Abstract
Aims
Diseases that abbreviate the cardiac action potential (AP) by increasing the strength of repolarizing transmembrane currents are highly arrhythmogenic. It has been proposed that optogenetic tools could be used to restore normal AP duration (APD) in the heart under such disease conditions. This study aims to evaluate the efficacy of an optogenetic treatment modality for prolonging pathologically shortened APs in a detailed computational model of short QT syndrome (SQTS) in the human atria, and compare it to drug treatment.
Methods and results
We used a human atrial myocyte model with faster repolarization caused by SQTS; light sensitivity was inscribed via the presence of channelrhodopsin-2 (ChR2). We conducted simulations in single cells and in a magnetic resonance imaging-based model of the human left atrium (LA). Application of an appropriate optical stimulus to a diseased cell dynamically increased APD, producing an excellent match to control AP (<1.5 mV deviation); treatment of a diseased cell with an AP-prolonging drug (chloroquine) also increased APD, but the match to control AP was worse (>5 mV deviation). Under idealized conditions in the LA (uniform ChR2-expressing cell distribution, no light attenuation), optogenetics-based therapy outperformed chloroquine treatment (APD increased to 87% and 81% of control). However, when non-uniform ChR2-expressing cell distribution and light attenuation were incorporated, optogenetics-based treatment was less effective (APD only increased to 55%).
Conclusion
This study demonstrates proof of concept for optogenetics-based treatment of diseases that alter atrial AP shape. We identified key practical obstacles intrinsic to the optogenetic approach that must be overcome before such treatments can be realized.
Keywords: Cardiac optogenetics, Short QT syndrome, Atrial arrhythmia, Computational modelling
What's new?
Our simulations indicate that optogenetic tools can be used to dynamically modulate action potential (AP) shape and duration in single atrial myocytes with high accuracy.
Optogenetics-based treatment can be used to correct pathological abbreviation of atrial APs caused by short QT syndrome. With uniform ChR2 expression and unattenuated illumination throughout the atria, the proposed approach can robustly restore APs to their non-diseased duration more efficiently than drug treatment.
Limitations at the organ level include poor light penetration in cardiac tissue and non-uniform distribution of light-sensitive cells in the atria. These constitute major barriers to the efficacy of light-based SQTS treatment, but advances in optogenetics and gene therapy are expected to overcome them in future.
Introduction
The emerging field of optogenetics has provided the research community with a new research tool: a population of ion channels that can be dynamically controlled by pulses of light. Optogenetics is based on a simple premise: light-sensitive proteins (opsins) can be expressed in mammalian tissue by gene transfer1 or cell delivery.2 When illuminated, opsins undergo a conformational change, enabling them to conduct transmembrane current that can polarize the cell membrane. While the main application of optogenetics has been in the field of neuroscience, cardiac applications have also come into focus over the last few years.3 Optogenetics-based solutions have been proposed as low-energy alternatives to electrical stimulation for cardiac pacing4 and cardioversion;5,6 these methods would involve direct illumination of opsin-expressing heart cells to produce regions of membrane depolarization. Another idea, which has been discussed previously,7 but not yet studied, is to utilize optogenetic tools to control the shape and duration of the cardiac action potential (AP). Diseases that affect AP duration (APD) are difficult to treat and lead to potentially lethal arrhythmias,8 making the idea of optogenetics-based alleviation of their effects attractive. In this study, we use biophysically detailed computer simulations to assess the feasibility of this novel therapeutic approach.
As a proof-of-concept case study, in this paper we simulate optogenetics-based treatment of short QT syndrome (SQTS). First reported in 2000,9 SQTS is a genetic disorder characterized by shortened APs throughout the atria and ventricles; the electrocardiographic signature of this syndrome is an abbreviated QT interval. The genetic basis of SQTS is highly heterogeneous—there are numerous possible causes of AP abbreviation, including gain-of-function mutations in genes that encode for channels that conduct the inward rectifier (IK1) and rapid delayed rectifier (IKr) potassium currents and L-type calcium channel loss-of-function mutations.10,11 Consequences of SQTS include atrial fibrillation (AF) and ventricular arrhythmias leading to sudden cardiac death.10 Currently, the treatment of choice is prophylactic implantable cardioverter-defibrillator (ICD) implantation; however, the complication rate for this procedure is extremely high in SQTS patients (58%, as reported by Giustetto et al.12). Moreover, many SQTS patients are children (≥25%, based on estimates of the paediatric13 and overall14 SQTS cohort sizes), in whom ICD implantation is challenging15 and incidence of inappropriate shocks is high.13 Drug treatment by hydroquinidine or quinidine is an alternative to prophylactic electrotherapy, but it is only effective in a subset of SQTS patients.12,14 Overall, there is a clear need for innovation in SQTS therapy.
Accordingly, the aim of this study is to ascertain whether optogenetics-based SQTS treatment focusing on the atria can lead to phenotype rescue of the abnormal AP under disease conditions (i.e. restoration to control length). Shortcomings in existing treatments indicate that a novel approach could prove to be of significant benefit. We perform in silico experiments with a physiologically accurate atrial myocyte model to study dynamic, light-based APD modulation using a realistic description of light-sensitive current. Then, we use a detailed three-dimensional (3D) geometrical model of the human left atrium (LA) to investigate whether restoration of length to the pathologically shortened SQTS AP can be achieved at the organ scale. To ascertain the relative efficacy of light therapy compared with drug-based approaches to APD elongation, we also simulate treatment with chloroquine, which has been proposed as a candidate drug for SQTS.16 Finally, we investigate how the optogenetics-based treatment modality would be affected by practical limitations such as light attenuation in cardiac tissue and heterogeneous opsin distribution resulting from sub-optimal opsin gene expression rates.
Methods
Human atrial models
Optogenetics-based APD modulation simulations were conducted at two different spatial scales. First, a realistic human atrial myocyte model was used to assess the feasibility of shaping the AP using controlled light pulses; then, a human LA model constructed from high-resolution magnetic resonance imaging images acquired in vivo from an AF patient17 was used to determine whether strategies developed at the cell level could be extended to the organ scale. We chose to focus on the atria because the relative thinness of the atrial walls compared with those of the ventricles allowed for optical stimulation of a greater proportion of tissue.
In both single myocyte and LA models, AP dynamics were governed by the Grandi–Pandit–Voigt (GPV) formulation,18 as modified by Deo et al.11 for the purpose of studying SQTS. Briefly, GPV sodium current (INa) was replaced by the formulation from the Luo–Rudy dynamic model19 (necessary to achieve propagating APs in atrial tissue models) and GPV IK1 was replaced by a ‘wild-type’ IK1 formulation based on new measurements made by Deo et al.11 We applied further modifications20 to simplify the Ca2+ and Na+ state buffers, a change that reduced computational complexity, enabling us to execute organ-level simulations in a reasonable amount of time without affecting the AP shape or repolarization characteristics,20 which were the focus of our study. Four unique electrophysiological model variants were used to represent healthy, diseased, and light- or drug-treated APs:
Control: AP represented by the modified GPV model, as described above.
Short QT syndrome (SQTS): AP same as control but with a mutant IK1 formulation based on measurements from an SQTS patient.11 This mutation was shown to abolish inward rectification (i.e. outward IK1 was radically increased at positive membrane potentials), causing premature AP repolarization.
Light-treated SQTS: AP same as SQTS, but with a detailed model of light-sensitive current mediated by channelrhodopsin-2 (ChR2)21 added to other membrane currents. Blue-light illumination (470 nm) of ChR2-expressing cells is known to elicit a strong depolarizing current when the membrane potential is below the ChR2 reversal potential (∼0 mV);21 thus, we surmised that a timely light pulse applied during the AP plateau phase could counteract the early repolarization due to increased IK1 in SQTS.
Drug-treated SQTS: AP same as SQTS, but with the addition of chloroquine, modelled as a voltage-dependent IK1 blocker.16 The assumed intracellular concentration of chloroquine was 1.8 μM, which is a typical level after administration of a standard clinical dosage.22
At the organ level, the propagation of electrical excitation in the LA was modelled by the monodomain formulation using the finite element method in the Cardiac Arrhythmia Research Package (CARP) software.23–25 Atrial tissue was assigned an effective isotropic conductivity value of 0.27 S/m to achieve a physiologically realistic conduction velocity of 62 cm/s.26 The average time required to simulate 1 s of activity in the LA model was 69 min on a high-performance computing cluster using 40 Intel Xeon CPU cores clocked at 2.83 GHz.
Simulation protocol
For each of the four AP cases described above, the single-cell model was pre-paced with nine electrical stimuli [strength: 60 μA/cm2, duration: 2 ms, basic cycle length (BCL): 1000 ms] to attain steady state (i.e. insignificant beat-to-beat changes in repolarization and APD properties). To identify an optimal light-based treatment for SQTS, the shape and duration of the optical pulse that elicited the ChR2 current were iteratively adjusted to optimize AP phenotype rescue (i.e. modification of the AP to approach the control AP). Root-mean-square deviation (RMSD) was used for quantitative comparison of AP properties between model variants:
where Vm,X(t) was the transmembrane voltage for variant X (SQTS, light-treated, or drug-treated) at a given time t during the AP, which was defined as the interval between onset of stimulation (t = 0) and the APD at 90% repolarization for the control AP (tAPD90 = 249 ms). Lower RMSD values indicated a better match between the AP in question and the control AP.
We conducted simulations with the LA model using the same four electrophysiological model variants as at the single-cell level. In each simulation, the LA was pre-paced four times to attain steady state, as described above. Sinus activation was simulated by pacing from the inter-atrial septum (strength: 60 μA/cm2, duration: 10 ms, BCL: 1000 ms). Action potential duration was calculated as the time interval from activation to repolarization events, which were defined, respectively, by the threshold crossings Vm>−35 mV and Vm<−60 mV; these values were chosen because reduction of IK1 in the drug-treated case caused abnormal elevation of resting potential.
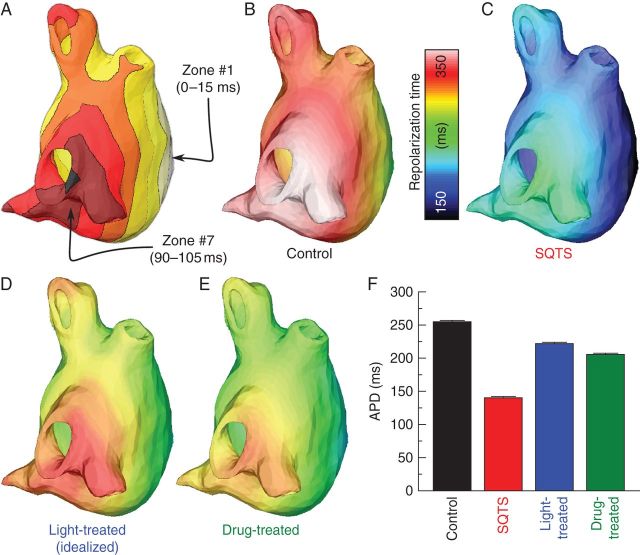
For simulations of light-based SQTS treatment in the LA model, the optimal light stimulus identified in the single-cell model was used; however, as atrial regions activated and consequently repolarized at different times during sinus rhythm, it would not have been possible to properly lengthen all APs throughout the LA with a single optical stimulus. Thus, we devised a multi-pulse illumination protocol. We divided the LA into eight zones based on the timing of excitation in the SQTS simulation; light delivery was controlled for each zone independently. Each zone, consisting of cells that activated during a particular 15 ms interval (0–15 ms, 15–30 ms, etc.), received illumination at a corresponding time (t = 0, 15 ms, etc.). These zones are shown in Figure 2A. The timing of the light stimulus for each zone was thus synchronized with the onset of local activation to ensure that the pathological repolarization due to increased IK1 was adequately counteracted. We assumed uniform endocardial illumination of a prescribed irradiance (Ee) within each of the above-described zones.
Figure 2.
Left atrium model simulation results. (A) Division of LA into eight zones based on activation times in the SQTS case; illumination onset in each zone was adjusted to coincide with local activation. (B–E) Left atrium repolarization times for control, SQTS, light-treated, and drug-treated cases. The light-treated case (D) shows results from simulations with idealized optogenetic conditions (uniform ChR2 distribution, no light attenuation). Repolarization times are measured with respect to the onset of pacing from the inter-atrial septum. (F) Comparison of APDs (mean ± standard deviation) for cases shown in (B–E).
Modelling practical limitations of optogenetics
For light treatment, we first modelled idealized optogenetics conditions (uniform ChR2 distribution, no light attenuation); then, we performed three additional simulations with one or more constraints based on practical limitations of optogenetics: non-uniform ChR2 distribution, light attenuation effects, or both. The purpose of this approach was to dissect the individual and combined impacts of each practical limitation.
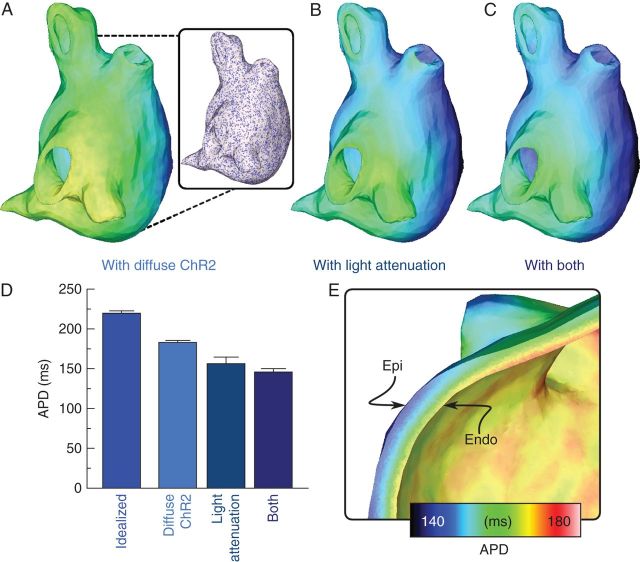
Previous analysis has suggested that the long-term result of in vivo cardiac gene therapy is a non-uniform distribution of transgene-expressing cells in the heart.27 To assess the impact of non-uniformity in ChR2 distribution on the efficacy of the proposed treatment, we tested two different spatial distributions of ChR2-expressing cells. First, we assumed uniform distribution with ChR2 expressed in all atrial myocytes, as a best-case model of gene delivery. Secondly, we used a previously described stochastic approach7 to generate a model with non-uniform light-sensitive cell distribution in the LA. The distribution algorithm was controlled by parameters for density (D) and patchiness (P);7 we used D = 0.4 and P = 0.95 to produce a model with ChR2 expression in 40% of the LA by volume with a diffuse spatial pattern (see Figure 3A), which approximates distributions observed in vivo.27
Figure 3.
Effects of practical limitations on optogenetics treatment in the SQTS LA. (A–C) Left atrium repolarization times (same scale as Figure 2) in the light-treated LA in cases of non-uniform distribution of light-sensitive cells, the presence of light attenuation, or both. Inset in (A) shows the model-generated pattern of ChR2-expressing cells (purple) and normal myocytes (white). (D) Comparison of APDs (mean ± standard deviation) for cases shown in (A–C); APD for idealized light-treated case (Figure 2D) is also shown. (E) Cutaway view showing transmural APD gradients for the light-treated LA with representation of light attenuation (B).
As light penetrates the atrial walls, it attenuates due to light scattering and tissue absorption.7 To model light attenuation, we used a depth-dependent exponential approximation to the steady-state solution of the photon diffusion equation for blue-light illumination of cardiac tissue (penetration depth δ = 570 μm),28 as described previously.7 The maximum irradiance of applied light was Ee = 0.2 mW/mm2, which was within the range typically used in optogenetics experiments.21
Results
Atrial myocyte simulation results
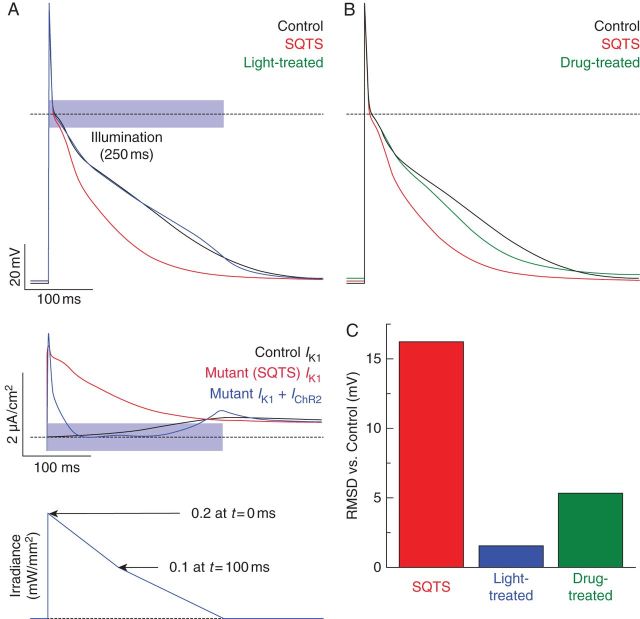
The SQTS AP was nearly identical to the control AP (compare red and black traces in top panel of Figure 1A) during the upstroke and notch phases (approximately t = 0–15 ms); Vm reduction was much faster in the early repolarization phase due to mutant IK1 properties. During the early phases of the AP, IK1 was large and positive because of the deranged channel behaviour (red trace in middle panel of Figure 1A); this was consistent with current–voltage relationships reported in the original SQTS study.11 Optogenetics-based treatment prolonged the SQTS-affected AP and produced a phenotype that closely resembled the control case (blue traces in top panel of Figure 1A). When light treatment was added, the combined effect of mutant IK1 and IChR2 approximated the control trace IK1 (compare blue and black traces in lower panel of Figure 1A). There was a significant deviation in the first 30 ms after AP onset due to the fact that IChR2 was very weak when Vm was near the ChR2 reversal potential (EChR2 ∼0 mV); however, this deviation did not result in a meaningful difference between light-treated and control APs, which is consistent with the earlier observation that pathologically reduced IK1 had little impact on early stages of the AP. The light-treated AP was elicited by an illumination pulse that was optimized, as described in the Methods section, by an iterative adjustment of its duration and shape (bottom panel of Figure 1A). The pulse began with a rapid rise (at t = 0) to peak irradiance (Ee = 0.2 mW/mm2), followed by a linear decrease to Ee = 0.1 mW/mm2 over the first 100 ms. Then, over the next 150 ms, there was a slower linear decrease to Ee = 0.
Figure 1.
Single-cell simulation results. (A) Top: Action potentials for the control (black), SQTS (red), and light-treated SQTS (blue) model variants. Dashed line indicates Vm = 0. Middle: Comparison of IK1 as a function of time for the control and SQTS model variants and IK1 + IChR2 for the light-treated case. Bottom: Optimized illumination pulse used to lengthen APD for light-treated case in top panel. (B) Same as top panel of (A) but with chloroquine-treated AP (green) instead of light-treated. (C) Quantitative comparison between control AP and APs for other model variants; lower values indicate a better match (see the text).
Chloroquine treatment also resulted in an AP similar to control (compare green and black traces in Figure 1B); however, Vm during repolarization deviated by up to 8 mV and the resting Vm was ∼2.2 mV higher. Quantitative comparison (Figure 1C) of untreated, light-treated, and drug-treated SQTS APs to the control AP confirmed that optogenetics-based therapy in a single cell produced superior AP phenotype rescue compared with chloroquine therapy.
Organ-level simulation results
Under control conditions, repolarization (Figure 2B) occurred later throughout the LA as compared with the case of SQTS (Figure 2C); average APD was markedly longer (250.5 ± 1.5 vs. 137.6 ± 0.9 ms for SQTS). Idealized light-based treatment (triggered local illumination sequence based on Figure 2A, optimal single-cell optical pulse properties as in Figure 1A, uniform ChR2 distribution, no light attenuation) successfully restored length to APs throughout the LA (Figure 2D), resulting in average APD values near control levels (218.4 ± 1.2 ms). Chloroquine therapy (assuming intracellular chloroquine concentrations of 1.8 μM throughout the LA) similarly delayed repolarization times (Figure 2E), although the average APD value was not as close to the control as in the light-treated case (202.5 ± 1.2 ms). A comparison of the APDs for each of the above cases is shown in Figure 2F. Activation times did not greatly vary between the four electrophysiological cases.
We then assessed how practical constraints of optogenetics-based therapy affected the efficacy of light treatment for SQTS. The quality of AP phenotype rescue throughout the LA was significantly decreased when a non-uniform spatial distribution of ChR2-expressing cells was incorporated in the atrial model; the pattern of light-sensitive cells, generated as described in the Methods section, is shown in the inset of Figure 3A. When the same optogenetics treatment protocol used in Figure 2D was applied to this version of the LA model, repolarization occurred earlier throughout the LA (Figure 3A) and average APD was only 180.7 ± 3.0 ms, which was lower than both the idealized light-treated and drug-treated cases shown (see Figure 2F). When light attenuation was simulated, the reduced efficacy of optogenetics treatment was even more dramatic because the irradiance of the light delivered to each LA point decreased exponentially with distance from the illuminated endocardial surface. This resulted in earlier repolarization times compared with all previously simulated light therapy configurations, particularly in sub-epicardial cells (Figure 2B); average APD was dramatically decreased (155.0 ± 8.5 ms). In the case where both practical limitations (non-uniform ChR2 distribution and light attenuation) were simulated, repolarization was further abbreviated (Figure 3C) and average APD was even shorter (144.8 ± 3.5 ms). A comparison of the average APDs throughout the LA for each of the above cases is shown in Figure 3D.
Finally, in both cases where light attenuation was included, differences in Ee across the LA gave rise to marked transmural APD gradients. The progressive decrease in light-induced APD lengthening with distance from the endocardium is highlighted in Figure 3E, which is a cutaway view of transmural APD distribution for the simulation highlighted in Figure 3B; a similar effect was observed for Figure 3C. Notably, even in cells close to the endocardium where attenuation was negligible, APD was reduced compared with values observed for unattenuated illumination—this was due to the strong electrotonic load imposed on these regions by faster-repolarizing cells deeper in the LA that were beyond the reach of the illumination applied at the endocardium.
Discussion
The aim of this study was to explore the therapeutic use of a light-sensitive ion channel as a means of manipulating cardiac APs at both the single cell and organ scales. To this end, we conducted biophysically detailed computational simulations in realistic cell- and organ-scale human atrial models to evaluate the efficacy of using optogenetic treatment to lengthen pathologically shortened atrial APs in SQTS patients. We compared light-based SQTS therapy to a drug-based alternative and we identified key limitations related to light penetration and spatial distribution of ChR2 transgene expression. Our major findings are as follows:
Optogenetic tools can be used to dynamically modulate AP shape and duration in atrial myocytes with a high degree of accuracy;
With uniform ChR2 expression and unattenuated illumination throughout the LA, optogenetics-based treatment to correct pathological AP abbreviation caused by IK1 gain-of-function mutation can robustly restore APs to their non-diseased duration more efficiently than drug treatment;
However, limitations related to poor blue-light penetration in cardiac tissue and possible non-uniform distribution of light-sensitive cells in the genetically modified LA are major barriers to the efficacy of optogenetics-based SQTS treatment.
Devising therapies for SQTS alternative to chloroquine drug treatment is of major clinical significance since pharmacological therapy for SQTS has numerous downsides. Pharmacological block of IK1 with chloroquine causes an increase in resting Vm in addition to the intended effect of APD lengthening. This side effect could have additional arrhythmogenic consequences unrelated to APD because depolarized resting potential leads to impaired excitability by reducing the number of available INa channels.29 Although we implemented a model of chloroquine which only simulates its effects on IK1, chloroquine also affects a number of currents other than IK1, including IKr and INa,30 so its use could lead to other unwanted changes in AP properties. Finally, according to clinical studies,22 a chloroquine therapy can cause a number of side effects. Thus, an optogenetic intervention would be an attractive alternative to drug therapy for SQTS.
Our modelling results demonstrate that light-based treatment under idealized conditions can achieve results superior to chloroquine drug-based treatment. Indeed, the atria are intrinsically a very attractive target for optogenetics-based treatment due to the fact that the atrial AP lacks a plateau that is prominently above 0 mV. Following the upstroke, Vm swiftly drops back below 0 mV, which is the range in which ChR2 channel activation results in membrane-depolarizing inward current. This means that light-sensitive current can be induced, that can compensate for the strong outward current from mutant IK1 channels in SQTS. In contrast, analogous optogenetics-based prolongation of ventricular APs to correct for this type of SQTS would be impossible due to the fact that intrinsic membrane voltage in ventricular cells stays well above zero (∼+30 mV) during the plateau phase;31 in this range, light-sensitive current would be outward and negligible in amplitude due to the reversal potential and rectification properties of ChR2, respectively.21 In addition, atrial wall thickness is considerably smaller than ventricular, which partially mitigates limitations due to light attenuation.
In our study, simulation of optogenetic treatment of the LA entailed the use of a light source capable of delivering multiple independent illumination pulses to different endocardial regions to ensure synchronization between the onset of optical stimuli and the onset of local activations. Notably, there is a tradeoff between the number of illumination zones and the organ-scale restoration of APD. Within each zone, there is intrinsic variation of activation times; however, all cells repolarize simultaneously when light stimulation ends, leading to local APD variability. Reducing the number of zones would result in a wider range of activation times within each zone, exacerbating local APD variability and leading to poorer approximation of control APs.
In practice, the light delivery system modelled here might be implemented with a series of implantable thin-film ribbons adhered to the atrial endocardium with light-emitting diode lights evenly distributed on each ribbon. We have previously speculated5 that optoelectronics on thin, flexible membranes as shown by Kim et al.32 could be used for this purpose. Appropriate stimulation also depends critically on knowledge of the atrial activation sequence. Light stimulation is sufficient to initiate excitation; therefore, inappropriately timed stimuli could easily lead to premature atrial activations. Real-time activation detection can be utilized to avert this issue. We speculate that a closed-loop detection/illumination system could be achieved using 3D-printed multifunctional integumentary membranes, as recently described by Xu et al.;33 these would detect local myocardial activation and deliver appropriately timed and spatially coordinated illumination pulses to a distributed set of target points.
Our study illustrates that light attenuation is a major limiting factor for optogenetics-based therapies seeking to modulate AP properties throughout the atria. The loss of optical energy due to absorption and photon scattering in the atrial wall leads to transmural APD gradients and potentially arrhythmogenic differences in repolarization times. This marked APD gradient occurs because atrial tissue thickness, which ranges between 2 and 6 mm, is large compared with both the penetration depth for blue light (δ = 570 μm) and the space constant for cardiac tissue (<1 mm).34 One potential approach to overcome this issue is the development of new opsins that generate larger currents in response to light of a wavelength that penetrates deeper into cardiac tissue (higher δ), diminishing the effect of attenuation. Efforts in this direction have already begun;35,36 for example, the ReaChR opsin is sensitive to red light (∼2× greater penetration depth in cardiac tissue than blue light) and elicits a dramatically increased photocurrent in response to illumination (IReaChR ∼2–5× IChR2). Further research along this trajectory could enable exciting new applications of optogenetics in the atria and provide an important stepping stone towards clinical translation.
Another option to overcome limitations caused by light attenuation might be to greatly increase the strength of the applied light stimulus to ensure that even the deepest parts of the tissue would be adequately illuminated. In vitro cardiac optogenetics experiments have used light of irradiance up to 5.50 mW/mm2 21 to elicit depolarization in light-sensitive cells, but it remains to be seen whether stimulation with high irradiance light in vivo is feasible or safe. A preliminary study suggests that safety may not be a major concern because significantly higher-energy light (hundreds of mW/mm2) is required to cause thermal damage.37 We used a relatively low peak Ee value (0.2 mW/mm2) for simulations in the LA model because it achieved optimal phenotype rescue in single-cell simulations and because minimization of required light energy was seen as beneficial. Future model-based research could explore whether stronger light stimuli could be used to achieve better phenotype rescue at the organ scale than what we observed in this study.
Conclusions
This study is an initial assessment of a novel treatment modality based on dynamic reshaping of pathologically shortened APs in the atria using optogenetic tools. Although the potential for phenotype rescue is highly promising at the cell level, our analysis shows that therapeutic benefit at the organ scale is limited by practical factors associated with optogenetics (namely, light attenuation and non-uniform distribution of light-sensitive cells). Our results demonstrate that computational modelling provides a useful set of tools to identify benefits and shortcomings of such proposed therapeutic strategies. We are confident that such simulations will play a key role in steering the design and development of optogenetics-based tools for treating cardiac arrhythmia.
Conflict of interest: none declared.
References
- 1.Abilez OJ, Wong J, Prakash R, Deisseroth K, Zarins CK, Kuhl E. Multiscale computational models for optogenetic control of cardiac function. Biophys J. 2011;101:1326–34. doi: 10.1016/j.bpj.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jia Z, Valiunas V, Lu Z, Bien H, Liu H, Wang HZ, et al. Stimulating cardiac muscle by light: cardiac optogenetics by cell delivery. Circ Arrhythm Electrophysiol. 2011;4:753–60. doi: 10.1161/CIRCEP.111.964247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Entcheva E. Cardiac optogenetics. Am J Physiol Heart Circ Physiol. 2013;304:H1179–91. doi: 10.1152/ajpheart.00432.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruegmann T, Malan D, Hesse M, Beiert T, Fuegemann CJ, Fleischmann BK, et al. Optogenetic control of heart muscle in vitro and in vivo. Nat Methods. 2010;7:897–900. doi: 10.1038/nmeth.1512. [DOI] [PubMed] [Google Scholar]
- 5.Boyle PM, Entcheva E, Trayanova NA. See the light: can optogenetics restore healthy heartbeats? And, if it can, is it really worth the effort? Expert Rev Cardiovasc Ther. 2014;12:1–4. doi: 10.1586/14779072.2014.864951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ambrosi CM, Entcheva E. Optogenetics' promise: pacing and cardioversion by light? Future Cardiol. 2014;10:1–4. doi: 10.2217/fca.13.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyle PM, Williams JC, Ambrosi CM, Entcheva E, Trayanova NA. A comprehensive multiscale framework for simulating optogenetics in the heart. Nat Commun. 2013;4:2370. doi: 10.1038/ncomms3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Napolitano C, Bloise R, Monteforte N, Priori SG. Sudden cardiac death and genetic ion channelopathies: long QT, Brugada, short QT, catecholaminergic polymorphic ventricular tachycardia, and idiopathic ventricular fibrillation. Circulation. 2012;125:2027–34. doi: 10.1161/CIRCULATIONAHA.111.055947. [DOI] [PubMed] [Google Scholar]
- 9.Gussak I, Brugada P, Brugada J, Wright RS, Kopecky SL, Chaitman BR, et al. Idiopathic short QT interval: a new clinical syndrome? Cardiology. 2000;94:99–102. doi: 10.1159/000047299. [DOI] [PubMed] [Google Scholar]
- 10.Cross B, Homoud M, Link M, Foote C, Garlitski AC, Weinstock J, et al. The short QT syndrome. J Interv Card Electrophysiol. 2011;31:25–31. doi: 10.1007/s10840-011-9566-0. [DOI] [PubMed] [Google Scholar]
- 11.Deo M, Ruan Y, Pandit SV, Shah K, Berenfeld O, Blaufox A, et al. KCNJ2 mutation in short QT syndrome 3 results in atrial fibrillation and ventricular proarrhythmia. Proc Natl Acad Sci USA. 2013;110:4291–6. doi: 10.1073/pnas.1218154110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giustetto C, Schimpf R, Mazzanti A, Scrocco C, Maury P, Anttonen O, et al. Long-term follow-up of patients with short QT syndrome. J Am Coll Cardiol. 2011;58:587–95. doi: 10.1016/j.jacc.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 13.Villafane J, Atallah J, Gollob MH, Maury P, Wolpert C, Gebauer R, et al. Long-term follow-up of a pediatric cohort with short QT syndrome. J Am Coll Cardiol. 2013;61:1183–91. doi: 10.1016/j.jacc.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 14.Bjerregaard P, Gussak I. Short QT syndrome. In: Gussak I, Antzelevitch C, editors. Electrical Diseases of the Heart. London: Springer; 2013. pp. 569–81. [Google Scholar]
- 15.Vardas PE, Auricchio A, Blanc JJ, Daubert JC, Drexler H, Ector H, et al. Guidelines for cardiac pacing and cardiac resynchronization therapy: the Task Force for Cardiac Pacing and Cardiac Resynchronization Therapy of the European Society of Cardiology. Developed in collaboration with the European Heart Rhythm Association. Europace. 2007;9:959–98. doi: 10.1093/europace/eum189. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Izquierdo A, Ponce-Balbuena D, Ferrer T, Sachse FB, Tristani-Firouzi M, Sanchez-Chapula JA. Chloroquine blocks a mutant Kir2.1 channel responsible for short QT syndrome and normalizes repolarization properties in silico. Cell Physiol Biochem. 2009;24:153–60. doi: 10.1159/000233241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDowell KS, Vadakkumpadan F, Blake R, Blauer J, Plank G, MacLeod RS, et al. Methodology for patient-specific modeling of atrial fibrosis as a substrate for atrial fibrillation. J Electrocardiol. 2012;45:640–5. doi: 10.1016/j.jelectrocard.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grandi E, Pandit SV, Voigt N, Workman AJ, Dobrev D, Jalife J, et al. Human atrial action potential and Ca2+ model: sinus rhythm and chronic atrial fibrillation. Circ Res. 2011;109:1055–66. doi: 10.1161/CIRCRESAHA.111.253955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo CH, Rudy Y. A dynamic model of the cardiac ventricular action potential. I. Simulations of ionic currents and concentration changes. Circ Res. 1994;74:1071–96. doi: 10.1161/01.res.74.6.1071. [DOI] [PubMed] [Google Scholar]
- 20.Chang KC, Bayer JD, Trayanova NA. Disruption of ryanodine receptor kinetics drives alternans in human atrial fibrillation. Heart Rhythm. 2014;11:S167. [Google Scholar]
- 21.Williams JC, Xu J, Lu Z, Klimas A, Chen X, Ambrosi CM, et al. Computational optogenetics: empirically-derived voltage- and light-sensitive channelrhodopsin-2 model. PLoS Comput Biol. 2013;9:e1003220. doi: 10.1371/journal.pcbi.1003220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mzayek F, Deng H, Mather FJ, Wasilevich EC, Liu H, Hadi CM, et al. Randomized dose-ranging controlled trial of AQ-13, a candidate antimalarial, and chloroquine in healthy volunteers. PLoS Clin Trials. 2007;2:e6. doi: 10.1371/journal.pctr.0020006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vigmond EJ, Aguel F, Trayanova NA. Computational techniques for solving the bidomain equations in three dimensions. IEEE Trans Biomed Eng. 2002;49:1260–9. doi: 10.1109/TBME.2002.804597. [DOI] [PubMed] [Google Scholar]
- 24.Vigmond EJ, Hughes M, Plank G, Leon LJ. Computational tools for modeling electrical activity in cardiac tissue. J Electrocardiol. 2003;36(Suppl):69–74. doi: 10.1016/j.jelectrocard.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 25.Vigmond EJ, Weber dos Santos R, Prassl AJ, Deo M, Plank G. Solvers for the cardiac bidomain equations. Prog Biophys Mol Biol. 2008;96:3–18. doi: 10.1016/j.pbiomolbio.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krummen DE, Bayer JD, Ho J, Ho G, Smetak MR, Clopton P, et al. Mechanisms of human atrial fibrillation initiation: clinical and computational studies of repolarization restitution and activation latency. Circ Arrhythm Electrophysiol. 2012;5:1149–59. doi: 10.1161/CIRCEP.111.969022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prasad KM, Smith RS, Xu Y, French BA. A single direct injection into the left ventricular wall of an adeno-associated virus 9 (AAV9) vector expressing extracellular superoxide dismutase from the cardiac troponin-T promoter protects mice against myocardial infarction. J Gene Med. 2011;13:333–41. doi: 10.1002/jgm.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bishop MJ, Rodriguez B, Eason J, Whiteley JP, Trayanova N, Gavaghan DJ. Synthesis of voltage-sensitive optical signals: application to panoramic optical mapping. Biophys J. 2006;90:2938–45. doi: 10.1529/biophysj.105.076505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaw RM, Rudy Y. Electrophysiologic effects of acute myocardial ischemia: a theoretical study of altered cell excitability and action potential duration. Cardiovasc Res. 1997;35:256–72. doi: 10.1016/s0008-6363(97)00093-x. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez-Chapula JA, Salinas-Stefanon E, Torres-Jacome J, Benavides-Haro DE, Navarro-Polanco RA. Blockade of currents by the antimalarial drug chloroquine in feline ventricular myocytes. J Pharmacol Exp Ther. 2001;297:437–45. [PubMed] [Google Scholar]
- 31.O'Hara T, Virag L, Varro A, Rudy Y. Simulation of the undiseased human cardiac ventricular action potential: model formulation and experimental validation. PLoS Comput Biol. 2011;7:e1002061. doi: 10.1371/journal.pcbi.1002061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim RH, Kim DH, Xiao J, Kim BH, Park SI, Panilaitis B, et al. Waterproof AlInGaP optoelectronics on stretchable substrates with applications in biomedicine and robotics. Nat Mater. 2010;9:929–37. doi: 10.1038/nmat2879. [DOI] [PubMed] [Google Scholar]
- 33.Xu L, Gutbrod SR, Bonifas AP, Su Y, Sulkin MS, Lu N, et al. 3D multifunctional integumentary membranes for spatiotemporal cardiac measurements and stimulation across the entire epicardium. Nat Commun. 2014;5:3329. doi: 10.1038/ncomms4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Otani NF. Deep entry of defibrillating effects into homogeneous cardiac tissue. IEEE Trans Biomed Eng. 2004;51:401–7. doi: 10.1109/TBME.2003.820995. [DOI] [PubMed] [Google Scholar]
- 35.Klapoetke NC, Murata Y, Kim SS, Pulver SR, Birdsey-Benson A, Cho YK, et al. Independent optical excitation of distinct neural populations. Nat Methods. 2014;11:338–46. doi: 10.1038/nmeth.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin JY, Knutsen PM, Muller A, Kleinfeld D, Tsien RY. ReaChR: a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nat Neurosci. 2013;16:1499–508. doi: 10.1038/nn.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Welch AJ. The thermal response of laser irradiated tissue. IEEE J Quantum Electron. 1984;20:1471–81. [Google Scholar]