| Chol | mg/dl | 190.0 (161.0–220.0) | 189.0 (161.0–218.0) | -2.0 (-18.0–17.0) | .409 |
| CRP | mg/l | 2.6 (1.2–5.5) | 2.3 (1.1–4.6) | -0.2 (-1.5–0.6) | .001 |
| TG | mg/dl | 123.5 (94.5–174.5) | 126.0 (90.0–173.0) | 1.0 (-27.0–24.0) | .900 |
| FPG | mg/dl | 100.0 (92.0–113.0) | 102.0 (94.0–115.0) | 1.0 (-6.0–10.0) | .003 |
| HbA1c | IFCC: mmol/mol | 38.8 (35.5–43.2) | 38.8 (35.5–43.2) | 0.0 (-1.9–2.2) | .190 |
| AHI | 26.5 (14.5–46.0) | 1.2 (0.4–2.5) | -25.0 (-44.1–-12.6) | <.001 | |
| Longest event | sec | 64.5 (47.7–90.0) | 30.0 (19.2–48.1) | -30.0 (-57.7–-11.0) | <.001 |
| Min O2 | % | 79.9 (72.9–83.9) | 90.0 (87.0–91.0) | 9.1 (6.0–15.0) | <.001 |
| ODI | 25.6 (12.9–45.0) | 1.2 (0.3–2.7) | -23.0 (-43.3–-10.9) | <.001 | |
| % TST<90 | 11.1 (2.6–37.1) | 0.0 (0.0–0.4) | -9.8 (-31.5–-1.9) | <.001 | |
| BP syst | mmHg | 141.0 (130.0–155.0) | 135.0 (125.0–150.0) | -5.0 (-17.0–8.0) | <.001 |
| BP diast | mmHg | 90.0 (81.0–97.0) | 85.0 (78.0–92.0) | -4.0 (-12.0–4.0) | <.001 |
| QoL | 75.0 (60.0–80.0) | 80.0 (70.0–90.0) | 0.0 (-5.0–20.0) | <.001 |
Table 5. Characteristics of the 50%success group; median (interquartile range).
| Success—50% | initial PSG | final PSG | Change | sign. change |
|---|---|---|---|---|
| Age | 60.6 (52.2–69.7) | 62.1 (53.2–71.1) | 1.1 (0.6–1.9) | <.001 |
| Height | 170.0 (164.0–176.0) | 170.0 (164.0–176.0) | 0.0 (0.0–0.0) | n/a |
| Weight | 93.0 (81.0–106.0) | 93.0 (82.0–106.0) | 0.0 (-2.0–3.0) | <.001 |
| BMI | 32.0 (28.0–36.2) | 32.2 (28.4–36.8) | 0.0 (-0.6–1.1) | <.001 |
| HDL | 49.0 (40.0–60.0) | 50.0 (41.0–60.0) | 0.0 (-5.0–5.0) | .788 |
| LDL | 113.0 (92.0–136.0) | 112.0 (90.0–136.0) | 0.0 (-16.0–14.0) | .467 |
| Chol | 189.0 (160.5–218.0) | 187.0 (159.0–217.0) | -2.0 (-18.0–16.0) | .228 |
| CRP | 2.9 (1.3–5.7) | 2.5 (1.2–4.9) | -0.2 (-1.5–0.7) | <.001 |
| TG | 125.5 (96.0–179.0) | 128.0 (91.0–181.0) | 1.0 (-27.0–28.0) | .601 |
| FPG | 101.0 (93.0–116.0) | 102.0 (95.0–116.0) | 1.0 (-7.0–9.0) | .025 |
| HbA1c | 38.8 (35.5–43.2) | 39.0 (35.5–44.0) | 0.0 (-2.1–2.2) | .184 |
| AHI | 29.9 (16.6–49.9) | 1.8 (0.6–4.2) | -26.6 (-45.1–-14.2) | <.001 |
| Longest event | 64.7 (47.5–90.8) | 35.0 (23.0–57.0) | -27.0 (-52.3–-3.8) | <.001 |
| Min O2 | 79.0 (71.9–83.9) | 89.0 (86.0–91.0) | 9.1 (5.1–15.1) | <.001 |
| ODI | 28.8 (15.6–50.3) | 1.8 (0.5–5.0) | -25.2 (-43.3–-12.6) | <.001 |
| % TST<90 | 13.2 (2.9–41.3) | 0.1 (0.0–0.9) | -11.9 (-36.8–-2.5) | <.001 |
| BP syst | 141.5 (130.0–155.0) | 135.0 (125.0–151.0) | -4.0 (-16.0–8.0) | <.001 |
| BP diast | 90.0 (81.0–98.0) | 85.0 (77.0–93.0) | -4.0 (-13.0–4.0) | <.001 |
| QoL | 75.0 (60.0–80.0) | 80.0 (60.0–90.0) | 0.0 (-5.0–20.0) | <.001 |
Table 6. Comparison between different levels of severity of sleep apnoea syndrome; p-values.
| Mild vs moderate | Severe vs mild | Severe vs moderate | ||
|---|---|---|---|---|
| Age | years | 0.016 | 0.385 | 0.435 |
| Height | cm | 0.834 | 1.000 | 0.868 |
| Weight | kg | 0.662 | 0.000 | 0.000 |
| BMI | kg/m² | 0.108 | 0.000 | 0.000 |
| HDL | mg/dl | 1.000 | 0.044 | 0.144 |
| LDL | mg/dl | 1.000 | 1.000 | 0.707 |
| Chol | mg/dl | 1.000 | 1.000 | 0.444 |
| CRP | mg/l | 0.153 | 0.000 | 0.001 |
| TG | mg/dl | 1.000 | 0.088 | 0.556 |
| FPG | mg/dl | 0.056 | 0.000 | 0.009 |
| HbA1c | IFCC: mmol/mol | 1.000 | 0.000 | 0.000 |
| AHI | 0.000 | 0.000 | 0.000 | |
| Longest event | sec | 0.077 | 0.000 | 0.397 |
| Min O2 | % | 0.011 | 0.000 | 0.000 |
| ODI | 0.000 | 0.000 | 0.000 | |
| % TST<90 | 0.002 | 0.000 | 0.000 | |
| BP syst | mmHg | 0.107 | 0.003 | 0.190 |
| BP diast | mmHg | 0.476 | 0.235 | 0.044 |
CRP, BMI and metabolic parameters according to SRBD severity
In severe SRBD, CRP showed the highest values (3.7 [1.8–7.0] mg/l) in comparison to moderate (2.5 [1.3–5.1] mg/l, p = 0.001) and mild SRBD (2.0 [1.0–4.4] mg/l, p<0.001). Severe SRBD patients also had the highest BMI (34.6 [30.2–38.8] kg/m2) in comparison to moderate (30.3 [27.4–34.1] kg/m2, p<0.001) and mild SRBD (29.3 [26.5–33.0] kg/m2, p<0.001). TG, FPG, HbA1c increase was also associated with SRBD severity, whereas Chol and LDL values did not show a significant association to severity.
Cardiovascular events and co-morbidities according to SRBD severity
Systemic arterial hypertension (treated or untreated) was significantly more often present in severe SRBD (72%, 80%, and 86% in mild, moderate, and severe SRBD, p<0.001). The number of cardiovascular events and the extent of co-morbidities were slightly higher in severe SRBD, but these differences between groups were not statistically significant. Atrial fibrillation was present in 9%, 9%, and 11% in mild, moderate and severe SRBD (p = 0.625). Coronary artery disease was present in 13%, 14%, and 18% in mild, moderate, and severe SRBD (p = 0.220). A history of myocardial infarction was present in 5%, 8%, and 10% in mild, moderate, and severe SRBD (p = 0.124). Apoplexy was present in 5%, 7%, and 7% in mild, moderate, and severe SRBD (p = 0.507). GFR according to MDRD showed a normal renal function in 25%, 24%, and 22% in mild, moderate, and severe SRBD (p = 0.696).
Results within groups of SRBD severity
SRBD therapy improved polysomnographic values in all groups. Non-satisfactory results in a few patients were due to final PSG without PAP due to lack of tolerance or due to on-going adaptation of treatment. Metabolic and inflammatory parameters showed variable changes (see Tables 1, 2 and 3):
Table 2. Characteristics of patients with moderate sleep apnoea syndrome; median (interquartile range).
| preAHI 15 bis < 30 | initial PSG | final PSG | Change | Significance of change | |
|---|---|---|---|---|---|
| Age | years | 63.0 (54.4–69.9) | 64.4 (55.8–71.5) | 1.1 (0.6–2.0) | <.001 |
| Height | cm | 170.0 (163.0–176.0) | 170.0 (163.0–176.0) | 0.0 (0.0–0.0) | n/a |
| Weight | kg | 87.0 (78.5–99.0) | 89.5 (79.0–100.0) | 1.0 (-1.0–4.0) | <.001 |
| BMI | kg/m² | 30.3 (27.4–34.1) | 30.8 (27.5–34.6) | 0.4 (-0.4–1.3) | <.001 |
| HDL | mg/dl | 50.0 (41.0–61.0) | 50.0 (43.0–61.0) | 0.0 (-5.0–6.0) | .779 |
| LDL | mg/dl | 114.0 (92.0–140.0) | 115.0 (95.0–142.0) | 3.0 (-20.0–18.0) | .784 |
| Chol | mg/dl | 192.0 (163.0–220.5) | 195.0 (167.0–223.0) | 0.0 (-17.0–20.0) | .385 |
| CRP | mg/l | 2.5 (1.3–5.1) | 2.3 (1.1–4.8) | -0.2 (-1.1–0.7) | .160 |
| TG | mg/dl | 124.0 (91.0–189.0) | 128.0 (84.0–187.0) | 3.0 (-24.5–30.5) | .201 |
| FPG | mg/dl | 100.0 (93.0–115.5) | 100.0 (95.0–115.0) | 1.0 (-7.0–9.0) | .197 |
| HbA1c | IFCC: mmol/mol | 37.7 (35.5–41.0) | 38.8 (35.5–42.1) | 0.2 (-1.1–2.2) | .006 |
| AHI | 22.1 (17.8–25.8) | 2.0 (0.8–5.1) | -18.3 (-22.7–-14.8) | <.001 | |
| Longest event | sec | 63.0 (45.0–93.0) | 37.0 (24.6–56.9) | -26.0 (-51.0–-2.2) | <.001 |
| Min O2 | % | 81.0 (76.0–84.9) | 89.0 (86.9–91.0) | 7.1 (4.0–11.1) | <.001 |
| ODI | 21.1 (17.1–25.9) | 1.8 (0.5–5.7) | -17.5 (-22.3–-12.8) | <.001 | |
| % TST<90 | 5.8 (2.0–19.4) | 0.0 (0.0–0.8) | -4.3 (-13.2–-1.1) | <.001 | |
| BP syst | mmHg | 141.0 (129.0–154.0) | 133.0 (126.0–149.0) | -3.5 (-15.0–9.0) | .011 |
| BP diast | mmHg | 88.0 (79.0–95.0) | 84.0 (77.0–92.0) | -3.0 (-12.0–5.0) | <.001 |
| QoL | 70.0 (50.0–90.0) | 80.0 (60.0–90.0) | 0.0 (0.0–15.0) | .001 |
CRP decreased significantly with therapy only in severe SRBD (p<0.001) who had also the highest baseline levels, while the changes in mild and moderate SRBD were not significant.
Systemic arterial blood pressure (BP) decreased in all groups (mild SRBD: systolic and diastolic p = 0.010 and diastolic p<0.001, moderate SRBD: p = 0.011 and p = 0.001, severe SRBD: p<0.001 and p<0.001). The same was true for quality of life (VAS 1–100) which improved in mild, moderate, and severe SRBD (72.5 to 80, p<0.001, 70 to 80, p = 0.001, 75 to 80, p = 0.004).
Patients with mild and moderate SRBD revealed a significant BMI increase during SRBD therapy (mild SRBD p = 0.032, moderate SRBD p<0.001), while in the severe SRBD group BMI was stable (p = 0.391). Accordingly, HbA1c increased in both mild (p = 0.026) and moderate SRBD (p = 0.006), no significant changes occurred in severe SRBD (p = 0.238). Fasting plasma glucose increased significantly in mild SRBD (p = 0.013), but not in moderate SRBD (p = 0.197) or in severe SRBD (p = 0.624). In contrast, total cholesterol decreased in mild SRBD (p = 0.044), while changes in moderate and severe SRBD were not significant. Triglycerides did not change during treatment in any group, neither did LDL (see Tables 1, 2 and 3).
Effects of normalization of AHI after SRBD therapy
When patients with satisfactory results (AHI <5/h) after sleep apnoea therapy were compared to patient with non-satisfactory results (AHI ≥5/h) after SRBD therapy, there were distinct differences with respect to changes in weight and laboratory values (Table 4, Table A and Table B in S1 File):
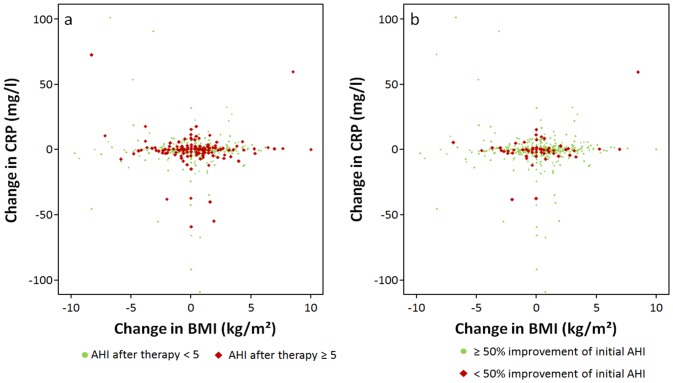
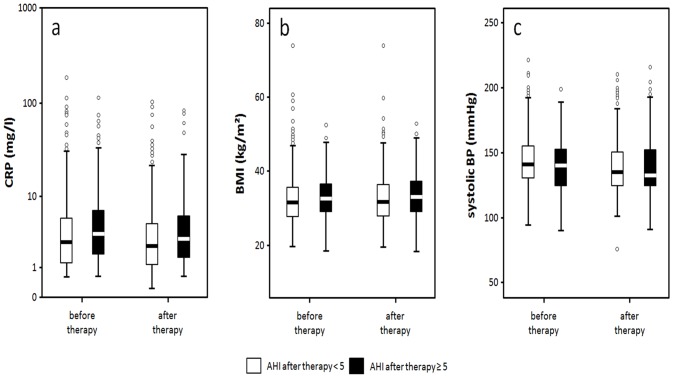
516 patients were assigned to the successful treatment group (initially mild SRBD n = 139, moderate SRBD n = 154, severe SRBD n = 223), 200 were assigned to the non-success group (mild SRBD n = 32, moderate SRBD n = 55, severe SRBD n = 113), although their PSG parameters improved significantly. Distribution of male and female patients in both groups were comparable (p = 0.582, success: f: 74%, m: 71%). After therapy, CRP decreased and changes were independent of BMI (Fig 1a). Decrease of CRP was significant in both treatment groups (p = 0.001, p = 0.047, Fig 2a) with greater changes in the success group (Table B in S1 File, p = 0.808). In contrast to CRP, BMI increased significantly in the success group (p<0.001, Fig 2b). BP improved in the success group (p<0.001 for systolic and diastolic values) and in the non-success group (p = 0.036 systolic, p<0.001 diastolic) within similar ranges (p = 0.184 systolic, p = 0.403 diastolic, Fig 2c).
Fig 1. Correlation of changes of BMI and CRP: Change of BMI is not associated with change in CRP.
a Success group (AHI<5/h) vs non-success. b 50%success group vs non-success.
Fig 2. Development of CRP (a), BMI (b) and systolic blood pressure (c) in success (AHI<5/h) vs non-success.
HbA1c and FPG showed no significant improvement or even worsening. Quality of life was significantly higher in the success group than in the non-success group (VAS = 80 vs 73, p = 0.002).
The period of time between initial and final examination was not the same for all patients. Patients with finally lower AHI had a longer period of time between the initial and the final examination (final AHI<5/h: 12.5 months [6.7–22.2], final AHI ≥5/h: 11.3 months [5.2–23.6], p = 0.036), but this yielded no correlation with CRP (AHI<5/h: ρ = -0.026; p = 0.560, AHI ≥5/h: ρ = 0.028; p = 0.692).
Overall correlation of time period to CRP did not yield statistically significant results, either (ρ = -0.005, p = 0.897).
Effects of 50% improvement of AHI
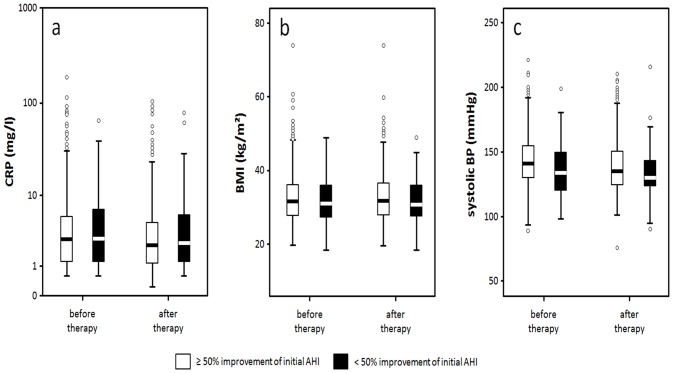
If treatment success was defined as improvement by at least 50% of initial AHI (success50%), 644 patients were in the success group, and 69 in the non-success group with no statistically significant difference regarding gender distribution (p = 0.346, success: f 89%, m 91%). The success group turned out to be younger, with initially more severe SRBD according to AHI, ODI, longest event, O2 saturation and had higher values of BP (see Table 5, and Table C and Table D in S1 File). As expected, the success group improved more distinctly in AHI, ODI, longest event, minimal O2 saturation, and TST<90%. CRP decreased significantly (p< 0.001) and independently of changes in BMI (see Figs 1b and 3a, Table 5), but CRP in the final PSG in the success50% group did not differ from CRP in the non-success group (see Table D in S1 File). Again, there were no significant changes in BMI or metabolic parameters. BMI (Fig 3b) and FPG even increased. BP improved significantly (p<0.001 systolic and diastolic) and reached similar values as compared to the non-success group although the baseline values were higher in the success group (Table D in S1 File, Fig 3c).
Fig 3. Development of CRP (a), BMI (b) and systolic blood pressure (c) in 50%success vs non-success.
The differences in the period of time between initial and final PSG had no influence on AHI (AHI success50%: 12.9 months [5.4–20.3]; AHI >50%: 13.3 months [6.6–22.4], p = 0.237) or on CRP (success50%: ρ = -0.011; p = 0.779; non-success: ρ = -0.010; p = 0.938).
Results within SRBD subtypes
Defining OSAS with obstructive respiratory events comprising ≥ 50% of the total AHI, and CSAS with central respiratory events being ≥ 50% of the total AHI, we analysed the results within these two subtypes. Of the 716 initially identified patients, 606 fulfilled the criteria for OSAS, 108 those for CSAS, in two patients the predominating respiration pattern was not distinct, so these were not included in this analysis. Patients in the OSAS group yielded similar results to the overall analysis with highest CRP and BMI in the severe SRBD group and significant results with success (Tables 7 and 8).
Table 7. Characteristics of the OSAS group.
| Total n = 606 | Mild SRBD | Moderate SRBD | Severe SRBD | p-value |
|---|---|---|---|---|
| (AHI≥ 5 to <15/h) | (AHI 15 to <30/h) | (AHI ≥30/h) | ||
| n | 155 (25%) | 181 (30%) | 270 (45%) | |
| CRP | 2.0 [1.0–4.5] | 2.7 [1.3–5.3] | 4.0 [2.0–7.3] | <0.001 severe vs moderate |
| <0.001 severe vs mild | ||||
| 0.07 moderate vs mild | ||||
| Art. Hypertension yes | 113 (23.2%) | 146 (29.9%) | 229 (46.9%) | 0.01(yes vs no) |
| Art. Hypertension no | 41 (36.0%) | 34 (29.8%) | 39 (34.2%) | 0.01(yes vs no) |
| BMI | 29.8 [26.4–33.5] | 30.8 [27.5–35.1] | 34.7 [31.1–39.3] | <0.001 severe vs moderate |
| <0.001 severe vs mild | ||||
| 0.02 moderate vs mild |
Table 8. Changes in CRP, QoL, systolic and diastolic blood pressure and BMI in the OSAS group for successfully treated patients.
| Success AHI<5/h | Success50% | |
|---|---|---|
| CRP | p<0.001 | p<0.001 |
| QoL | p<0.001 | p<0.001 |
| BPsyst | p<0.001 | p<0.001 |
| BPdiast | p<0.001 | p<0.001 |
| BMI | p<0.001 | p<0.001 |
In CSAS, the initial distribution of mild, moderate, and severe SRBD was significantly different to the one in OSAS (p = 0.01) with more severe SRBD in CSAS compared to OSAS (59% vs. 45%). CRP values were without significant differences within the severity groups, and results concerning CPR and BMI with SRBD treatment lacked statistical significance, however, quality of life as well as blood pressure values yielded similar improvement as in the overall analysis (Tables 9 and 10).
Table 9. Characteristics in the CSAS group.
| Total n = 108 | Mild SRBD | Moderate SRBD | Severe SRBD | p-value |
|---|---|---|---|---|
| (AHI≥ 5 to <15/h) | (AHI 15 to <30/h) | (AHI ≥30/h) | ||
| N | 16 (15%) | 28 (26%) | 64 (59%) | |
| CRP | 1.1 [1.0–2.3] | 1.8 [1.0–3.5] | 2.0 [1.0–5.2] | 0.054 severe vs moderate |
| 0.108 severe vs mild | ||||
| 0.259 moderate vs mild | ||||
| Art. Hypertension yes | 9 (10.3%) | 21 (24.1%) | 57 (65.5%) | 0.008(yes vs no) |
| Art. Hypertension no | 7 (33.3%) | 7 (33.3%) | 7 (33.3%) | 0.008(yes vs no) |
| BMI | 28.1 [27.4–29.7] | 28.3 [26.4–30.9] | 31.7 [27.8–36.2] | 0.002 severe vs moderate |
| 0.01 severe vs mild | ||||
| 0.81 moderate vs mild |
Table 10. Changes of CRP, Qol, systolic and diastolic blood pressure and BMI in the CSAS group for successfully treated patients.
| Success AHI<5/h | Success50% | |
|---|---|---|
| CRP | p = 0.941 | p = 0.979 |
| QoL | p = 0.029 | p = 0.053 |
| BPsyst | p = 0.020 | p = 0.007 |
| BPdiast | p<0.001 | p<0.001 |
| BMI | p = 0.687 | p = 0.708 |
Results with regard to type of treatment
Analysis with regard to treatment with CPAP/APAP yielded similar results to the overall analysis with respect to improvement of CRP, quality of life, blood pressure values, and increasing BMI. Significance of CRP baseline levels comparing the various severity groups was comparable to the overall results as well. Differences in prevalence of blood pressure were less distinct. In the BPAP and ASV groups, the results did not follow the trend as found in CPAP/APAP and overall (Tables 11–16). However, in BPAP, diastolic blood pressure improved in the 50%success group as did quality of life, the latter just missing statistical significance. In ASV, quality of life and blood pressure improved significantly, but not CRP. Quality of life improved more distinctly in the group reaching AHI<5/h than in the 50%success group.
Table 11. Characteristics in the CPAP/APAP group.
| Total n = 407 | Mild SRBD | Moderate SRBD | Severe SRBD | p-value |
|---|---|---|---|---|
| (AHI≥ 5 to <15/h) | (AHI 15 to <30/h) | (AHI ≥30/h) | ||
| n | 81 (20%) | 127 (31%) | 199 (49%) | |
| CRP | 2.0 [1.1–4.2] | 2.5 [1.1–5.3] | 3.4 [1.7–6.6] | 0.004 severe vs moderate |
| <0.001 severe vs mild | ||||
| 0.299 moderate vs mild | ||||
| Art. Hypertension yes | 64 (19.3%) | 102 (30.8%) | 165 (49.8%) | 0.648(yes vs no) |
| Art. Hypertension no | 16 (21.9%) | 25 (34.2%) | 32 (43.8%) | 0.648(yes vs no) |
| BMI | 30.2 [27.7–33.5] | 30.8 [27.4–35.1] | 34.7 [31.1–38.8] | <0.001 severe vs moderate |
| <0.001 severe vs mild | ||||
| 0.455 moderate vs mild |
Table 16. Changes of CRP, QoL, systolic and diastolic blood pressure and BMI in the ASV group for successfully treated patients.
| Success AHI<5/h | Success50% | |
|---|---|---|
| CRP | p = 0.326 | p = 0.072 |
| QoL | p = 0.022 | p = 0.004 |
| BPsyst | p = 0.027 | p = 0.004 |
| BPdiast | p<0.001 | p<0.001 |
| BMI | p = 0.017 | p = 0.017 |
Table 12. Changes of CRP, QoL, systolic and diastolic blood pressure and BMI in the CPAP/APAP group for successfully treated patients.
| Success AHI<5/h | Success50% | |
|---|---|---|
| CRP | p = 0.007 | p = 0.011 |
| QoL | p<0.001 | p = 0.001 |
| BPsyst | p<0.001 | p<0.001 |
| BPdiast | p<0.001 | p<0.001 |
| BMI | p<0.001 | p<0.001 |
Table 13. Characteristics in the BPAP group.
| Total n = 32 | Mild SRBD | Moderate SRBD | Severe SRBD | p-value |
|---|---|---|---|---|
| (AHI≥ 5 to <15/h) | (AHI 15 to <30/h) | (AHI ≥30/h) | ||
| n | 6 (19%) | 6 (19%) | 20 (62%) | |
| CRP | 2.9 [2.0–4.5] | 6.0 [2.1–9.4] | 6.2 [2.9–9.4] | 0.976 severe vs moderate |
| 0.219 severe vs mild | ||||
| 0.485 moderate vs mild | ||||
| Art. Hypertension yes | 5 (17.2%) | 6 (20.7%) | 18 (62.1%) | 0.605 (yes vs no) |
| Art. Hypertension no | 1 (33.3%) | 0 (0%) | 2 (66.7%) | 0.605 (yes vs no) |
| BMI | 29.2 [25.6–33.5] | 40.3 [34.6–43.4] | 39.7 [33.3–44.6] | 1.000 severe vs moderate |
| 0.013 severe vs mild | ||||
| 0.041 moderate vs mild |
Table 14. Changes of CRP, QoL, systolic and diastolic blood pressure and BMI in the BPAP group for successfully treated patients.
| Success AHI<5/h | Success50% | |
|---|---|---|
| CRP | p = 0.538 | p = 0.161 |
| QoL | p = 0.320 | p = 0.058 |
| BPsyst | p = 0.936 | p = 0.657 |
| BPdiast | p = 0.083 | p = 0.012 |
| BMI | p = 0.165 | p = 0.178 |
Table 15. Characteristics in the ASV group.
| Total n = 148 | Mild SRBD | Moderate SRBD | Severe SRBD | p-value |
|---|---|---|---|---|
| (AHI≥ 5 to <15/h) | (AHI 15 to <30/h) | (AHI ≥30/h) | ||
| n | 12 (8%) | 38 (26%) | 98 (66%) | |
| CRP | 2.3 [1.7–5.4] | 2.1 [1.1–4.6] | 3.8 [1.7–6.9] | 0.042 severe vs moderate |
| 0.407 severe vs mild | ||||
| 0.682 moderate vs mild | ||||
| Art. Hypertension yes | 10 (7.8%) | 30 (23.3%) | 89 (69.0%) | 0.164(yes vs no) |
| Art. Hypertension no | 2 (10.5%) | 8 (42.1%) | 9 (47.4%) | 0.164(yes vs no) |
| BMI | 31.0 [27.4–33.6] | 30.4 [27.1–34.1] | 33.3 [29.4–37.3] | 0.026 severe vs moderate |
| 0.176 severe vs mild | ||||
| 0.946 moderate vs mild |
Results according to gender
Of all 716 patients, 512 were male (71.5%) and 204 female (28.5%). Most male patients had severe SRBD (50%), whereas only 22.9% had mild and 27.1% moderate SRBD, respectively.
Most female patients also had severe SRBD (46.9%), 26.5% had mild, and 29.2% moderate SRBD. The distribution differed significantly between male and female (p = 0.030).
Similar to the initial distribution with more male than female patients at baseline, the gender distribution according to treatment effect showed a higher percentage of male patients in all groups. However, treatment effects did not show statistically significant differences within the sexes (Table 17) with p = 0.597 in the group with final AHI<5/h and p = 0.342 in the 50%success group, respectively.
Table 17. Differences in gender within the groups of treatment effect.
| Treatment effect | Male | Female |
|---|---|---|
| AHI <5/h | 73% | 27% |
| AHI ≥ 5/h | 71% | 29% |
| Success50% | 72% | 28% |
| nonSuccess50% | 67% | 33% |
Initial and final CRP was higher in female patients. However, change was similar in female and male patients (Table 18).
Table 18. CRP within gender.
| Male | Female | p | |
|---|---|---|---|
| Initial CRP | 2.5 [1.2–5.2] | 3.8 [1.7–6.9] | 0.001 |
| Final CRP | 2.4 [1.1–4.6] | 2.6 [1.5–5.9] | 0.008 |
| Change of CRP | -0.2 [-1.4–.8] | -0.2 [-2.0–.65] | 0.656 |
Results with respect to medication and its changes
We analysed some major medication groups with possible influence on respiration, metabolic and cardiovascular parameters. Antihypertensive drugs and antidepressants comprised the highest percentage of medication used (Table 19). Significant changes occurred within groups of antihypertensive medication as well as in opioids, although the latter did not comprise a large group neither in the initial nor in the final examination (Table 20). Patients with continuous medication, those without, and those with changes (either medication added or removed) in the analysed medication groups had different results in CRP, systolic and diastolic blood pressure (Table 21). Interestingly, in a post hoc analysis of significant medication, angiotensin-receptor-blockers only influenced CRP, but not blood pressure parameters. However, this effect was apparent only in comparison of newly added ARB to no usage at all. Continuous medication with this substance did not lead to differences in comparison to no usage nor in comparison to newly added ARB (Table 22). ARB usage did not influence success rates, whereas betablockers, antidepressants, and central antihypertensives did (Table 23). Comparing the successful treatment group (AHI<5/h) with the non-successful group (AHI≥5/h), a higher percentage of patients with AHI<5/h never had betablockers (55.9% vs 48.7%) or had additional betablockers at the final PSG (6.6% vs 5.5%). Interestingly, in the success groups, a higher percentage of patients never had antidepressants (AHI<5/h: 77.9%) nor central antihypertensives (success50%: 93.2%).
Table 19. Number of patients receiving medication.
| Medication | Initial PSG | Final PSG |
|---|---|---|
| Betablockers | 284 (40%) | 302 (42%) |
| ACE-inhibitors | 235 (33%) | 255 (36%) |
| Angiotensin-receptor-blockers | 114 (16%) | 135 (19%) |
| Central antihypertensives | 35 (5%) | 37 (5%) |
| Antidepressants | 147 (21%) | 158 (22%) |
| Neuroleptics | 33 (5%) | 32 (5%) |
| Tranquilizer | 22 (3%) | 25 (4%) |
| Opioids | 13 (2%) | 23 (3%) |
| Antiparkinson therapy | 20 (3%) | 27 (4%) |
| Thyroid replacement therapy | 124 (17%) | 118 (17%) |
| PD5-Inhibitors | 2 (0%) | 3 (0%) |
| Endothelin-receptor-antagonists | 5 (1%) | 7 (1%) |
| Systemic Glucocorticoids | 2 (0%) | 4 (1%) |
Table 20. Change of medication from initial to final PSG.
| Medication | n | Initial | Final | added | removed | significance |
|---|---|---|---|---|---|---|
| Betablockers | 711 | 283 (39,8%) | 301 (42,3%) | 45 (6%) | 27 (4%) | 0.044 |
| ACE-inhibitors | 710 | 233 (32,8%) | 255 (35,9%) | 47 (7%) | 25 (4%) | 0.013 |
| Angiotensin-receptor-blockers | 712 | 114 (16,0%) | 135 (19,0%) | 39 (5%) | 18 (3%) | 0.008 |
| Central antihypertensives | 708 | 35 (4,9%) | 37 (5,2%) | 17 (2%) | 15 (2%) | 0.860 |
| Antidepressants | 711 | 144 (20,3%) | 158 (22,2%) | 31 (4%) | 17 (2%) | 0.059 |
| Neuroleptics | 706 | 32 (4,5%) | 32 (4,5%) | 8 (1%) | 8 (1%) | 1.000 |
| Tranquilizer | 711 | 22 (3,1%) | 25 (3,5%) | 11 (2%) | 8 (1%) | 0.648 |
| Opioids | 706 | 13 (1,8%) | 23 (3,3%) | 12 (2%) | 2 (0%) | 0.013 |
| Antiparkinson therapy | 710 | 20 (2,8%) | 27 (3,8%) | 14 (2%) | 7 (1%) | 0.189 |
| Thyroid replacement therapy | 706 | 122 (17,3%) | 117 (16,6%) | 9 (1%) | 14 (2%) | 0.405 |
| PD5-inhibitors | 704 | 2 (0,3%) | 2 (0,3%) | 1 (0%) | 1 (0%) | 1.000 |
| Endothelin-receptor-antagonists | 699 | 5 (0,7%) | 6 (0,9%) | 1 (0%) | 0 (0%) | 1.000 |
| Systemic Glucocorticoids | 699 | 2 (0,3%) | 4 (0,6%) | 4 (1%) | 2 (0%) | 0.687 |
Table 21. Differences in CRP, QoL, systolic and diastolic blood pressure and BMI in patients with continuous/no/removed/added medication.
| Medication | CRP | QoL | systolic | diastolic | BMI |
|---|---|---|---|---|---|
| Betablockers | 0.871 | 0.952 | 0.011 | 0.097 | 0.174 |
| ACE-inhibitors | 0.495 | 0.418 | 0.175 | 0.299 | 0.197 |
| Angiotensin-receptor-blockers | 0.025 | 0.673 | 0.362 | 0.254 | 0.647 |
| Central antihypertensives | 0.554 | 0.829 | 0.734 | 0.866 | 0.363 |
| Antidepressants | 0.436 | 0.225 | 0.113 | 0.002 | 0.332 |
Table 22. Post hoc analysis of significant medication groups.
| Medication | Post hoc | No vs added | Continuous vs added | No vs cont. | Cont. vs removed | No vs removed | Added vs removed |
|---|---|---|---|---|---|---|---|
| Betablockers | Systolic BP | p<0.001 | p = 0.009 | p = 0.497 | p = 0.825 | p = 0.540 | p = 0.101 |
| ARB | CRP | p = 0.005 | p = 0.060 | p = 0.191 | p = 0.966 | p = 0.564 | p = 0.303 |
| Antidepressants | Diastolic BP | p = 0.020 | p = 0.005 | p = 0.100 | p = 0.005 | p = 0.012 | p = 0.658 |
Table 23. Medication and success.
| Success AHI<5/h | Success50% | |
|---|---|---|
| Betablockers | 0.045 | 0.353 |
| ACE-inhibitors | 0.567 | 0.237 |
| Angiotensin-receptor-blockers | 0.816 | 0.052 |
| Central antihypertensives | 0.206 | 0.031 |
| Antidepressants | 0.039 | 0.158 |
Discussion
Our data are in accordance to previous studies (16, 17, 27, 28), describing an association of SRBD with inflammation. Patients with severe SRBD had the highest BMI, highest CRP and HbA1c, highest BP, and a trend towards higher prevalence of atrial fibrillation, coronary artery disease, myocardial infarction, apoplexy and renal insufficiency, according to the hypothesis that SRBD causes or interacts with cardiovascular and metabolic sequelae. Lack of statistical significance concerning the differences in co-morbidities (AF, CAD, Apo, renal insufficiency) regarding different levels of SRBD severity may be due to the fact that most patients were referred by respiratory specialists, whereas only a few were referred by cardiologists, nephrologists, and neurologists, which may skew the prevalence of associated co-morbidities.
During SRBD specific therapy, patients did not improve their BMI. Nevertheless, patients with AHI ≥30/h at baseline showed a significant reduction in CRP without changes in their metabolic parameters (BMI, HbA1c, FPG, TG, LDL, Chol). This suggests that sleep apnoea specifically caused increased CRP values which were corrected by specific therapy. This is in accordance to the previous findings by Arnardottir et al. [27] who concluded that CRP levels were independently associated with SRBD severity with a greater impact of obstructive sleep apnoea on inflammatory biomarkers in obese patients. Our results suggest that severity of SRBD has a greater influence on inflammatory biomarkers than weight, however, the average of all groups in our study was obese (29.2kg/m², 30.2kg/m², 34.6kg/m²). Improvement of SRBD had an impact on CRP only in patients with an initial AHI ≥30/h, even in the absence of weight loss or improvement of BMI. The findings of Punjabi &Beamer [28] support the improvement of CRP independently of BMI.
In previous studies that failed to find an obesity-independent association between SRBD and CRP [29, 30, 31], patients with severe co-morbidities were excluded. Thus, only early stages of influence of SRBD on cardiovascular diseases via inflammation may have been covered. The inflammatory impact of SRBD may be manifest and detectable only in more advanced disease, though. The same may be true for a study where unstable cardiovascular disease was an exclusion criteria and the basis of data collection was a mailed survey [32]. Both facts may have led to the exclusion of patients with more advanced diseases. This is reflected by the overall percentage of cardiovascular disease of only 5.3% in that study.
In our study, the analysis of SRBD treatment with regard to success (AHI <5/h) yielded a significant decrease of CRP along with treatment success. Results for HbA1c and FPG were controversial. However, CRP improvement occurred despite increase in BMI. This strongly supports the causal role of SRBD for systemic inflammation and the improvement of inflammatory biomarkers with SRBD therapy, independent of BMI. The influence on cardiovascular disorders is reflected by the overall and significant decrease of BP levels. These findings are in accordance to previous findings [20, 21, 33, 34, 35]. Others were not able to support these findings [36], but they used CPAP for only four weeks, which may be too short to yield beneficial effects. In our analysis, the period of time between initial and final PSG was considerably longer, but not the same for all patients. Although success rate regarding success with AHI<5/h differed with time, it did not differ with regard to 50%success. Therefore, we conclude that the difference in AHI over the given time period was still close to AHI<5/h. Nevertheless, time period had no influence on CRP, improvement of AHI yielded improvement of CRP regardless of time, given a median time period close to 12 months.
Previous studies on CRP in SRBD as comparison are outlined in Table E and Table F in S1 File [16–19, 21, 22, 27–33, 35, 37–41].
Inflammatory markers improved if success was defined as improvement of AHI of at least 50%, but without a significant difference to non-success. Apparently reduction of AHI, even markedly, without reaching the limits of normal is not sufficient to influence markers of inflammation. This suggests that only normalisation of AHI may prevent inflammatory activation.
We analysed subtypes of SRBD as well as different treatment groups. The results for OSAS as well as for CPAP/APAP are in accordance to the overall findings. However, CSAS and other treatment forms (BPAP and ASV) did not show statistical significance in all the examined parameters. But these results must be interpreted with caution, as the groups of CSAS as well as these of BPAP or ASV were considerably smaller than those of OSAS or CPAP/APAP, respectively.
Percentage of male patients was higher in the overall study population according to known prevalence in general population [2]. As a consequence, the portion of male patients remained higher in all groups. However, treatment effect did not differ between male and female patients.
The analysis of medication usage and changes yielded controversial results which should be interpreted with caution as the analysis was done post hoc and data regarding medication were not available for all patients at both time points. Not surprisingly, addition of betablockers influenced systolic blood pressure. However, changes in other antihypertensive drugs did not. Addition of antidepressants had a similar effect on diastolic blood pressure. Interestingly, a higher percentage of successfully treated SRBD patients never had antidepressants or central antihypertensives. But as the non-success groups did not have a higher percentage of these medications, a direct link to success or non-success of SRBD treatment cannot be assumed.
Addition of ARB had an impact on CRP. However, continuous treatment with ARB or their removal did not. So again, an association is difficult to establish. A multiplied effect of newly added ARB and SRBD treatment could be possible.
The only constant result in all analyses seems to be a direct link between SRBD and CRP. This offers a possibility to influence consequences of inflammation such as cardiovascular disease with SRBD treatment. Thus, despite the relationship of obesity and SRBD [42], SRBD must be addressed on its own irrespective of weight and weight changes. This must be done thoroughly, as only a tight control of SRBD with reaching normal limits of AHI seems to be able to reduce the inflammatory burden due to SRBD.
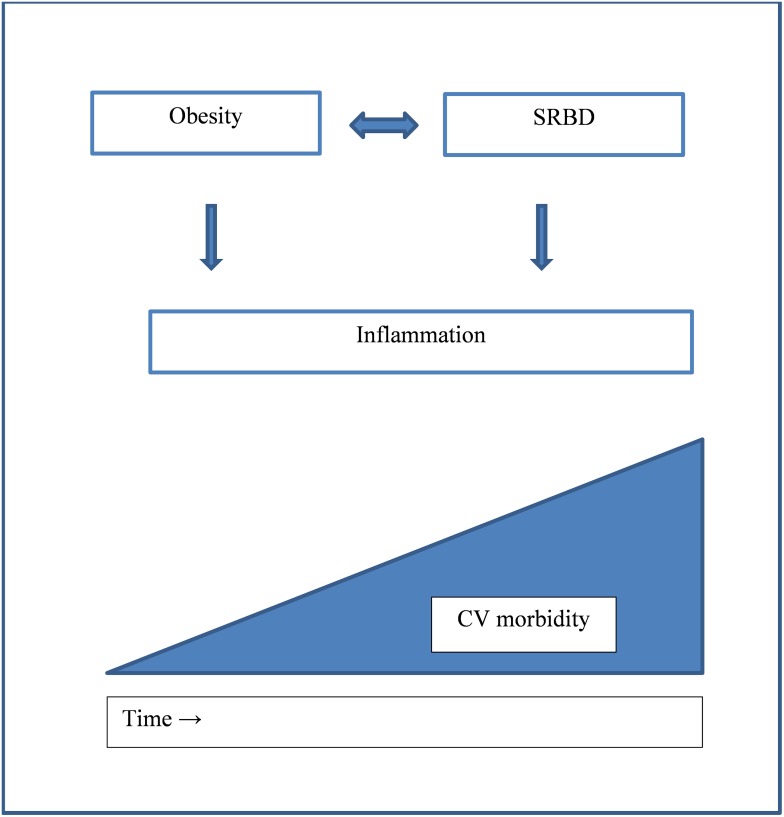
The more distinct findings of association of SRBD and CRP in patients with cardiovascular co-morbidities warrant a higher awareness of SRBD and the necessity of normalization of AHI in these conditions, regarding SRBD not as a side-effect, but as the backbone of morbidity and mortality. On the other hand, our findings point out the aggravating effect of SRBD in its natural course and therefore emphasize the need for early treatment to prevent cardiovascular sequelae (Fig 4).
Fig 4. Relationship of SRBD with CRP, obesity, and cardiovascular morbidity.
Limitations
This study was conducted as a retrospective analysis of data and is therefore prone to confounding. However, the data had been prospectively collected in a comprehensive database and were based on polysomnography rather than polygraphy. We included all patients who fulfilled the inclusion criteria and all patients went through the follow-up, only few data were missing. Therefore, the bias may be rather small. We did not investigate a control group with a sham SRBD therapy and compared just patients based on their baseline SRBD severity and their response to SRBD therapy. This precludes the proof of a causal relationship between SRBD and systemic inflammation. However, we show some evidence that makes such a causal relationship appear very likely.
We do not consider the lack of stratification or exclusion of co-morbidities as a limitation but rather as strength of our study. Only this approach allowed for our findings in more severely affected patients and reflects everyday life and conditions.
We did not use hs-CRP analysis although the median CRP level was within normal limits. Employing hs-CRP might have led to more distinct values. Nevertheless, we found a statistically significant association which speaks in favour of a robust effect of SRBD on inflammation.
As non-success groups were distinctly smaller than success groups, comparability might have been impaired and significant differences between these groups may have been missed.
The same is true for the CSAS subgroups as well as BPAP and ASV subgroups.
Conclusion
CRP is associated with SRBD independently of obesity. SRBD specific therapy (PAP in any form, positional therapy) can reduce inflammatory processes if normalisation of AHI and associated parameters is achieved. The association of CRP and SRBD is more distinct in severe SRBD and in patients with SRBD-related co-morbidities. As SRBD seems to act independently on morbidity and mortality, a higher awareness and effective treatment of SRBD is essential to prevent and stabilize or ameliorate cardiovascular morbidity.
Supporting Information
Table A. Values at initial and final PSG. Table B. Comparison of success (AHI<5/h) and non-success group. Table C. Values in the non-success group at initial and final PSG. Table D. Comparison success (50% reduction) to non-success. Table E. Studies on CRP in SRBD. Table F. Studies on effect of CPAP.
(DOCX)
Acknowledgments
Special thanks to Gabor Kovacs, Division of Pulmonology, Department of Internal Medicine, Medical University Graz, Austria, and Ludwig Boltzmann Institute for Lung Vascular Research, Graz, Austria, for critical revision of the manuscript.
Special thanks to Markus Dominco, Division of Pulmonology, Department of Internal Medicine, Medical University Graz, Austria, for invaluable help with data management.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1. Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009. January; 373 (9657): 82–93. Epub 2008 Dec 26. doi: 10.1016/S0140-6736(08)61622-0 [DOI] [PubMed] [Google Scholar]
- 2. Young T, Palta M, Dempsey J, Peppard PE, Nieto FJ, Hla KM. Burden of sleep apnea: rationale, design, and major findings of the Wisconsin Sleep Cohort study. WMJ. 2009; 108(5): 246. [PMC free article] [PubMed] [Google Scholar]
- 3. International Classification of Sleep Disorders, 3rd ed, American Academy of Sleep Medicine, Darien, IL: 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005; 365: 1046–53. [DOI] [PubMed] [Google Scholar]
- 5. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of association between sleep-disordered breathing and hypertension. NEJM. 2000; 342: 1378–84. [DOI] [PubMed] [Google Scholar]
- 6. Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Nieto FJ, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001; 163:19–25. [DOI] [PubMed] [Google Scholar]
- 7. The Emerging Risk Factors Collaboration. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010; 375: 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. [DOI] [PubMed] [Google Scholar]
- 9. Lagrand WK, Visser CA, Hermens WT, Niessen HW, Verheugt FW, Wolbink GJ, et al. C-reactive protein as a cardiovascular risk factor: more than an epiphenomenon? Circulation. 1999; 100:96–102. [DOI] [PubMed] [Google Scholar]
- 10. Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102:2165–68. [DOI] [PubMed] [Google Scholar]
- 11. Torzewski M, Rist C, Mortensen R. C-reactive protein in the arterial intima: role of C-reactive protein receptor-dependent monocyte recruitment in atherogenesis. Arterioscler Thromb Vasc Biol. 2000; 20:2094–98. [DOI] [PubMed] [Google Scholar]
- 12. Zwaka TP, Hombach V, Torzewski J. C-reactive protein-mediates low density lipoprotein uptake by macrophages: implications for atherosclerosis. Circulation. 2001; 103:1194–97. [DOI] [PubMed] [Google Scholar]
- 13. Hatipoglu U, Rubinstein I. Inflammation and obstructive sleep apnea syndrome pathogenesis: a working hypothesis. Respiration. 2003; 70(6):665–71. [DOI] [PubMed] [Google Scholar]
- 14. McNicholas WT. Obstructive sleep apnea syndrome and inflammation. ProgCardiovasc Dis. 2009; 51(5):392–99. [DOI] [PubMed] [Google Scholar]
- 15. Quercioli A, Mach F, Montecucco F. Inflammation accelerates atherosclerotic processes in obstructive sleep apnea syndrome (OSAS). Sleep Breath. 2010; 14(3):261–269. [DOI] [PubMed] [Google Scholar]
- 16. FiratGuven S, Turkkani MH, Ciftci B, Ciftci TU, Erdogan Y. The relationship between high-sensitivity C-reactive protein levels and the severity of obstructive sleep apnea. Sleep Breath. 2012; 16:217–21. [DOI] [PubMed] [Google Scholar]
- 17. Lui MM, Lam JC, Mak HK, Xu A, Ooi C, Lam DC. et al. C-reactive protein is associated with obstructive sleep apnea independent of visceral obesity. Chest. 2009; 135:950–56. [DOI] [PubMed] [Google Scholar]
- 18. Shamsuzzaman AS, Winnicki M, Lanfranchi P, Wolk R, Kara T, Accurso V, et al. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation. 2002; 105:2462–2464. [DOI] [PubMed] [Google Scholar]
- 19. Yao M, Tachibana N, Okura M, Ikeda A, Tanigawa T, Yamagishi K, et al. The relationship between sleep-disordered breathing and high-sensitivity C-reactive protein in Japanese men. Sleep. 2006; 29(5):661–665. [PubMed] [Google Scholar]
- 20. Ishida K, Kato M, Kato Y, Yanagihara K, Kinugasa Y, Kotani K, et al. Appropriate use of nasal continuous positive airway pressure decreases elevated C-reactive protein in patients with obstructive sleep apnea. Chest. 2009; 136:125–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schiza SE, Mermigkis C, Panagiotis P, Bouloukaki I, Kallergis E, Tzanakis N, et al. C-reactive protein evolution in obstructive sleep apnoea patients under CPAP therapy. Eur J Clin Invest. 2010; 40(11): 968–75. [DOI] [PubMed] [Google Scholar]
- 22. Yokoe T, Minoguchi K, Matsuo H, Oda N, Minoguchi H, Yoshino G, et al. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation. 2003; 107:1129–34. [DOI] [PubMed] [Google Scholar]
- 23. Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006; 17(1): 4–12. [PubMed] [Google Scholar]
- 24. Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011; 121(6): 2111–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sahlman J, Miettinen K, Peuhkuinen K, Seppä J, Peltonen M, Herder C, et al. for the Kuopio Sleep Apnoea Group The activation of inflammatory cytokines in overweight patients with mild obstructive sleep apnea. J Sleep Res. 2010; 19: 341–38. [DOI] [PubMed] [Google Scholar]
- 26. Iber C, Ancoli-Israel S, Chesson A,Quan SF for the American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, 1st ed: Westchester, Illinois: American Academy of Sleep Medicine, 2007. [Google Scholar]
- 27. Arnardottir ES, Maislin G, Schwab RJ, Staley B, Benediktsdottir B, Olafsson I, et al. The interaction of obstructive sleep apnea and obesity on the inflammatory markers C-reactive protein and interleukin-6: The Iclandic Sleep Apnea Cohort. Sleep. 2012; 35(7):921–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Punjabi NM, Beamer Ba. C-reactive protein is associated with sleep disordered breathing independent of adiposity. Sleep. 2007; 30(1): 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guilleminault MD, Kirisoglu C, Ohayon MM. C-reactive protein and sleep-disordered breathing. Sleep. 2004; 27(8): 1507–11. [DOI] [PubMed] [Google Scholar]
- 30. Ryan S, Nolan GM, Hannigan E, Cunningham S, Taylor C, McNicholas WT. Cardiovascular risk markers in obstructive sleep apnoea syndrome and correlation with obesity. Thorax. 2007; 62:509–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Su MC, Chen YC, Huang KT, Wang CC, Lin MC, Lin HC. Association of metabolic factors with high-sensitivity C-reactive protein in patients with sleep-disordered breathing. Eur Arch Oto-Rhino-Laryngol. 2013; 270(2): 749–54. [DOI] [PubMed] [Google Scholar]
- 32. Taheri S, Austin D, Lin L, Nieto FJ, Young T, Mignot E. Correlates of C-reactive protein (CRP)–no association with sleep duration or sleep disordered breathing. Sleep. 2007; 30(8): 991–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Friedman M, Samuelson CG, Hamilton C, Fisher M, Kelley K, Joseph NJ, et al. Effect of continuous positive airway pressure on C-reactive protein levels in sleep apnea: a meta-analysis. Otolaryngology—Head and Neck Surgery. 2012; 147(3): 423–33. [DOI] [PubMed] [Google Scholar]
- 34. Nural S, Günay E, Halici B, Celik S, Ünlü M. Inflammatory processes and effects of continuous positive airway pressure (CPAP) in overlap syndrome. Inflammation. 2013; 36(1):66–74. [DOI] [PubMed] [Google Scholar]
- 35. Panoutsopoulos A, Kallianos A, Kostopoulos K, Seretis C, Koufogiorga E, Protogerou A, et al. Effect of CPAP treatment on endothelial function and plasma CRP levels in patients with sleep apnea. Med Sci Mont. 2012; 18(12):CR747–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kohler M, Ayers L, Pepperell JC, Packwood KL, Ferry B, Crosthwaite N, et al. Effects of continuous positive airway pressure on systemic inflammation in patients with moderate to severe obstructive sleep apnoea: a randomised controlled trial. Thorax. 2009; 64:67–73. [DOI] [PubMed] [Google Scholar]
- 37. Boudjeltia KZ, Van Meerhaege A, Doumit S, Guillaume M, Cauchie P, Brohée D, et al. Sleep Apnoea-Hypopnoea Index is an independent predictor of high-sensitivity C-reactive protein elevation. Respiration. 2006; 73:243–46. [DOI] [PubMed] [Google Scholar]
- 38. Can M, Açigöz S, Mungan G, Bayraktaroğlu T, Koçak E, Güven B, et al. Serum cardiovascular risk factors in obstructive sleep apnea. Chest. 2006; 129:233–37. [DOI] [PubMed] [Google Scholar]
- 39. Hayashi M, Fujimoto K, Urushibata K, Takamizawa A, Kinoshita O, Kubo K. Hypoxia-sensitive molecules may modulate the development of atherosclerosis in sleep apnoea syndrome. Respirology. 2006; 11:24–31. [DOI] [PubMed] [Google Scholar]
- 40. Kokturk O, Ciftci TU, Mollarecep E, Ciftci B. Elevated C-reactive protein levels and increased cardiovascular risk in patients with obstructive sleep apnea syndrome. Int Heart J. 2005; 46: 801–809. [DOI] [PubMed] [Google Scholar]
- 41. Saletu M, Nosiska D, Kapfhammer G, Lalouschek W, Saletu B, Benesch T, et al. Structural and serum surrogate markers of cerebrovascular disease in obstructive sleep apnea. J Neurol. 2006; 253: 746–52. [DOI] [PubMed] [Google Scholar]
- 42. Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000; 284(23): 3015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table A. Values at initial and final PSG. Table B. Comparison of success (AHI<5/h) and non-success group. Table C. Values in the non-success group at initial and final PSG. Table D. Comparison success (50% reduction) to non-success. Table E. Studies on CRP in SRBD. Table F. Studies on effect of CPAP.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.