Abstract
Background
Observational studies and small intervention studies suggest alcohol raises gamma-glutamyltransferase (GGT). We used Mendelian randomization to assess the causal effect of alcohol use on GGT in older Chinese people.
Methods
An instrumental variable (IV) analysis in 2,321 men and 2,757 women aged 50+ years from phase 3 of the Guangzhou Biobank Cohort Study with ALDH2 (rs671) genotyped, alcohol use and GGT available was used to assess the causal effect of alcohol use on GGT. Rs671 was used as an IV and F-statistics was used to test for weak instrument hypothesis. An F-statistic of ≥10 indicates the IV is not weak.
Results
In men, the F-statistic for rs671 on alcohol use was 70. Using IV analysis alcohol use increased GGT by 10.60 U/L per alcohol unit (10 gram ethanol) per day (95% confidence interval (CI) 6.58 to 14.62). The estimate was lower in observational multivariate regression: 3.48 U/L GGT per alcohol unit per day (95% CI 2.84 to 4.11) adjusted for age, education, physical activity and smoking. In women, rs671 was not associated with alcohol or GGT and the F-statistic was 7 precluding IV analysis.
Conclusion
In Mendelian randomization, we found confirmative evidence that alcohol use increases GGT among Southern Chinese men. Moreover, we found that the ALDH2 variant rs671 was not associated with GGT among Southern Chinese women who generally consume very low levels of alcohol. Taken together our findings strongly suggest that alcohol increases GGT, although we cannot rule out the possibility that other unknown factors may cause a different relation between alcohol and GGT in other populations.
Introduction
Gamma-glutamyltransferase (GGT) has been widely used as a marker for alcohol use in epidemiologic studies [1]. Observational studies have shown a positive association of alcohol use with blood levels of GGT [2–6]. However, observational studies are prone to biases from confounding, and may not be well suited to evaluating the causal effects of alcohol use. Randomized placebo controlled trials have shown that effective treatments for alcohol dependence or abuse which reduce alcohol use also reduce serum GGT concentrations [7, 8]. However, whether the reduction in GGT was mediated by the reduction in alcohol use or was due to other aspects of the treatments is unclear. Short-term intervention studies based on very small selected samples have shown that alcohol use increases GGT [9–11] but the results may not be applicable to the general population and cannot confirm the health effect of long-term alcohol use.
Given the limitations of the observational studies and ethical concerns regarding the carcinogenic effects precluding large scale randomized controlled trials of alcohol use, whether the association of alcohol use with GGT is causal or due to residual confounding remains to be determined. Mendelian randomization (MR) takes advantage of genetic variants present from conception and allocated randomly according to Mendel’s second law [12, 13]. Gene variants determining alcohol use can be used in instrumental variable analysis to elucidate the causal effects of alcohol on health [14]. However, a recent large MR study using functional polymorphisms in the alcohol dehydrogenase gene (ADH1B) as a genetic determinant of alcohol use did not show an effect of alcohol on GGT [15], probably because ADH1B is not only associated with alcohol use but also with the speed of alcohol metabolism [16, 17], which means that MR studies using ADH1B as a genetic instrument for alcohol use may give biased estimates of the effect of alcohol because effects may be due to genetic variation in ADH1B rather than to alcohol. Instead, a genetic marker, aldehyde dehydrogenase 2 (ALDH2) with single nucleotide polymorphism (SNP) rs671, has been shown by us to be a credible genetic instrument for alcohol use in southern Chinese men [14]. People with slow metabolization of acetaldehyde due to inactive ALDH2 alleles may feel ill after drinking alcohol, and so in settings where alcohol use is discretionary tend to drink less. The ALDH2 polymorphism rs671 has previously been used by us and others in MR studies to assess the effects of moderate alcohol use on cardiovascular disease risk factors and cognitive function [18–20].
We hypothesized that higher alcohol used would increase GGT, and the effect is due to alcohol but not the genetic variation in ALDH2. In the Guangzhou Biobank Cohort Study, taking advantage of this unique Southern Chinese setting in which alcohol use is generally low to moderate and may reflect genetic differences among men [14], we used ALHD2 as a genetic instrument to obtain an unbiased estimate of the effect of alcohol use on GGT. We also assessed whether ALHD2 alleles were associated with GGT in Southern Chinese women who rarely use alcohol.[21] A lack of association in such women would indicate that any effects seen in men are due to alcohol but not genetics. Finally, for comparison we assessed the associations of alcohol use with GGT using multivariable regression adjusted for confounders based on the same participants in an observational study design.
Materials and Methods
Participants
The details of the Guangzhou Biobank Cohort Study (GBCS) and MR and alcohol related studies have been reported elsewhere [14, 18, 19, 22]. Briefly, GBCS is a 3-way collaboration of Guangzhou 12th Hospital and the Universities of Hong Kong and Birmingham, UK. The GBCS baseline examination was conducted in three phases from 2003 to 2008, and participants were then followed up from 2008 to 2012. Participants were recruited from “The Guangzhou Health and Happiness Association for the Respectable Elders” (GHHARE), a community social and welfare organization. GHHARE is unofficially aligned with the municipal government. Membership is open to Guangzhou permanent residents aged 50 years or above for a nominal fee of 4 CNY (≈50 US cents) per month. GHHARE included about 7% of Guangzhou residents in this age group, with branches in all 10 districts of Guangzhou, the capital city of Guangdong province in southern China. The health examination included interview concerning lifestyle, family and personal medical history and assessment of anthropometric and clinical factors. Information on socioeconomic position and lifestyle including age, sex, education, smoking and alcohol use was collected by a computer based standardized questionnaire. Alcohol use was recorded in terms of frequency, type of beverage and usual amount per occasion. GGT was only measured in phase 3. The Guangzhou Medical Ethics Committee of the Chinese Medical Association approved the study and all participants gave written, informed consent before participation.
DNA extraction and SNP analysis
Biological samples for DNA extraction used in the present study were obtained in GBCS phase 3 at baseline and in recruitment phases 1 and 2 at follow-up [14]. DNA was extracted at Guangzhou No. 12 Hospital either from fresh blood using a standard phenol-chloroform extraction procedure and stored at -80°C or from blood or buffy coat previously stored at -80°C using a standard magnetic bead extraction procedure. Genotyping of SNP rs671 to identify ALDH2 genotypes (AA, GA or GG) was performed using the MassARRAY system (Sequenom, San Diego, CA, USA) and the iPLEX assay at a commercial company (Beijing CapitalBio Corporation, Beijing, China). For logistic reasons genotyping for the three GBCS phases was performed at three different times, 953 men and 942 women had ALDH2 genotyped in 2010, 2,748 men had ALDH2 genotyped in 2011, then an additional 3,406 men and 1,535 women had ALDH2 genotyped in 2012.
Alcohol use
The exposure was continuous alcohol units (10 gram (g) ethanol) per day based on total alcohol consumption obtained from the frequency, quantity and type recorded at recruitment. Details of the assessment in alcohol use were reported previously [14, 23]. Specifically, we asked the participants how often they drank alcohol (once or twice per year, once every couple of months, <1 day/ week, 1–2 days/week, 3–4 days/week, 5–6 days/week, daily or almost every day), the type of alcohol usually consumed, and how much of each type of alcohol (beer, western grape wine, spirits, Chinese rice wine or Chinese spirits (high strength)) usually consumed per occasion, from which we calculated units per day. Participants who reported use of >30 alcohol units per day were considered as infeasible and were excluded [14]. Never drinkers were those who did not drink any alcoholic beverage throughout their life. Occasional drinkers were those who drank less than once per week, or drank only on special occasions, such as at a wedding party or festival, in the past 12 months. Moderate drinkers were people who drank at least once per week with less than or equal to 140 gram of ethanol for women and 210 gram of ethanol for men. Heavy drinkers were those who weekly drank more than 140 gram of ethanol in women and 210 gram of ethanol in men.[14] Former and occasional alcohol users were included as non-drinkers as reported previously [14]. We also conducted sensitivity analysis excluding former users and/or heavy users.
Outcome
Serum GGT concentrations was the outcome. GGT were measured by Shimadzu CL-8000 Clinical Chemistry Analyzer (Shimadzu, Kyoto, Japan).
Statistical analysis
We used linear regression to assess the strength of the association of ALDH2 variants (rs671) with alcohol units, from which we reported the F statistic and r2, to assess whether ALDH2 variants were associated with GGT and to assess whether the association was fully mediated by alcohol units. We used 2 stage least squares (2SLS) to estimate the possible causal effect of alcohol on GGT, i.e., the change in GGT per unit increase in alcohol intake per day. As a sensitivity analysis, we also used a 2-sample IV analysis in which the association between rs671 and alcohol use was estimated in participants with ALDH2 genotyped and the association of rs671 with GGT was estimated in participants from phase 3. The ratio of the estimates and the confidence interval were calculated by seemingly unrelated regression and the Wald estimator [24] using the “suest” common in STATA. We did not adjust for confounders in the instrumental variable analysis because ALDH2 genotypes randomly allocated at conception cannot be confounded by age or other environmental exposures. For comparison, we also present the associations of alcohol units with GGT under multivariable linear regression models adjusted for potential confounders including age, education, physical activity and smoking in the same participants. Few Southern Chinese women use alcohol [25]. ALDH2 alleles correspond poorly with alcohol use among women, so we would expect rs671 to be a poor instrument for alcohol use among women [14]. Hence we present sex-stratified analysis. A two-sided significance level of α = 0.05 was used. All statistical analysis was done using STATA/IC 13.1 (Stata Corp LP, College Station, TX, USA).
Results
Of 2,569 men and 7,519 women from phase 3 of GBCS, 2,321 (90%) men and 2,757 (37%) women with GGT and ALDH2 genotyping were included in the analysis. An additional sample of 2,631 men from phases 1 and 2 with ALDH2 genotyping but without GGT were included in a two-sample instrumental variable analysis.
About half of men (54%) and a minority of women (37%) were current alcohol users. Table 1 shows that in men ALDH2 was strongly associated with alcohol use, such that men with two alleles for fast metabolization of acetaldehyde consumed 1.1 units of alcohol per day compared with 0.09 units in men with two alleles for slow metabolism of acetaldehyde. However, for women the association was much weaker because alcohol consumption was much less common. Rs671 was not associated with age, education, smoking or physical activity in men or women. ALHD2 was associated with GGT in men but not in women.
Table 1. Alcohol consumption and socio-demographic characteristics by ALDH2 polymorphism rs671 in men and women from the Phase 3 of Guangzhou Biobank Cohort Study (2006–8).
| ALDH2 polymorphism rs671 | ||||
|---|---|---|---|---|
| Two inactive alleles (AA) | One inactive allele (GA) | No inactive alleles (GG) | †P value | |
| Men | ||||
| Number of participants | 220 | 971 | 1,130 | |
| Alcohol units, 10g ethanol per day, Geometric mean (95% CI) | 0.55 (0.08–3.96) | 1.28 (0.97–1.69) | 2.49 (2.17–2.87) | <0.001 |
| Gamma-glutamyltransferase, U/L, Geometric mean (95% CI) | 25.0 (23.4–26.6) | 26.1 (25.2–27) | 30.2 (29.1–31.4) | <0.001 |
| Age, years, Mean (SD) | 63.3 (7.7) | 63.8 (7.6) | 63.4 (7.5) | 0.40 |
| Education, % | ||||
| Primary or below | 25.5 | 30.4 | 27.6 | 0.45 |
| Middle School | 58.6 | 55.7 | 56.8 | |
| College or above | 15.9 | 13.9 | 15.6 | |
| Smoking status, % | ||||
| Never | 38.2 | 37.0 | 37.4 | 0.62 |
| Former | 29.6 | 25.5 | 26.8 | |
| Current | 32.3 | 37.5 | 35.8 | |
| Physical activity, % | ||||
| Inactive | 8.2 | 7.9 | 7.8 | 0.32 |
| Moderate | 28.6 | 33.5 | 35.8 | |
| Active | 63.2 | 58.6 | 56.4 | |
| Women | ||||
| Number of participants | 231 | 1,091 | 1,435 | |
| Alcohol units, 10g ethanol per day, Geometric mean (95% CI) | 0.31 (0.004–25.43) | 0.36 (0.24–0.54) | 0.48 (0.36–0.63) | 0.22 |
| Gamma-glutamyltransferase, U/L, Geometric mean (95% CI) | 20.2 (19.0–21.4) | 21.5 (20.8–22.2) | 20.4 (19.8–21.0) | 0.25 |
| Age, years, Mean (SD) | 59.9 (7.0) | 60 (7.3) | 60.1 (7.3) | 0.91 |
| Education, % | ||||
| Primary or below | 42.4 | 41.7 | 40.4 | 0.62 |
| Middle School | 50.7 | 50.1 | 52.6 | |
| College or above | 6.9 | 8.3 | 7.0 | |
| Smoking status, % | ||||
| Never | 98.7 | 97.2 | 97.8 | 0.37 |
| Former | 0.9 | 0.9 | 1.1 | |
| Current | 0.4 | 1.9 | 1.2 | |
| Physical activity, % | ||||
| Inactive | 4.8 | 5.9 | 7.8 | 0.11 |
| Moderate | 27.7 | 24.5 | 22.7 | |
| Active | 67.5 | 69.7 | 69.5 | |
†P-value from analysis of variance (ANOVA) for continuous variables and from a χ2 test for categorical variables, 2 sided; Alcohol unit and GGT were log-transformed before the ANOVA analysis.
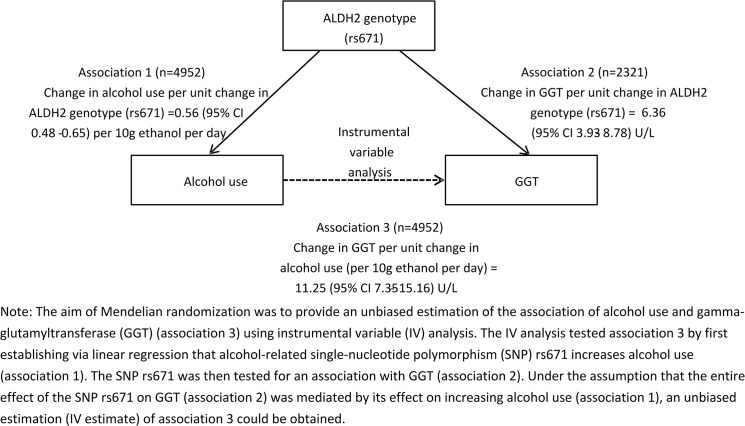
The F-statistic for the association of rs671 with alcohol use was 70 in men, indicating that rs671 is a valid instrument for alcohol use in men. The r2 for rs671 on alcohol use was 0.03 in men. Table 2 shows that, in men, alcohol use increased GGT in instrumental variable analysis (10.60 U/L per alcohol unit, 95% confidence interval (CI) 6.58 to 14.62). After excluding heavy and former drinkers, the estimate became larger (45.89 U/L per alcohol unit, 95% CI 23.65 to 68.13) (Table 2). Moreover, the estimate was larger in men aged <65 years (15.66 U/L per alcohol unit, 95% CI 8.22 to 23.10) than those aged 65+years (5.89 U/L per alcohol unit, 95% CI 1.53 to 10.24). In two-sample instrumental variable analysis, alcohol use also increased GGT in men (11.25 U/L per alcohol unit, 95% CI 7.35 to 15.16) (Fig 1). After excluding heavy and/or former users, alcohol still increased GGT in men (Table 2). Observational multivariate regression also showed a positive association of alcohol with GGT in men (3.48 U/L per alcohol unit, 95% CI 2.84 to 4.11).
Table 2. Associations of alcohol (per 10g ethanol per day) with gamma-glutamyltransferase (GGT, U/L) using a Mendelian randomization design and an observational multivariable linear regression analysis among participants from the Phase 3 of Guangzhou Biobank Cohort Study (2006–2008).
| Mendelian randomization instrumental variable analysis | Observational multivariable regression analysis † | |||||
|---|---|---|---|---|---|---|
| Selection | Number | F-statistic | β | 95% confidence interval | β | 95% confidence interval |
| Men | ||||||
| Total | 2,321 | 70 | 10.60 | 6.58 to 14.62 | 3.48 | 2.84 to 4.11 |
| Excluding heavy users | 2,155 | 51 | 49.34 | 25.3 to 73.39 | 3.65 | 0.55 to 6.75 |
| Excluding former users | 2,166 | 73 | 10.15 | 6.33 to 13.97 | 3.51 | 2.87 to 4.15 |
| Excluding heavy and former users | 2,000 | 54 | 45.89 | 23.65 to 68.13 | 3.78 | 0.67 to 6.90 |
| Women | ||||||
| Total | 2,757 | 6.8 | -15.1 | -75.61 to 45.42 | 0.99 | -1.98 to 3.95 |
| Excluding heavy users | 2,748 | 5.4 | -42.9 | -220.17 to 134.36 | 11.15 | 3.51 to 18.80 |
| Excluding former users | 2,610 | 7.2 | -9.35 | -66.5 to 47.81 | 0.94 | -2.04 to 3.93 |
| Excluding heavy and former users | 2,601 | 5.8 | -25.8 | -189.5 to 137.87 | 10.99 | 3.29 to 18.70 |
† Adjusted for age, education, physical activity and smoking.
Fig 1. Two-sample Mendelian randomization of alcohol use and gamma-glutamyltransferase (GGT) in the Guangzhou Biobank Cohort Study.
The F-statistic for the association of rs671 with alcohol use was 7 in women, indicating that rs671 is not a valid instrument for alcohol use in women in this population. The r2 for rs671 on alcohol use was 0.003. For completeness, Table 2 shows the instrumental variable and observational analysis for women.
Discussion
Using IV analysis in a setting where alcohol use may reflect genetic differences in men [14], genetically higher alcohol use was associated with higher GGT. In contrast, among women where alcohol use is much less clearly a reflection of genetic differences, because of social pressure for Southern Chinese women to abstain from alcohol [25], these same genetic variants were not associated with GGT. Taken together these findings indicate convincingly that alcohol use increases GGT.
Using ADH1B as a determinant for alcohol use, a recent Mendelian randomization study did not show a significant effect of alcohol on GGT in alcohol drinkers even with a first-stage F-statistic of 59 [15], probably because ADH1B is not only associated with alcohol use but also with the speed of alcohol metabolism [16, 17], which means that ADH1B may not only be associated with GGT through alcohol use but ADH1B may also be directly associated with GGT through alcohol processing speed thereby giving a biased estimate of the effect of alcohol use on GGT. As such using ADH1B as a genetic instrument for alcohol use may violate the exclusion restriction assumption in Mendelian randomization, i.e., no link between the genetic instrument and the health outcome other than via the exposure. Moreover, weak instrument bias for ADH1B also cannot be completely ruled out because the high F-statistic was mainly driven by the very large sample size (n = 58,313) with a weak association of ADH1B with alcohol use (r2 = 0.001). Another recent Mendelian randomization study using ADH1B as genetic determinant for alcohol use also failed to show a significant effect of alcohol on high-density lipoprotein cholesterol [26], although the effect of alcohol on high-density lipoprotein cholesterol has been confirmed in the most up-to-date meta-analysis of experimental studies [27], supporting and suggesting ADH1B may not be an adequate instrumental variable for alcohol use. Instead, ALDH2 has been shown to be an adequate and validated instrument (F statistic 75.1) for alcohol use in Southern Chinese men [14].
Using ALDH2 as an instrumental variable in the current Mendelian randomization study, we found that the genetic effect of a unit change in alcohol use on GGT in men was larger than the association seen in observational linear regression. There are several possible explanations. First, observational study designs may be subject to residual confounding that biases associations toward the null. For example, hepatitis C virus (HCV) infection leads to higher GGT [28] and individuals with HCV infection tend to reduce alcohol consumption. Genetically determined alcohol use should not be confounded, especially by confounders which may mask the association of alcohol with GGT [29]. Second, the stronger association in Mendelian randomization after excluding heavy users could be due to canalization and developmental adaptation [30]. The genetic effect of alcohol on GGT may be modified via compensatory responses to environmental influences, i.e. up-regulating ALDH2 gene expression, to protect heavy alcohol users from increased GGT. This would also attenuate the association of genetically determined alcohol use with GGT. Third, the Mendelian randomization estimate for the effect of a unit change in alcohol use on GGT depends on the assumption that alcohol use is assessed without random measurement error, otherwise the association of genetic variants with alcohol use will be understated and correspondingly the instrumental variable estimate inflated [31]. Alcohol use is notoriously difficult to measure accurately, which may explain the higher estimate for the instrumental variable analysis than seen in observational studies [32]. Given the limited experimental data, it is difficult to know whether the effect of alcohol on GGT seen here from Mendelan randomization differs from what would be expected from an intervention. [10, 33]. Nevertheless, our findings suggest that the effect of alcohol on GGT is probably larger than that usually observed.
There were several limitations for our study. First, Mendelian randomization studies may be prone to confounding from linkage disequilibrium (LD) and the existence of pleiotropy. However, as ALDH2 encodes mitochondrial ALDH2, the primary liver isoenzyme involved in the metabolism of acetaldehyde to acetate, ALDH2 variants are unlikely to affect GGT independent of alcohol use, or be in LD with gene(s) affecting GGT [14]. Notably, ALDH2 was not associated with GGT among the women in our sample who consume very little alcohol, suggesting ALDH2 is only associated with GGT via alcohol use. Second, ALDH2 mainly varies in East Asians. The effects of alcohol on health may vary with some as yet unknown differences between East Asians and other populations, although this is unlikely. Confounding by population stratification cannot be completely ruled out. However, our sample is restricted to permanent residents of one city in China, who are ethnically homogenous as it is difficult for internal migrants to obtain permanent residency. Our sample is not totally representative, however that would only create a bias if the association of ALDH2 with alcohol use was different in our sample than in the general population. Third, alcohol use was self-reported, but we have previously validated self-reported alcohol use against high density lipoprotein-cholesterol.[34] Finally, given the low prevalence of alcohol use in Southern Chinese women, Mendelian randomization analysis of alcohol use, may be less optimal for women in this setting. However, causal effects should be consistent across sex, unless there is a biological reason, such as hormonal effects, for effects varying by sex.
Conclusions
In conclusion, using Mendelian randomization, we found confirmative evidence for a direct causal effect of alcohol use on GGT. Our results underscore the value of Mendelian randomization to infer causality in observational epidemiology and to unravel underlying pathophysiologic mechanisms of alcohol-induced health effects.
Acknowledgments
This work was supported by the University of Hong Kong Foundation for Development and Research, Hong Kong; The University of Hong Kong University Research Committee Strategic Research Theme Public Health, Hong Kong; Guangzhou Public Health Bureau, and Guangzhou Science and Technology Committee, Guangzhou, China; and The University of Birmingham, Birmingham, UK. This sub-study was supported by the Hong Kong Health and Health Services Research Fund (Grant 06070981), the Bureau of Guangzhou Science and Technology (Grant 2013J4100031), the Key technology collaboration project funded by the Bureau of Guangzhou Science and Technology (Grant number: 2012J5100041) and Natural Science Foundation of Guangdong Province, China. The authors have indicated no financial conflicts of interest.
Data Availability
Due to ethical restrictions protecting patient privacy, data are available on request from the GBCS Data Access Committee. Please send requests for the data to gbcsdata@hku.hk.
Funding Statement
This work was supported by the University of Hong Kong Foundation for Development and Research, Hong Kong; The University of Hong Kong University Research Committee Strategic Research Theme Public Health, Hong Kong; Guangzhou Public Health Bureau, and Guangzhou Science and Technology Committee, Guangzhou, China; and The University of Birmingham, Birmingham, UK. This sub-study was supported by the Hong Kong Health and Health Services Research Fund (Grant 06070981), the Bureau of Guangzhou Science and Technology (Grant 2013J4100031), the Key technology collaboration project funded by the Bureau of Guangzhou Science and Technology (Grant number: 2012J5100041) and Natural Science Foundation of Guangdong Province, China. The authors have indicated no financial conflicts of interest.
References
- 1. Whitfield JB. Gamma glutamyl transferase. Critical reviews in clinical laboratory sciences. 2001;38:263–355. [DOI] [PubMed] [Google Scholar]
- 2. Lee DH, Ha MH, Kim JR, Gross M, Jacobs DR Jr. Gamma-glutamyltransferase, alcohol, and blood pressure. A four year follow-up study. Annals of epidemiology. 2002;12:90–6. [DOI] [PubMed] [Google Scholar]
- 3. Gluud C, Andersen I, Dietrichson O, Gluud B, Jacobsen A, Juhl E. Gamma-glutamyltransferase, aspartate aminotransferase and alkaline phosphatase as markers of alcohol consumption in out-patient alcoholics. European journal of clinical investigation. 1981;11:171–6. [DOI] [PubMed] [Google Scholar]
- 4. Papoz L, Warnet JM, Pequignot G, Eschwege E, Claude JR, Schwartz D. Alcohol consumption in a healthy population. Relationship to gamma-glutamyl transferase activity and mean corpuscular volume. JAMA: the journal of the American Medical Association. 1981;245:1748–51. [DOI] [PubMed] [Google Scholar]
- 5. Nishmura M, Teschke R. Effect of chronic alcohol consumption on the activities of liver plasma membrane enzymes: gamma-glutamyltransferase, alkaline phosphatase and 5'-nucleotidase. Biochemical pharmacology. 1982;31:377–81. [DOI] [PubMed] [Google Scholar]
- 6. Gjerde H, Amundsen A, Skog OJ, Morland J, Aasland OG. Serum gamma-glutamyltransferase: an epidemiological indicator of alcohol consumption? British journal of addiction. 1987;82:1027–31. [DOI] [PubMed] [Google Scholar]
- 7. Chick J, Anton R, Checinski K, Croop R, Drummond DC, Farmer R, et al. A multicentre, randomized, double-blind, placebo-controlled trial of naltrexone in the treatment of alcohol dependence or abuse. Alcohol and alcoholism. 2000;35:587–93. [DOI] [PubMed] [Google Scholar]
- 8. Persson J, Magnusson PH. Early intervention in patients with excessive consumption of alcohol: a controlled study. Alcohol. 1989;6:403–8. [DOI] [PubMed] [Google Scholar]
- 9. Belfrage P, Berg B, Cronholm T, Elmqvist D, Hägerstrand I, Johansson B, et al. Prolonged administration of ethanol to young, healthy volunteers: effects on biochemical, morphological and neurophysiological parameters. Acta medica Scandinavica Supplementum. 1972;552:1–44. [PubMed] [Google Scholar]
- 10. Frimpong NA, Lapp JA. Effects of moderate alcohol intake in fixed or variable amounts on concentration of serum lipids and liver enzymes in healthy young men. The American journal of clinical nutrition. 1989;50:987–91. [DOI] [PubMed] [Google Scholar]
- 11. Randell E, Diamandis EP, Goldberg DM. Changes in serum carbohydrate-deficient transferrin and gammaglutamyl transferase after moderate wine consumption in healthy males. Journal of clinical laboratory analysis. 1998;12:92–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smith GD, Ebrahim S. 'Mendelian randomization': can genetic epidemiology contribute to understanding environmental determinants of disease? International journal of epidemiology. 2003;32:1–22. [DOI] [PubMed] [Google Scholar]
- 13. Smith GD, Ebrahim S. Mendelian randomization: prospects, potentials, and limitations. International journal of epidemiology. 2004;33:30–42. [DOI] [PubMed] [Google Scholar]
- 14. Au Yeung SL, Jiang C, Cheng KK, Liu B, Zhang W, Lam TH, et al. Is aldehyde dehydrogenase 2 a credible genetic instrument for alcohol use in Mendelian randomization analysis in Southern Chinese men? Int J Epidemiol. 2013;42:318–28. 10.1093/ije/dys221 [DOI] [PubMed] [Google Scholar]
- 15. Lawlor DA, Benn M, Zuccolo L, De Silva NM, Tybjaerg-Hansen A, Smith GD, et al. ADH1B and ADH1C Genotype, Alcohol Consumption and Biomarkers of Liver Function: Findings from a Mendelian Randomization Study in 58,313 European Origin Danes. PloS one. 2014;9:e114294 10.1371/journal.pone.0114294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Macgregor S, Lind PA, Bucholz KK, Hansell NK, Madden PA, Richter MM, et al. Associations of ADH and ALDH2 gene variation with self report alcohol reactions, consumption and dependence: an integrated analysis. Human molecular genetics. 2009;18:580–93. 10.1093/hmg/ddn372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Salujha SK, Chaudhury S, Menon PK, Srivastava K, Gupta A. Allelic variants of ADH, ALDH and the five factor model of personality in alcohol dependence syndrome. Industrial psychiatry journal. 2014;23:44–51. 10.4103/0972-6748.144956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Au Yeung SL, Jiang CQ, Cheng KK, Liu B, Zhang WS, Lam TH, et al. Evaluation of moderate alcohol use and cognitive function among men using a Mendelian randomization design in the Guangzhou biobank cohort study. American journal of epidemiology. 2012;175:1021–8. 10.1093/aje/kwr462 [DOI] [PubMed] [Google Scholar]
- 19. Au Yeung SL, Jiang C, Cheng KK, Cowling BJ, Liu B, Zhang W, et al. Moderate alcohol use and cardiovascular disease from Mendelian randomization. PloS one. 2013;8:e68054 10.1371/journal.pone.0068054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Au Yeung SL, Jiang C, Cheng KK, Adab P, Lam KB, Liu B, et al. Aldehyde dehydrogenase 2—a potential genetic risk factor for lung function among southern Chinese: evidence from the Guangzhou Biobank Cohort Study. Annals of epidemiology. 2014;24:606–11. 10.1016/j.annepidem.2014.05.015 [DOI] [PubMed] [Google Scholar]
- 21. Wei H, Derson Y, Xiao S, Li L, Zhang Y. Alcohol consumption and alcohol-related problems: Chinese experience from six area samples, 1994. Addiction. 1999;94:1467–76. [DOI] [PubMed] [Google Scholar]
- 22. Jiang C, Thomas GN, Lam TH, Schooling CM, Zhang W, Lao X, et al. Cohort profile: The Guangzhou Biobank Cohort Study, a Guangzhou-Hong Kong-Birmingham collaboration. Int J Epidemiol. 2006;35:844–52. [DOI] [PubMed] [Google Scholar]
- 23. Jiang CQ, Xu L, Lam TH, Thomas GN, Zhang WS, Cheng KK, et al. Alcohol consumption and aortic arch calcification in an older Chinese sample: the Guangzhou Biobank Cohort Study. International journal of cardiology. 2013;164:349–54. 10.1016/j.ijcard.2011.07.046 [DOI] [PubMed] [Google Scholar]
- 24. Didelez V, Sheehan N. Mendelian randomization as an instrumental variable approach to causal inference. Statistical methods in medical research. 2007;16:309–30. [DOI] [PubMed] [Google Scholar]
- 25. Au Yeung SL, Jiang CQ, Zhang WS, Lam TH, Cheng KK, Leung GM, et al. Systematic differences among never, occasional and moderate alcohol users in southern China, and its use in alcohol research: a cross-sectional study. Journal of epidemiology and community health. 2013;67:1054–60. 10.1136/jech-2013-202807 [DOI] [PubMed] [Google Scholar]
- 26.Bouchard C. Genetic epidemiology association and sib-pair linkage: results from the Quebec Family Study. 1996. p. 470–81.
- 27. Brien SE, Ronksley PE, Turner BJ, Mukamal KJ, Ghali WA. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: systematic review and meta-analysis of interventional studies. BMJ. 2011;342:d636 10.1136/bmj.d636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Silva IS, Ferraz ML, Perez RM, Lanzoni VP, Figueiredo VM, Silva AE. Role of gamma-glutamyl transferase activity in patients with chronic hepatitis C virus infection. Journal of gastroenterology and hepatology. 2004;19:314–8. [DOI] [PubMed] [Google Scholar]
- 29. Steenland K, Deddens J, Salvan A, Stayner L. Negative bias in exposure-response trends in occupational studies: modeling the healthy workers survivor effect. American journal of epidemiology. 1996;143:202–10. [DOI] [PubMed] [Google Scholar]
- 30. Rutherford SL. From genotype to phenotype: buffering mechanisms and the storage of genetic information. BioEssays: news and reviews in molecular, cellular and developmental biology. 2000;22:1095–105. [DOI] [PubMed] [Google Scholar]
- 31. Schooling CM, Yeung SL, Freeman G. Mendelian Randomization Estimates May Be Inflated. Journal of the American College of Cardiology. 2013;61:1931. [DOI] [PubMed] [Google Scholar]
- 32. Nakajima T, Ohta S, Fujita H, Murayama N, Sato A. Carbohydrate-related regulation of the ethanol-induced increase in serum gamma-glutamyl transpeptidase activity in adult men. The American journal of clinical nutrition. 1994;60:87–92. [DOI] [PubMed] [Google Scholar]
- 33. Freer DE, Statland BE. The effects of ethanol (0.75 g/kg body weight) on the activities of selected enzymes in sera of healthy young adults: 1. Intermediate-term effects. Clinical chemistry. 1977;23:830–4. [PubMed] [Google Scholar]
- 34. Schooling CM, Jiang CQ, Lam TH, Zhang WS, Cheng KK, Leung GM. Alcohol use and fasting glucose in a developing southern Chinese population: the Guangzhou Biobank Cohort Study. Journal of epidemiology and community health. 2009;63:121–7. 10.1136/jech.2008.077602 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Due to ethical restrictions protecting patient privacy, data are available on request from the GBCS Data Access Committee. Please send requests for the data to gbcsdata@hku.hk.